- *Corresponding Author:
- Yanli Du
Department of Neurosurgery, Hulunbuir People’s Hospital, Hulunbuir, Inner Mongolia 021000,China
E-mail: lilili8908@163.com
This article was originally published in a special issue, “Recent Developments in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(4) Spl Issue “220-226” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Aneurysmal subarachnoid hemorrhage is a severe neurological disease that is characterized by acute onset, multiple complications and poor prognosis. The aim of this study was to analyze the relationship between the expression level of micro ribonucleic acid-30c in plasma and prognosis in patients with aneurysmal subarachnoid hemorrhage. A total of 181 patients with aneurysmal subarachnoid hemorrhage were prospectively selected as study subjects. Modified Rankin scale was used to evaluate the prognosis of the subjects and detecting the level of micro ribonucleic acid-30c by real-time fluorescence quantitative polymerase chain reaction to analyze the relationship between micro ribonucleic acid-30c and prognosis. The levels of micro ribonucleic acid-30c in poor prognosis group (T0-micro ribonucleic acid-30c, T1-micro ribonucleic acid-30c and T2-micro ribonucleic acid-30c) were significantly lower than those in good prognosis group (p<0.05). The logistic regression analysis showed that the T2-micro ribonucleic acid-30c was an independent protective factor for the prognosis of aneurysmal subarachnoid hemorrhage patients (p<0.05). The results of restricted cubic spline fitting logistic regression analysis showed that T2-micro ribonucleic acid-30c had a non-linear relationship with the prognosis of patients with aneurysmal subarachnoid hemorrhage. The level of micro ribonucleic acid-30c in patients with aneurysmal subarachnoid hemorrhage is closely related to their prognosis. The low level of T2-micro ribonucleic acid-30c suggested a higher risk of poor prognosis.
Keywords
Aneurysmal subarachnoid hemorrhage, micro ribonucleic acid-30c, modified Rankin scale, prognosis
Aneurysmal Subarachnoid Hemorrhage (aSAH) is a disease with high morbidity, disability and mortality, which threatens the life of patients seriously[1,2]. Although there is some progress in treatment of aSAH in recent years, the prognosis of patients with aSAH is still not improved significantly. Its 1 y mortality rate is around 50 % and only 33.3 % of those who survived are able to live independently and only about 25 % of those who recovered fully[3]. Studies showed that prognostic indicators for patients with aSAH were important for clinicians to make treatment plans and improve prognosis. At present, most biomarkers have been found to be used in assessing the prognosis of aSAH, such as periosteal protein[4] and peptide[5]. However, there were some deficiencies in the above indicators that the sensitivity or specificity of prognosis assessment was low.
Micro Ribonucleic acid (miRNA) is a single-stranded RNA which consists of 18~24 nucleotides. It does not have the function of coding protein, but it can regulate the process of cell proliferation, apoptosis and necrosis[6]. Many miRNAs had been shown to be involved in the pathogenesis and progression of aSAH, such as miR- 15a[7], miR-132-3p[8] and miR-324-3p[8]. The miR-30c gene, as a member of miRNA family, has been showed to down-regulate the expression of Nod-Like Receptor Protein-3 (NLRP3) to inhibit cell apoptosis[9-11]. NLRP3 played a key role in the aseptic inflammatory response which is induced by intracerebral hemorrhage and it could aggravate the inflammatory response[12]. In addition, miR-30c was involved in the development and functional regulation of the brain and Cerebral Ischemia/Reperfusion (CI/R) injury[13-15]. Based on the above studies, it could be speculated that miR-30c was involved in brain injury that is induced by aSAH. The level of miR-30c may be an indicator of prognosis. Therefore, this study was carried out in order to provide a reference for prognostic evaluation of patients with aSAH.
Materials and Methods
Materials:
A total of 181 patients with aSAH that were admitted to the First Affiliated Hospital of Baotou Medical College, Inner Mongolia University of science and technology from October 2019 to September 2021 were prospectively selected as study subjects. They were diagnosed and treated of aSAH according to “Chinese guidelines for diagnosis and treatment of subarachnoid hemorrhage 2019”[16].
Inclusion criteria: Age ranges from 18-70 y; the aSAH was confirmed by Computed Tomography Angiography (CTA) or digital subtraction angiography; admission to hospital for surgery or interventional treatment within 12 h of onset immediately and family signed an informed consent form.
Exclusion criteria: Subarachnoid hemorrhage caused by nonaneurysmal rupture; aSAH induced by trauma, infection; severe coagulopathy, platelet count below 100×109/l, the international standard ratio is higher than 1.4 or there were obvious signs of bleeding; there were serious heart, liver, kidney and other organ dysfunction; pregnant or lactating women; vitals were unstable; previous stroke or other neurological disease.
Among the 181 patients with aSAH, 70 were males and 111 were females, the age ranged from 22 to 64 (42.80±8.27) y. The study was approved by the medical ethics committee of the First Affiliated Hospital of Baotou Medical College, Inner Mongolia University of science and technology.
Clinical data collection:
Collecting the data of all subjects in the group, the indicators were age, sex, Body Mass Index (BMI), history of disease (hypertension, hyperlipidemia, diabetes), location of aneurysm, Hunt-Hess grade, Fisher grade, cerebral vasospasm (average flow rate of middle cerebral artery>120 cm/s and ipsilateral Lindegaard index≥3), treatment (craniotomy clipping, endovascular intervention), Glasgow Coma Scale (GCS) and C-Reactive Protein (CRP), etc.
MiR-30c level detection:
The level of miR-30c in the plasma was detected by real-time fluorescent quantitative Polymerase Chain Reaction (PCR)[17]. 10 ml peripheral venous blood samples were collected at three different times, that before treatment (T0), after treatment (T1) and 1 w after treatment (T2). The samples were centrifuged and the supernatant was taken and stored in the refrigerator at -80° for examination. Total RNA was extracted with TRIzol (Takara, Japan, no. 9108- 1), then reverse-transcribed into complementary Deoxyribonucleic Acid (cDNA) by 6011A PrimeScriptTM 1st strand cDNA synthesis kit (Takara, Japan 6110A-1). Amplification of cDNA products was done by PCR. The miR-30c sequence, Forward (F): 5’-GCCGCTGTAAACATCCTACACT-3’;Reverse(R): 5’-GTGCAGGGTCCGAGGT-3’; internal reference U6 sequence, F: 5’-CTCGCTTCGGCAGCACA-3’; R: 5’-AACGCTTCACGAATTTGCGT-3’. Blood samples were taken from 20 healthy people of the same age and sex, and the mean level of miR-30c expression in the healthy plasma was used as the corrected control. The expression level of the miR-30c was measured by 2-ΔΔCt.
Follow-up and prognostic evaluation:
The subjects were followed by outpatient and telephone visits to know their prognosis within 3 mo. The follow- up was taken once a month and the deadline for it was January 21, 2022. The prognosis was assessed with the modified Rankin Scale (mRS). It scored 1 point: The patients had mild symptoms, no significant disability and could complete daily activities by themselves; 2 points were scored: They were mildly disabled and able to manage daily life on his own, but they were unable to fully engage in pre-illness activities; 3 points were scored: Moderately disabled, able to walk alone, but needs help with other complex daily activities; 4 points were scored: Severely disabled, cannot walk alone and other daily activities need to be looked after; 5 points were scored: Severe disability, patients continue to stay in bed, urinary and fecal incontinence, need continuous bedside care. 6 points indicates death. A score of 0~2 was regarded as a good prognosis and a score of 3~6 was considered to be a poor prognosis[18].
Statistical methods:
R3.6.3 software was used to analyze the data. Measurement data of normal distribution was expressed as the mean±Standard Deviation (SD), independent sample t test was used for comparison between the two groups; repeated measure data were analyzed by repeated measure variance. The count data was expressed as number of subjects [n (%)], Chi square (χ2) test was used for intergroup comparison. Evaluation of the efficiency of miR-30c for diagnosing the prognosis of patients with aSAH was done by Receiver Operating Characteristic (ROC) curve. The restricted cubic spline fitting logistic regression analysis was used to analyze the relationship between miR-30c and the prognosis of the patients. Lasso regression was used to screen the risk factors of the prognosis of the patients. The p<0.05 was considered as statistically significant.
Results and Discussion
Comparison of baseline data of two groups was explained here. Patients with aSAH were divided into two groups according to their prognosis: Poor prognosis group (n=49) and good prognosis group (n=132). The age, Hunt-Hess Grade IV+V, Fisher Grade III+IV, cerebral vasospasm, GCS and CRP in the poor prognosis group were higher than those in the good prognosis group and the differences were statistically significant (p<0.05). The results were shown in Table 1.
| Factors | Poor prognosis group (n=49) | Good prognosis group (n=132) | t/χ2 | p |
|---|---|---|---|---|
| Age (mean±SD, years) | 45.37±9.82 | 41.85±7.44 | 2.277 | 0.026* |
| Sex (male/female) | 20/29 (40.8 %/59.2 %) | 50/82 (37.9 %/62.1 %) | 0.13 | 0.718 |
| BMI (mean±SD, kg/m2) | 25.82±1.97 | 25.40±2.44 | 1.204 | 0.231 |
| Hypertension | 28 (57.1 %) | 65 (49.2 %) | 0.893 | 0.345 |
| Hyperlipidemia | 18 (36.7 %) | 50 (37.9 %) | 0.02 | 0.888 |
| Diabetes | 10 (20.4 %) | 29 (22.0 %) | 0.052 | 0.82 |
| Location of the aneurysm (anterior circulation/posterior circulation/anterior circulation and posterior circulation) | 24/13/12 (50.0 %/26.5 %/24.5 %) | 59/29/44 (44.7 %/22.0 %/33.3 %) | 1.367 | 0.505 |
| Hunt-Hess grade (Ⅰ+Ⅱ/Ⅲ/Ⅳ+Ⅴ) | 4/31/14 (8.2 %/63.3 %/28.6 %) | 98/26/8 (74.2 %/19.7 %/6.1 %) | 64.126 | <0.001* |
| Fisher grade (Ⅰ+Ⅱ/Ⅲ+Ⅳ) | 14/35 (28.6 %/71.4 %) | 128/4 (97.0 %/3.0 %) | 26.03 | <0.001* |
| Cerebral vasospasm | 25 (51.0 %) | 9 (6.8 %) | 45.766 | <0.001* |
| Treatment (craniotomy clipping/endovascular intervention) | 19/30 (38.8 %/61.2 %) | 47/85 (35.6 %/64.4 %) | 0.155 | 0.694 |
| GCS (mean±SD, scores) | 7.37±2.07 | 5.33±1.76 | 6.583 | <0.001* |
| CRP (mean±SD, mg/l) | 11.24±3.37 | 7.87±1.86 | 6.63 | <0.001* |
Note: *Statistically significant; BMI: Body Mass Index; GCS: Glasgow Coma Scale, CRP: C-Reactive Protein; SD: Standard Deviation and χ2: Chi Square
Table 1: Comparison of Baseline Data of two Groups
Comparison of miR-30c levels in two groups were explained here. The T1-miR-30c and T2-miR-30c levels were 0.49±0.11 and 0.80±0.18 in patients with aSAH treated with craniotomy clipping was compared with T1- miR-30c (0.50±0.13) and T2-miR-30c (0.85±0.19) levels in patients with aSAH who are treated with endovascular intervention, there was no significant difference (t=0.657, p=0.512; t=1.544, p=0.124). The difference of miR-30c level between the two groups had time effect (F=282.360, p<0.001), intergroup effect (F=92.642, p<0.001), time and intergroup interaction effect (F=12.546, p<0.001). The level of miR-30c in the two groups was increased with time. The levels of T0-miR-30c, T1-miR-30c and T2-miR- 30c in the poor prognosis group were significantly lower than those in the good prognosis group (p<0.05). The results were shown in Table 2.
| Groups | Number of patients | T0-miR-30c | T1-miR-30c | T2-miR-30c |
|---|---|---|---|---|
| Poor prognosis group | 49 | 0.41±0.07 | 0.43±0.10 | 0.67±0.10 |
| Good prognosis group | 132 | 0.47±0.13 | 0.52±0.12 | 0.89±0.18 |
| t | 4.192 | 4.861 | 10.300 | |
| p | <0.001* | <0.001* | <0.001* |
Note: *Statistically significant
Table 2: Comparison of miR-30C Levels in two Groups (MEAN±SD)
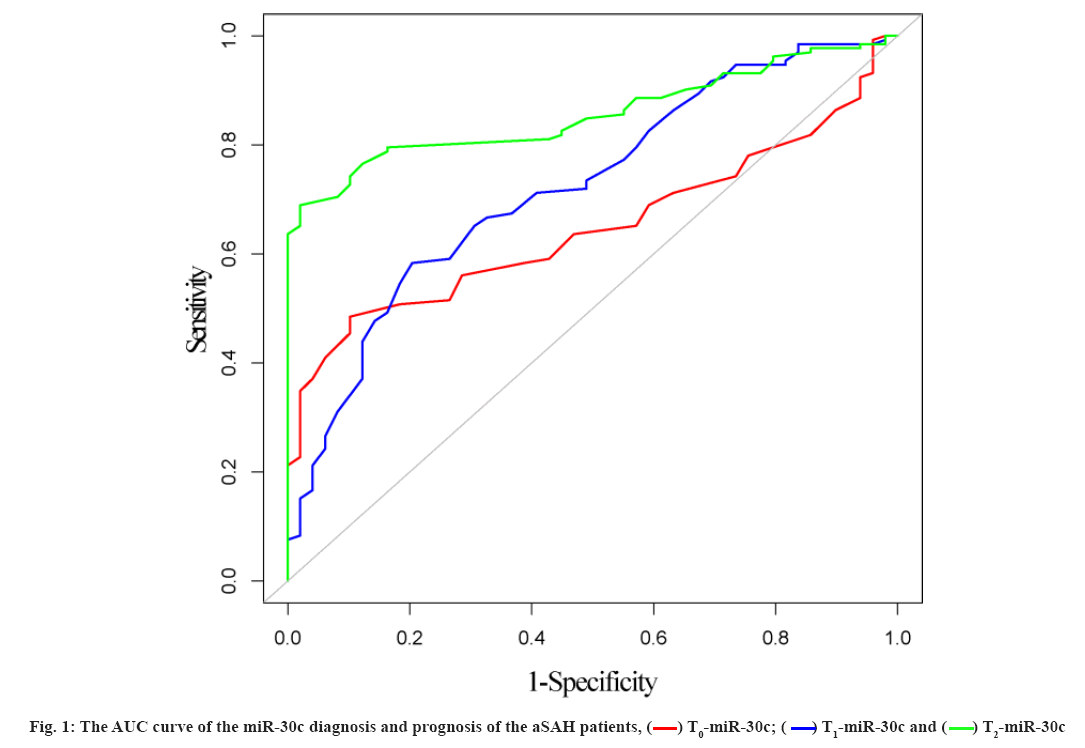
The relationship between miR-30c and prognosis was explained here. Using the T0-miR-30c, T1-miR-30c and T2-miR-30c to diagnose the prognosis of the aSAH patients was shown here. By T0-miR-30c: The Area Under the ROC curve (AUC), the best cut-off point, the sensitivity and the specificity were 0.647 (95 % Confidence Interval (CI): 0.569~0.725), 0.47, 89.80 % and 48.48 %, respectively. By T1-miR-30c: The AUC, the best cut-off point, the sensitivity and the specificity were 0.723 (95 % CI: 0.642~0.804), 0.50, 79.59 % and 58.33 %, respectively. By T2-miR-30c: The AUC, the best cut-off point, the sensitivity and the specificity were 0.856 (95 % CI: 0.803~0.908), 0.80, 97.96 % and 68.94 %, respectively. The efficacy of T2-miR-30c in diagnosis and the prognosis of the aSAH patients were significantly higher than that of T0-miR-30c and T1-miR-30c. The differences were statistically significant (Z=4.586, p<0.001; Z=2.761, p=0.006). The results were shown in fig. 1.
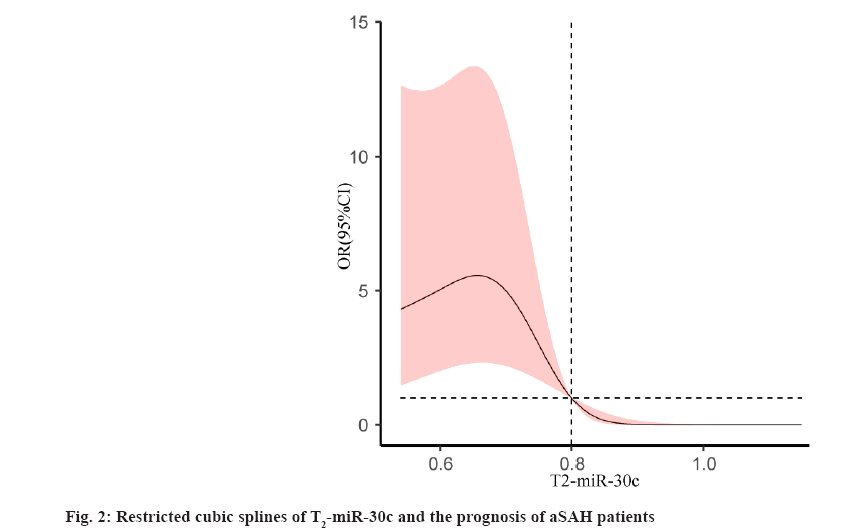
Risk factors affecting the prognosis of aSAH were explained here. The prognosis of patients with aSAH was used as a dependent variable. Age, Hunt-Hess grade, Fisher grade, cerebral vasospasm, GCS, CRP and T2- miR-30c were used as independent variables. All above factors were included in the logistic regression analysis. The results showed that Hunt-Hess Grade IV+V, cerebral vasospasm, GCS and CRP were independent risk factors for the prognosis of aSAH patients (p<0.05) and T2-miR- 30c was an independent protective factor for the prognosis of aSAH patients (p<0.05). The results were shown in Table 3. The restricted cubic spline fitting logistic regression analysis was used to analyze the relationship between T2-miR-30c and prognosis of the aSAH patients. When the number of nodes was 3, the AUC value was the smallest (AUC=138.374). The results showed that T2-miR-30c was associated with the prognosis of the aSAH patients (χ2=14.89, p=0.001), and it had a non- linear relationship (χ2=9.54, p=0.002). The results were shown in fig. 2. When T2-miR-30c<0.80, the risk of poor prognosis in aSAH patients was increased and when T2- miR-30c>0.80, the risk was decreased.
| Factors | β | SE | Wald | p | OR | 95 %CI |
|---|---|---|---|---|---|---|
| Age | -0.008 | 0.039 | 0.038 | 0.844 | 0.992 | 0.920~1.070 |
| Hunt-Hess grade | 0.971 | 0.471 | 4.256 | 0.039 | 2.641 | 1.050~6.647 |
| Fisher grade | -0.318 | 1.386 | 0.053 | 0.819 | 0.728 | 0.048~11.007 |
| Cerebral vasospasm | 3.165 | 0.939 | 11.353 | 0.001 | 23.700 | 3.759~149.434 |
| GCS | 0.700 | 0.202 | 12.045 | 0.001 | 2.014 | 1.356~2.990 |
| CRP | 0.610 | 0.143 | 18.212 | <0.001 | 1.840 | 1.391~2.435 |
| T2-miR-30c | -6.814 | 2.655 | 6.587 | 0.010 | 0.001 | 0.001~0.200 |
Note: β: Beta Coefficient; SE: Standard Error; OR: Odds Ratio and CI: Confidence Interval
Table 3: Risk Factors for Prognosis of ASAH Patients
Effective preoperative evaluation of patients with aSAH who is undergoing surgery is important for clinicians to make treatment plans and to evaluate the prognosis. Both the Hunt-Hess grade and the World Federation of Neurosurgical Societies (WFNS) grade could not accurately assess the prognosis of patients with severe aSAH before surgery[19]. Therefore, sensitive and accurate predictors are urgently needed to evaluate the prognosis of patients with aSAH. At present, few studies have analyzed the relationship between miR-30c and the prognosis of patients with aSAH, and the efficacy of miR-30c in evaluating prognosis was fewer to be analyzed.
In the 181 subjects, 27.1 % had a poor prognosis. The result is similar to those of Lee et al.[20]. In this study, the levels of T0-miR-30c, T1-miR-30c and T2-miR-30c were compared between patients with poor prognosis and those with good prognosis. The levels of T0-miR-30c, T1-miR-30c and T2-miR-30c in patients with poor prognosis were lower than those in patients with good prognosis. These results suggest that miR-30c may be related to the prognosis of aSAH patients. It may be used to evaluate the prognosis of patients with aSAH. Then, the AUC of the miR-30c diagnosis and the prognosis of the aSAH patients were constructed. The result which diagnosed with T2-miR-30c showed that the AUC, the sensitivity and the specificity were 0.856, 97.96 % and 68.94 %, respectively. The diagnostic efficacy of T2-miR-30c is higher than that of T0- miR-30c and T1-miR-30c. The above results indicate that T2-miR-30c is more effective in evaluating the prognosis of aSAH patients. However, the specificity is low, so it can be used to evaluate the prognosis of aSAH patients.
Previous studies showed that age, Hunt-Hess grade, Fisher grade, cerebral vasospasm, GCS and CRP were closely related to the prognosis of patients with aSAH[20-23]. In this study, the results showed that Hunt-Hess grade, cerebral vasospasm, GCS and CRP were related to the prognosis of patients with aSAH. While the age and Fisher grade were not related to the prognosis. It is speculated that the difference may be related to the sample size and further large sample study is needed to verify the conclusion of this study. In addition, T2-miR-30c was found to be associated with the prognosis of aSAH patients and the result of restricted cubic spline showed a non-linear relationship with the prognosis of aSAH patients. The reason maybe that, miR-30c regulated15 NLRP3 or Sex- Determining Region Y-type (SRY)-related High Mobility Group (HMG) Box gene 9 (SOX9) in aSAH progression and its level may reflect the prognosis of aSAH patients. When the aneurysm ruptured, the blood entered the subarachnoid space. It induced the acute hydrocephalus, the cerebral crest fluid outflow tract obstruction and so on. Then, the intracranial pressure increased, the cerebral blood flow reduced. Finally, it induced cerebral edema and delayed cerebral ischemia and so on[24]. In this process, NLRP3-induced inflammatory response which will aggravate the brain neuron death[25]; elevated SOX9 levels reduce neuronal cell viability and promote apoptosis[15]; miR-30c can regulate NLRP3 expression and participate in cell apoptosis[9-11]. In addition, miR-30c also targets SOX9 to protect neurological function in rats with CI/R injury[15]. Therefore, miR-30c may play a key role in the progression of aSAH, and its level may reflect the prognosis of aSAH. The mechanism of miR-30c participated in the progression of aSAH and its upstream and downstream pathways still need to be confirmed by further research.
There are still some deficiencies in this study. First, the sample size of this study is relatively small, while more factors were included in logistic regression analysis which can easily increase the probability of type II errors in statistical analysis. Second, the miR-30c levels were only measured at three time point (T0, T1 and T2), so the best time to evaluate the prognosis of patients with aSAH cannot be determined. Third, no basic research was conducted to analyze whether miR-30c affected the prognosis of aSAH patients by modulating NLRP3. In the next step, we will aim at the above shortcomings and carry out research again to enrich the content of this study.
In summary, the level of miR-30c in patients with aSAH is closely related to their prognosis and detection of miR- 30c level, which can be used to evaluate the prognosis of them. The low level of T2-miR-30c suggests a higher risk of poor prognosis.
Conflict of interests:
The authors declared no conflict of interest.
References
- Dai JX, Cai JY, Sun J, Lin Q, Yu ZQ. Serum soluble tumor necrosis factor-like weak inducer of apoptosis is a potential biomarker for outcome prediction of patients with aneurysmal subarachnoid hemorrhage. Clin Chim Acta 2020;510:354-9.
[Crossref] [Google scholar] [PubMed]
- Li B, McIntyre M, Gandhi C, Halabi M, Long A, van Hoof A, et al. Low total cholesterol and high density lipoprotein are independent predictors of poor outcomes following aneurysmal subarachnoid hemorrhage: A preliminary report. Clin Neurol Neurosurg 2020;197:106062.
[Crossref] [Google scholar] [PubMed]
- Kiiski H, Langsjö J, Tenhunen J, Ala-Peijari M, Huhtala H, Hämäläinen M, et al. S100B, NSE and MMP-9 fail to predict neurologic outcome while elevated S100B associates with milder initial clinical presentation after aneurysmal subarachnoid hemorrhage. J Neurol Sci 2018;390:129-34.
[Crossref] [Google scholar] [PubMed]
- Luo W, Wang H, Hu J. Increased concentration of serum periostin is associated with poor outcome of patients with aneurysmal subarachnoid hemorrhage. J Clin Lab Anal 2018;32(5):e22389.
[Crossref] [Google scholar] [PubMed]
- Zuo Z, Ji X. Prognostic value of copeptin in patients with aneurysmal subarachnoid hemorrhage. J Neuroimmunol 2019;330:116-22.
[Crossref] [Google scholar] [PubMed]
- Chen N, Xiao B, Wang S, Wei B. Bioinformatics analysis of microRNA linked to ubiquitin proteasome system in traumatic osteonecrosis of the femoral head. Medicine 2020;99(33):e21706.
[Google scholar] [PubMed]
- Kikkawa Y, Ogura T, Nakajima H, Ikeda T, Takeda R, Neki H, et al. Altered expression of microRNA-15a and Kruppel-like factor 4 in cerebrospinal fluid and plasma after aneurysmal subarachnoid hemorrhage. World Neurosurg 2017;108:909-16.
[Crossref] [Google scholar] [PubMed]
- Su XW, Chan AH, Lu G, Lin M, Sze J, Zhou JY, et al. Circulating microRNA 132-3p and 324-3p profiles in patients after acute aneurysmal subarachnoid hemorrhage. PLoS One 2015;10(12):e0144724.
[Crossref] [Google scholar] [PubMed]
- Xu J, Ma L, Fu P. MicroRNA-30c attenuates contrast-induced acute kidney injury by suppressing NLRP3 inflammasome. Int Immunopharmacol 2020;87:106457.
[Crossref] [Google scholar] [PubMed]
- Li P, Zhong X, Li J, Liu H, Ma X, He R, et al. MicroRNA-30c-5p inhibits NLRP3 inflammasome-mediated endothelial cell pyroptosis through FOXO3 down-regulation in atherosclerosis. Biochem Biophys Res Commun 2018;503(4):2833-40.
[Crossref] [Google scholar] [PubMed]
- Liu C, Zhuo H, Ye MY, Huang GX, Fan M, Huang XZ. LncRNA MALAT1 promoted high glucose‐induced pyroptosis of renal tubular epithelial cell by sponging miR‐30c targeting for NLRP3. Kaohsiung J Med Sci 2020;36(9):682-91.
[Crossref] [Google scholar] [PubMed]
- Wang C, Jia Q, Sun C, Jing C. Calcium sensing receptor contribute to early brain injury through the CaMKII/NLRP3 pathway after subarachnoid hemorrhage in mice. Biochem Biophys Res Commun 2020;530(4):651-7.
[Crossref] [Google scholar] [PubMed]
- Sun T, Li T, Davies H, Li W, Yang J, Li S, et al. Altered morphologies and functions of the olfactory bulb and hippocampus induced by miR-30c. Front Neurosci 2016;10:207.
[Crossref] [Google scholar] [PubMed]
- Sun T, Li W, Ling S. miR‐30c and semaphorin 3A determine adult neurogenesis by regulating proliferation and differentiation of stem cells in the subventricular zones of mouse. Cell Prolif 2016;49(3):270-80.
[Crossref] [Google scholar] [PubMed]
- Zhang M, Zhu Y, Wei M, Liu H. Neuroprotective effects of miR-30c on rats with cerebral ischemia/reperfusion injury by targeting SOX9. Pathol Res Pract 2020;216(12):153271.
[Crossref] [Google scholar] [PubMed]
- Chinese Society of Neurology, Chinese Stroke Society, Neurovascular Intervention Group of Chinese Society of Neurology. Chinese guidelines for diagnosis and treatment of subarachnoid hemorrhage 2019. Chin J Neurol 2019;52(12):1006-21.
- Su Y, Li Q, Zheng Z, Wei X, Hou P. Integrative bioinformatics analysis of miRNA and mRNA expression profiles and identification of associated miRNA-mRNA network in aortic dissection. Medicine 2019;98(24):e16013.
[Crossref] [Google scholar] [PubMed]
- Jeong HG, Kim BJ, Choi JC, Hong KS, Yang MH, Jung C, et al. Post treatment national institutes of health stroke scale is superior to the initial score or thrombolysis in cerebral ischemia for 3-month outcome. Stroke 2018;49(4):938-44.
[Crossref] [Google scholar] [PubMed]
- Zhao JW, Luo XY, Zhang Z, Chen K, Shi GZ, Zhou JX. Use of somatosensory evoked potentials for preoperative assessment in patients with severe aneurysmal subarachnoid hemorrhage before surgical or interventional treatment: A prospective observational cohort study. Chin Crit Care Med 2018;30(3):251-6.
[Crossref] [Google scholar] [PubMed]
- Lee S, Kim YO, Ryu JA. Clinical usefulness of early serial measurements of C-reactive protein as outcome predictors in patients with subarachnoid hemorrhage. BMC Neurol 2020;20(1):1-10.
[Crossref] [Google scholar] [PubMed]
- Zhang D, Zhuang Z, Wei Y, Liu X, Li W, Gao Y, et al. Association of admission serum glucose-phosphate ratio with severity and prognosis of aneurysmal subarachnoid hemorrhage. World Neurosurg 2019;127:e1145-51.
[Crossref] [Google scholar] [PubMed]
- Zhu Y, Jiang H, Li Y, Weng Y, Xu K, Zhou L, et al. Serum alkaline phosphatase level is associated with angiographic vasospasm, delayed cerebral ischemia-caused clinical deterioration, and functional outcome after aneurysmal subarachnoid hemorrhage. Neurocrit Care 2019;31(3):466-75.
[Crossref] [Google scholar] [PubMed]
- Lin Q, Ba HJ, Dai JX, Sun J, Lu C, Chen MH, et al. Serum soluble lectin-like oxidized low-density lipoprotein receptor-1 concentrations and prognosis of aneurysmal subarachnoid hemorrhage. Clin Chim Acta 2020;500:54-8.
[Crossref] [Google scholar] [PubMed]
- Xu W, Li T, Gao L, Zheng J, Yan J, Zhang J, et al. Apelin-13/APJ system attenuates early brain injury via suppression of endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation and oxidative stress in a AMPK-dependent manner after subarachnoid hemorrhage in rats. J Neuroinflammation 2019;16(1):1-4.
[Crossref] [Google scholar] [PubMed]
- Zhuang K, Zuo YC, Sherchan P, Wang JK, Yan XX, Liu F. Hydrogen inhalation attenuates oxidative stress related endothelial cells injury after subarachnoid hemorrhage in rats. Front Neurosci 2020;13:1441. [ Crossref]
[Google scholar] [PubMed]