- *Corresponding Author:
- Xuan Zhao
Department of Orthopaedics, Xuanwu Hospital, Capital Medical University, Xicheng, Beijing 100053, China
E-mail: zhaoxuan@stu.njmu.edu.cn
| This article was originally published in a special issue, “Drug Development in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(5) Spl Issue “110-118” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Traumatic spinal cord injury can result in severe motor and sensory impairment. Accumulating evidence suggests that traumatic spinal cord injury triggers a systemic immune response involving circulating immunocytes. We sought to investigate the circulating immunocytes composition during the acute phases of spinal cord injury and its association with clinical parameters. Flow cytometry was used to perform detailed immunoassays in spinal cord injury mice and patients with acute disease. We found that the frequencies of circulating immunocytes were significantly different among mice with different baseline degrees of injury severity. Moreover, the severity of injury in human spinal cord injury patients was found to be positively correlated with neutrophil counts and the proportion of cluster of differentiation 8+ T cells in the acute phase but inversely correlated with lymphocytes, cluster of differentiation 4+ T cells and the cluster of differentiation 4+/cluster of differentiation 8+ T-cell ratio. In addition, multivariate analysis revealed that acute spinal cord injury induced increases in neutrophils (odds ratio 1.215, 95 % confidence interval=1.005-1.468), lymphocytes (odds ratio=28.208, 95 % confidence interval=2.190-363.390) and cluster of differentiation 8+ T cells (odds ratio=1.186, 95 % confidence interval=1.033-1.362) were independent predictors of association impairment scale grade conversion failure and the predictive model displayed robust sensitivity (88.0 %) and specificity (88.2 %). Our findings not only elucidate the association between early circulating immune cells and injury severity, but also indicate the prognostic value of modeling early circulating neutrophil and lymphocyte counts with proportions of cluster of differentiation 8+ T cells for predicting association impairment scale grade conversion at 6 mo post spinal cord injury.
Keywords
Traumatic spinal cord injury, acute phase, neutrophils, lymphocytes, T cells, hemorrhage, AIS grade conversion
Traumatic Spinal Cord Injury (SCI) causes permanent damage to the neural tissue of the spinal cord, resulting in the loss of motor and sensory functions at and below the injury level and a significant decline in quality of life[1,2]. Globally, an estimated 27 million people are now suffering from the impacts of SCI[3]. Early prediction of the outcome might lead to increased patient satisfaction and compliance with treatment, as well as classification of patients for post-acute care treatment based on their likelihood of neurological remission[4]. The prognosis of individuals with acute SCI is mostly based on the neurological evaluation of residual function resulting from lesion features[5]. Despite a reliable baseline following SCI, spontaneous recovery is diverse across SCI patients with the same American Spinal Injury Association Impairment Scale (AIS) grade[6]. Therefore, metrics for stratifying the severity of the damage and correctly predicting neurological recovery would be of great assistance to SCI patients. Further understanding of these factors is needed to find viable strategies and enhance clinical diagnosis.
It has been proven that peripheral blood biomarkers reflect tissue damage as well as host immune activity and are readily accessible for routine blood sampling[5,7]. Prior research has demonstrated that monitoring blood protein levels can be utilized to determine the degree of Central Nervous System (CNS) damage (such as traumatic brain injury, cerebral infarction, intracranial hemorrhage and SCI)[8-10]. Nonetheless, few of these biomarkers have been recognized and integrated into standard therapeutic practice[11,12]. Recent research has demonstrated the significance of circulating leukocytes as a prognostic indicator of CNS disease[13-15]. These results imply that systemic peripheral immunity is involved in the development of CNS diseases. A deeper knowledge of immune cell population alterations and their probable connections with clinical features is needed to improve the clinical evaluation and therapeutic management of SCI.
In an effort to describe systemic responses to traumatic SCI in mice and humans, we investigated the hypothesized relationship among changes in early circulating immunocytes, injury severity and neurological outcomes in mice and SCI patients. In the acute phase of SCI (0-2 d post-SCI), the frequencies of circulating immune cell subsets in SCI mice were altered with increased neutrophils and Cluster of Differentiation (CD)-8+ T cells and reduced lymphocytes, total T cells, CD4+ T cells and CD4+/CD8+ T-cell ratios. While alterations in the number or frequency of circulating immune cell subsets in SCI patients were partially distinct from those in mice compared to Negative Controls (NC), shown by an increase in neutrophils. In addition, the data suggested that an increase in neutrophils and CD8+ T cells and a reduction in lymphocytes, CD4+ T cells, and the CD4+/CD8+ T-cell ratio were positively linked with injury severity. In addition, the levels of neutrophils, lymphocytes, CD4+ T cells, and CD8+ T cells differed between SCI individuals who experienced AIS grade conversion and those who did not. A combination of neutrophils, lymphocytes, and CD8+ T cells was found to be a potential predictor of AIS grade conversion by multifactor analysis. Our findings investigate an injury severity indicator and a predictor of AIS grade conversion at 6 mo post injury in individuals with traumatic SCI.
Materials and Methods
General information:
Our study included 20 NCs and 42 acute SCI patients recruited from April 2021 to May 2022 in our spinal cord disease research center.
Inclusion criteria: Age from 18 y to 65 y; admission time less than 24 h after SCI; imaging (Magnetic Resonance Imaging (MRI)/Computed Tomography (CT)) evidence to clearly diagnose injuries (injury site included the cervical spinal cord, thoracic spinal cord and lumbar spinal cord); evidence of a history of trauma and informed consent of the patient.
Exclusion criteria: Injury of the cauda equina or cauda equina root; combined injury of the brain demonstrated by cranial magnetic resonance imaging; concurrent infection in the injured site of the spinal cord or other sites; pure spinal shock, spinal concussion or other functional impairment; other concurrent malignant tumors; spinal cord degeneration or injury in the past; pregnancy immune system disease and incomplete data or preoperative samples were not obtained. The NC group comprised patients who had acute spinal fracture with no SCI confirmed by MRI and no disturbance in movement and sensation of limbs. This work received institutional review board approval and complies with all applicable national legislation and norms.
Each patient underwent routine blood tests and lymphocyte set examinations on admission within 1 d after injury. The clinical and laboratory information was collected like age, sex, AIS grade, neutrophils, lymphocytes, monocytes, total T cells, CD4+ T cells, CD8+ T cells and the CD4+/CD8+ T-cell ratio. The International Standards for Neurological Classification of SCI (ISNCSCI) of the American Spinal Injury Association (ASIA) was used to classify the severity of patient’s functional injuries (the AISA represents the worst neurological function). Acknowledging that AIS grade conversion is a gross measure of neurological recovery, clinical trials of novel neurorestorative agents are increasingly utilizing improvement in the Motor Score (MS) at 6 mo post injury as an outcome measure, accepting that the majority of motor recovery occurs during this period[16,17]. Patients in the SCI group were scored by a same experienced attending doctor at the time of admission and at outpatient follow-up 6 mo after the injury, follow-up ISNCSCI assessments were conducted 6 mo post injury in 42/44 patients (95 %). Based on the patients' AIS grade and motor recovery situation 6 mo after the injury, the samples were divided into two groups; conversion group (1 or more than 1 AIS grade improved 6 mo after the injury) and no conversion group (AIS grades slightly changed (<1 AIS grade) 6 mo after the injury).
Animals and surgical procedures:
All animal tests were conducted with the approval of the committee on the ethics of animal experiments at Nanjing Medical University. Eighteen C57BL/6 male mice (Gem Pharmatech, China) aged 8-10 w were utilized to establish a model of SCI. A laminectomy was conducted under anesthesia to expose the spinal cord at T10. The contusion injuries were caused by the Infinite Horizon SCI device (Precision Systems and Instrumentation, Lexington, Kentucky, United States of America (USA)). The device was adjusted to three intensities (40 kdyn, 75 kdyn, and 90 kdyn) that caused mild, moderate and severe contusions, respectively[18,19]. Successful SCI modeling was assessed by the trembling of the body, the swinging of the tail, the trembling and retraction of the hind limbs and body upon contact. Until bladder function improved after SCI, the bladder was squeezed daily to facilitate urination. A laminectomy group without suffering spinal cord damage was used as the NC. Mice with asymmetrical SCI were excluded from the experimental analysis.
Functional assessment in mice:
After injury, the motor function of mice was assessed by the Basso Mouse Scale (BMS); each mouse was individually trained and placed in an open field[20]. On 1, 3, 7, 14, 21, and 28 d following SCI, all mice were monitored by two scientists who were blinded to the different groups. The BMS score ranges from 0 (no ankle movement) to 9 (full functional recovery) and evaluates locomotion on the basis of hind limb joint motions, trunk posture and stability, stepping coordination, paw placement, toe clearance and tail position. Animals with a higher than 2-point discrepancies between their two hind limbs were removed from the experimental analysis.
Flow cytometry:
For the analysis of circulating immunocytes in mice, whole blood was collected by removing the mouse eyeball, followed by lysed erythrocyte, washed twice with Phosphate Buffer Solution (PBS), and then stained with APC-CY7-FVD, Percp CY5.5-anti-CD45, PE-CY7-anti-CD11b, Fluorescein Isothiocyanate (FITC)-anti-CD3, BV510-anti-CD4, PE-anti-CD8a, APC-anti-Ly6g, and BV421-anti-CD14 for 30 min. Cells were analyzed by flow cytometry (FACSVerse 8, BD Biosciences, Piscataway, New Jersey, USA) and data analysis was performed using FlowJo software (Version 7.6.1, Treestar, Ashland, Oregon, USA).
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) version 26.0 (IBM Corporation, Armonk, NY) was utilized to analyze statistical data. Continuous variables are presented as mean±standard deviations. Categorical variables are presented as counts (n) and percentages (%). The independent sample t test was used to evaluate differences in continuous variables between groups, and the Chi-square (γ²) test was applied for categorical variables. Multivariate logistic regression analysis was performed to calculate the Odds Ratio (OR) and the 95 % Confidence Interval (CI). Additionally, the Receiver Operating Characteristic (ROC) curve was constructed and the Area under the Curve (AUC) was calculated to illustrate the value of the immunocytes combination for predicting AIS grade conversion p<0.05 was considered statistically significant.
Results and Discussion
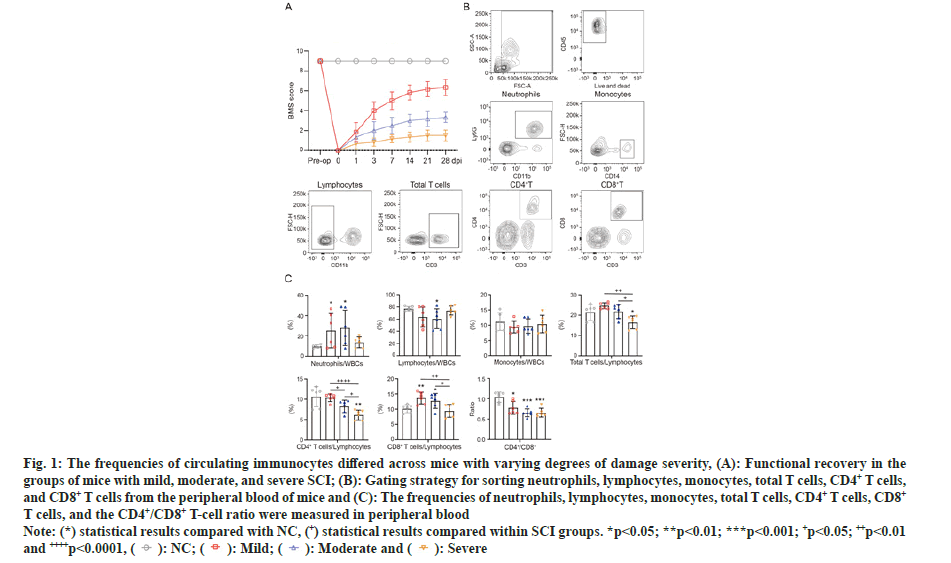
To illustrate the circulating immunocyte composition during the acute stages of SCI and its link with injury parameters, a mouse SCI model with three degrees of damage severity was established. After 28 d post injury, the motor outcome in the mild SCI group (average BMS score=6.3) was superior to that in the moderate SCI group (average BMS score=3.3; p<0.0001) and severe SCI group (average BMS score=1.5; p<0.0001) (fig. 1A). The above data indicated the stability and repeatability of the SCI model.
Fig. 1: The frequencies of circulating immunocytes differed across mice with varying degrees of damage severity, (A): Functional recovery in the
groups of mice with mild, moderate, and severe SCI; (B): Gating strategy for sorting neutrophils, lymphocytes, monocytes, total T cells, CD4+ T cells,
and CD8+ T cells from the peripheral blood of mice and (C): The frequencies of neutrophils, lymphocytes, monocytes, total T cells, CD4+ T cells, CD8+ T cells, and the CD4+/CD8+ T-cell ratio were measured in peripheral blood.
Note: (*) statistical results compared with NC, (+) statistical results compared within SCI groups. *p<0.05; **p<0.01; ***p<0.001; +p<0.05; ++p<0.01
and ++++p<0.0001, 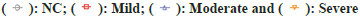
Subsequently, we tested whether the frequencies of circulating immunocytes in the acute phase correlated with the severity of SCI. Flow cytometry was performed 1 d post-SCI to investigate alterations in the frequencies of circulating immunocytes in six biological replicates of the NC, mild, moderate and severe groups. We measured the frequencies of neutrophils (CD45+CD11b+Ly6G+), lymphocytes (CD45+CD11b-), monocytes (CD45+ and CD14+), total T cells (CD45+CD11b-CD3+), CD4+ T cells (CD45+CD11b-CD3+CD4+), and CD8+ T cells (CD45+CD11b-CD3+CD8+) and the CD4+/CD8+ T-cell ratio in peripheral blood (fig. 1B). Compared with the control counterparts, the frequencies of neutrophils and CD8+ T cells were increased, while the lymphocytes, total T cells and CD4+ T cells, as well as the ratio of CD4+ and CD8+ T cells, were decreased in SCI groups. Moreover, we further evaluated the frequencies of circulating immunocytes in the mild, moderate and severe SCI groups. The results showed that the frequencies of total T cells, CD4+ T cells and CD8+ T cells were significantly different among mice with different baseline degrees of injury severity. All detailed data of mice including the frequencies of circulating immunocytes in the four groups are illustrated in as shown in fig. 1C.
To examine whether circulating immunocytes are also associated with injury parameters in the population, the immunocyte composition in NC and acute SCI patients with different injury severity was further analyzed. All patients had routine blood and lymphocyte examinations upon admission. Evaluations were performed within 1 d after injury and the laboratory department provided the clinical information for these indices. The baseline data of the study participants are displayed in Table 1. There were no significant differences in sex or age between the NC and SCI groups. Compared with the NC group, patients in SCI groups showed significantly increased levels of neutrophils (p<0.001).
| NC (n=20) | SCI (n=42) | p value | |
|---|---|---|---|
| Gender (n males, %) | 14 (70.0) | 31 (73.8) | 0.753 |
| Age (years, SD) | 52.5±12.0 | 50.1±15.6 | 0.549 |
| Neutrophil | 5.5±2.1 | 9.8±5.0 | <0.001 |
| Lymphocyte | 1.2±0.5 | 1.2±0.4 | 0.893 |
| Monocyte | 0.5±0.2 | 0.7±0.3 | 0.099 |
| Total T (%) | 70.9±7.2 | 71.1±7.8 | 0.921 |
| CD4+ T (%) | 42.6±6.0 | 41.1±9.9 | 0.551 |
| CD8+ T (%) | 25.4±7.7 | 27.4±8.5 | 0.383 |
| CD4/8+ T | 1.9±0.9 | 1.7±0.9 | 0.509 |
Note: p<0.05 was considered statistically significant
Table 1: Demographic data of each experimental group.
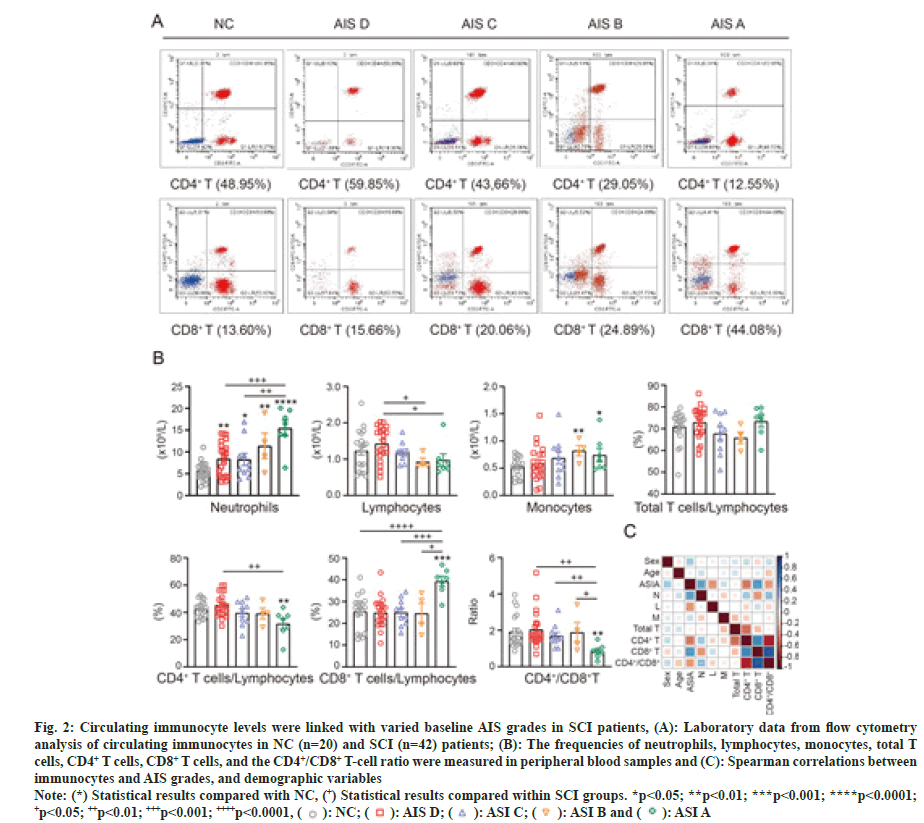
In addition, the results showed statistically significant between-group differences for AIS grades A, B, C and D for neutrophils, lymphocytes, CD4+ T cells, CD8+ T cells, and the CD4+/CD8+ T-cell ratio in SCI patients (fig. 2A and fig. 2B). All detailed data of patients including the frequencies of circulating immunocytes in the five groups are illustrated, as shown in fig. 2B.
Fig. 2: Circulating immunocyte levels were linked with varied baseline AIS grades in SCI patients, (A): Laboratory data from flow cytometry
analysis of circulating immunocytes in NC (n=20) and SCI (n=42) patients; (B): The frequencies of neutrophils, lymphocytes, monocytes, total T
cells, CD4+ T cells, CD8+ T cells, and the CD4+/CD8+ T-cell ratio were measured in peripheral blood samples and (C): Spearman correlations between
immunocytes and AIS grades, and demographic variables.
Note: (*) Statistical results compared with NC, (+) Statistical results compared within SCI groups. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001; +p<0.05; ++p<0.01; +++p<0.001; ++++p<0.0001, 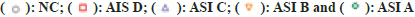
We then estimated the correlations between immunocytes (neutrophils, lymphocytes, monocytes, total T cells, CD4+ T cells, CD8+ T cells and the CD4+ /CD8+ T-cell ratio) and AIS grades (A, B, C, D) and demographic variables (sex and age) in SCI participants (fig. 2C). Consistent with the above results, AIS grades showed positive correlations with neutrophils and CD8+ T cells but negative correlations with lymphocytes, CD4+ T cells, and the CD4+/CD8+ T-cell ratio. Moreover, we noticed that the AIS grades and cellular proportions could be affected by age and sex.
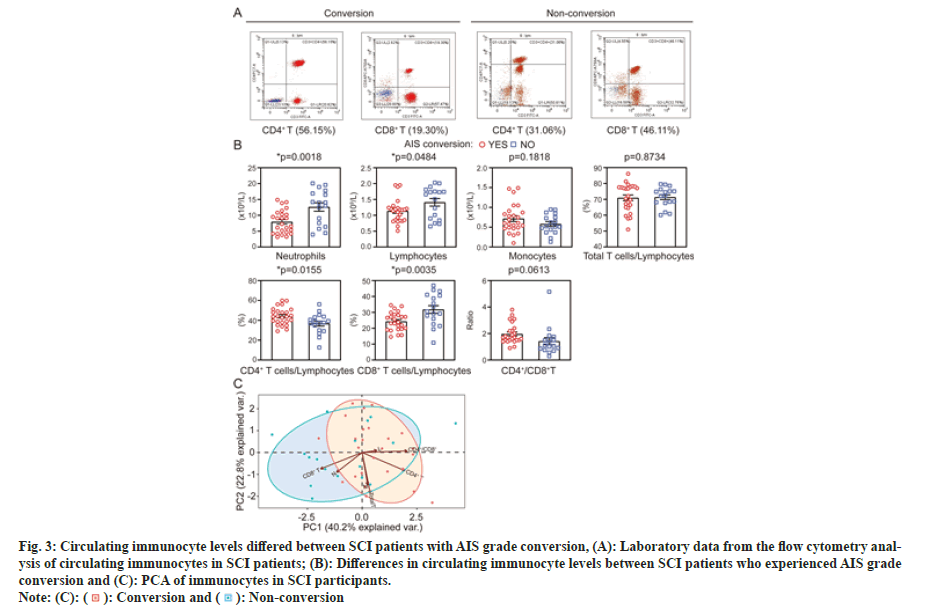
Next, we determined whether the circulating immunocytes at 1 d post injury were different between those who experienced AIS grade conversion at 6 mo and those who did not. As shown in fig. 3A and fig. 3B, for the cohort of 42 SCI patients, neutrophils (p=0.0018), lymphocytes (p=0.0484), and CD8+ T cells (p=0.0035) were significantly lower in the converted group than in the non-converted group, while CD4+ T cells (p=0.0155) were higher. The levels of other parameters, including monocytes, total T cells and the CD4+/CD8+ T-cell ratio, were comparable between the two groups. The above results indicated that there may be an association between circulating immunocyte levels and neurological improvement at 6 mo post-SCI.
Fig. 3: Circulating immunocyte levels differed between SCI patients with AIS grade conversion, (A): Laboratory data from the flow cytometry analysis
of circulating immunocytes in SCI patients; (B): Differences in circulating immunocyte levels between SCI patients who experienced AIS grade
conversion and (C): PCA of immunocytes in SCI participants.
To investigate the relationships among immunocytes described above, a Principal Component Analysis (PCA) was performed across all individual participants. As shown in fig. 3C, the first two PCs cumulatively explained 63.0 % of the total variance, with PC1 explaining 40.2 % and PC2 explaining 22.8 %. PC1 was most strongly driven by CD4+ T cells, CD8+ T cells and neutrophils, and PC2 was mainly driven by total T cells. Notably, the samples of conversion and no conversion groups separated along PC1. In summary, the PCA scatterplot revealed that the immune cells could characterize differences between groups.
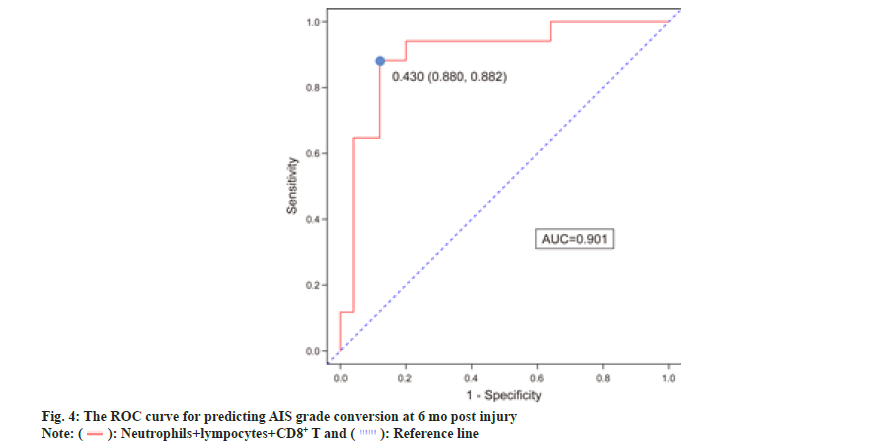
We used multivariate logistic regression analysis to predict conversion by choosing four variables (neutrophils, lymphocytes, CD4+ T cells, and CD8+ T cells) that were substantially different between the conversion and non-conversion groups. After multivariable adjustment, three predictors were still considered significant in the multivariate logistic regression model. Table 2 shows the independent predictors of AIS grade conversion after SCI, which include neutrophils, lymphocytes and CD8+ T cells. Neutrophils (OR=1.215, 95 % CI 1.005–1.468), lymphocytes (OR=28.208, 95 % CI 2.190–363.390), and CD8+ T cells (OR=1.186, 95 % CI 1.033–1.362) predicted an increased risk of AIS grade conversion failure. As presented in fig. 4, the ROC curve of neutrophils combined with the other two factors is represented by a red line with an AUC of 0.901. The sensitivity was 88.0 %, and the specificity was 88.2 %.
| Variable | B | SEM | Wald | p value | OR (95 % CI) |
|---|---|---|---|---|---|
| Neutrophil | 0.195 | 0.097 | 4.063 | 0.044 | 1.215 (1.005-1.468) |
| Lymphocyte | 3.34 | 1.304 | 6.558 | 0.010 | 28.208 (2.190-363.390) |
| CD8+ T | 0.171 | 0.071 | 5.854 | 0.016 | 1.186 (1.033-1.362) |
Table 2: Multivariate logistic regression analysis of risk factors for predicting AIS grade conversion.
In this study, we characterized the immunological profiles of SCI mice and patients in the acute disease phase using flow cytometry. The early alterations in circulating immunocytes in mice with contusion SCI were documented and significant results from the mouse data were then validated in an independent validation population to strengthen the reliability of our findings. The most significant alterations were in the neutrophil, lymphocyte and T-cell subset compartments, where AIS grades correlated positively with neutrophils and CD8+ T cells but negatively with lymphocytes, CD4+ T cells, and the CD4+/CD8+ T-cell ratio. A combination of neutrophils, lymphocytes and CD8+ T cells was also discovered as a potential predictor of AIS grade conversion at 6 mo post-SCI. In general, our findings provide new insights into early circulating immunocyte alterations in individuals with traumatic SCI and uncover a potential relationship between these changes and injury severity and neurological outcomes at 6 mo post injury.
Neutrophils' function in SCI recovery has been widely studied in experimental animal models, although their precise significance remains controversial[21-23]. We revealed that the proportion of neutrophils in the peripheral blood of SCI mice with varying degrees of damage was not significantly different. Additional testing of peripheral blood neutrophils in the population revealed that the number of circulating neutrophils was elevated during the acute phase following SCI and was substantially connected with damage severity. Subgroup analysis found that patients with lower neutrophil counts after SCI were more likely to experience improvements in AIS grade, indicating a possible correlation between circulating neutrophils and neurological outcomes. Further research revealed that the neutrophil count was an independent risk factor for AIS grade conversion. Therefore, neutrophils remain an important therapeutic target for intervention in acute SCI and future research should investigate how their phenotype is altered by SCI relative to normal physiological conditions to achieve immune regulation.
In line with previous descriptions of SCI in humans and animals[24-29], a reduction in circulating lymphocyte counts was observed 1 d post-SCI, which has been regarded as a sign of systemic deficiency in adaptive immunity and may be driven by neuroendocrine abnormalities[26]. In addition, we discovered that the number of circulating lymphocytes in the acute phase after SCI was inversely linked with the severity of the damage, which contradicts the findings of prior research. We believe this discrepancy is due to the difference in the number of patients enrolled. Prior research has demonstrated that individuals with an isolated cervical SCI and acute clinical lymphopenia are more likely to experience an AIS grade conversion from admission to discharge[30]. This finding is similar with those of our cohort and prior animal studies, which found that SCI mice lacking T cells had better functional recovery[31]. It is necessary to further investigate lymphocyte subsets and their role in SCI recovery.
T cells are crucial for adaptive immune system activity. Compared to the NC group, we noticed a decline in the frequency of CD4+ T cells during the acute phase of SCI in mice, particularly in those with more severe injuries and similar results were found in the population. Previous research has demonstrated that the biology of T cells is altered following SCI and that the frequency and function of CD4+ T cells are decreased in individuals with acute SCI[28,32]. The findings of this study further reveal that the frequency of CD4+ T cells has a substantial inverse correlation with injury severity. Notably, for CD8+ T-cell frequencies in individuals with varying degrees of damage during the acute phase post-SCI, we obtained inconsistent results in mice and patients, which may be due to species differences. In addition, we discovered that the frequency of CD8+ T cells was strongly linked with injury severity and was an independent risk factor for AIS grade conversion. Further research is required to determine the applicability of these findings to the broader SCI population and to examine the functional activities of altered T-cell subsets.
We have provided insights into how circulating immunocytes respond to and/or are modulated by SCI. Nonetheless, several limitations of this study should be discussed. First, the levels of circulating immunocytes in this study were measured before surgery, and the counts and frequencies of cells during the ensuing procedure in patients were not examined longitudinally. Second, the mechanism by which circulating immunocytes affect the prognosis of SCI should be further explored. Finally, a large-sample, multicenter research is required to confirm the findings from this investigation.
In general, through immune profiling of both mice and patients with SCI, we found associations between early alterations in circulating immunocytes and injury severity, and neurological outcomes in patients with traumatic SCI. The results from this study provided a new immunocyte combination for which the prognostic value of SCI should be further studied. To further understand how these circulating immune cell subtypes respond to and/or are modulated by SCI, the next step will be to investigate them at higher resolution in order to better identify their functional roles, capacities and predictive values in connection to SCI recovery.
Ethical approval:
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the First Affiliated Hospital of Nanjing Medical University (Ethics Number: 2021-SR-124). The animal study protocol was approved by the Ethics Committee of Animal Experiments at Nanjing Medical University.
Author’s contributions:
Conceptualization was done by Xuan Zhao; formal analysis ,investigation, methodology and project administration was done by Xuan Zhao, Yin Li and Ao Xue; funding acquisition by Xuan Zhao; supervision by Xuan Zhao; validation by Xuan Zhao and Yin Li; writing-original draft by Xuan Zhao; review, editing, and writing by Xuan Zhao. All authors have read and agreed to the published version of the manuscript.
Acknowledgment:
Xuan Zhao, Yin Li and Ao Xue have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Ahuja CS, Wilson JR, Nori S, Kotter M, Druschel C, Curt A, et al. Traumatic spinal cord injury. Nat Rev Dis Primers 2017;3(1):1-21.
- McDonald JW, Sadowsky C. Spinal-cord injury. Lancet 2002;359(9304):417-25.
- Feigin VL, Nichols E, Alam T, Bannick MS, Beghi E, Blake N, et al. Global, regional and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18(5):459-80.
[Crossref] [Google Scholar] [PubMed]
- Heller RA, Seelig J, Crowell HL, Pilz M, Haubruck P, Sun Q, et al. Predicting neurological recovery after traumatic spinal cord injury by time-resolved analysis of monocyte subsets. Brain 2021;144(10):3159-74.
[Crossref] [Google Scholar] [PubMed]
- Jogia T, Kopp MA, Schwab JM, Ruitenberg MJ. Peripheral white blood cell responses as emerging biomarkers for patient stratification and prognosis in acute spinal cord injury. Curr Opin Neurol 2021;34(6):796-803.
[Crossref] [Google Scholar] [PubMed]
- Priebe MM, Chiodo AE, Scelza WM, Kirshblum SC, Wuermser LA, Ho CH. Spinal cord injury medicine. 6. Economic and societal issues in spinal cord injury. Arch Phys Med Rehabil 2007;88(3):S84-8.
[Crossref] [Google Scholar] [PubMed]
- Schwab JM, Zhang Y, Kopp MA, Brommer B, Popovich PG. The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Exp Neurol 2014;258:121-9.
[Crossref] [Google Scholar] [PubMed]
- Hayakawa K, Okazaki R, Ishii K, Ueno T, Izawa N, Tanaka Y, et al. Phosphorylated neurofilament subunit NF-H as a biomarker for evaluating the severity of spinal cord injury patients, a pilot study. Spinal Cord 2012;50(7):493-6.
[Crossref] [Google Scholar] [PubMed]
- Kuhle J, Gaiottino J, Leppert D, Petzold A, Bestwick JP, Malaspina A, et al. Serum neurofilament light chain is a biomarker of human spinal cord injury severity and outcome. J Neurol Neurosurg Psychiatry 2015;86(3):273-9.
[Crossref] [Google Scholar] [PubMed]
- Olivecrona M, Rodling-Wahlström M, Naredi S, Koskinen LD. S-100B and neuron specific enolase are poor outcome predictors in severe traumatic brain injury treated by an intracranial pressure targeted therapy. J Neurol Neurosurg Psychiatry 2009;80(11):1241-8.
[Crossref] [Google Scholar] [PubMed]
- Snyder R, Verla T, Ropper AE. Practical application of recent advances in diagnostic, prognostic and therapeutic modalities for spinal cord injury. World Neurosurg 2020;136:330-6.
[Crossref] [Google Scholar] [PubMed]
- Streijger F, Skinnider MA, Rogalski JC, Balshaw R, Shannon CP, Prudova A, et al. A targeted proteomics analysis of cerebrospinal fluid after acute human spinal cord injury. J Neurotrauma 2017;34(12):2054-68.
[Crossref] [Google Scholar] [PubMed]
- Kyritsis N, Torres-Espín A, Schupp PG, Huie JR, Chou A, Duong-Fernandez X, et al. Diagnostic blood RNA profiles for human acute spinal cord injury. J Exp Med 2021;218(3):e20201795.
[Crossref] [Google Scholar] [PubMed]
- Zhao JL, Lai ST, Du ZY, Xu J, Sun YR, Yuan Q, et al. Circulating neutrophil-to-lymphocyte ratio at admission predicts the long-term outcome in acute traumatic cervical spinal cord injury patients. BMC Musculoskeletal Disord 2020;21:1-7.
[Crossref] [Google Scholar] [PubMed]
- Wang D, Fan Y, Fan Y, Wang Z, Yang L, Huang J, et al. Peripheral monocyte percentage as a potential indicator of prognosis in patients with chronic subdural hematoma receiving conservative therapy. World Neurosurg 2022;165:e92-101.
[Crossref] [Google Scholar] [PubMed]
- Waters RL, Yakura JS, Adkins RH, Sie I. Recovery following complete paraplegia. Arch Phys Med Rehabil 1992;73(9):784-9.
[Google Scholar] [PubMed]
- Waters RL, Adkins RH, Yakura JS, Sie I. Motor and sensory recovery following complete tetraplegia. Arch Phys Med Rehabil 1993;74(3):242-7.
[Google Scholar] [PubMed]
- Zhong G, Yang Y, Huang X, Chen J, Feng D, Wei K, et al. The serum SIRT1 protein is associated with the severity of injury and neurological recovery in mice with traumatic spinal cord injury. Neuroscience 2021;469:103-9.
[Crossref] [Google Scholar] [PubMed]
- Chen H, Ji H, Zhang M, Liu Z, Lao L, Deng C, et al. An agonist of the protective factor SIRT1 improves functional recovery and promotes neuronal survival by attenuating inflammation after spinal cord injury. J Neurosci 2017;37(11):2916-30.
[Crossref] [Google Scholar] [PubMed]
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, Mctigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 2006;23(5):635-59.
[Crossref] [Google Scholar] [PubMed]
- Neirinckx V, Coste C, Franzen R, Gothot A, Rogister B, Wislet S. Neutrophil contribution to spinal cord injury and repair. J Neuroinflammation 2014;11(1):150.
[Crossref] [Google Scholar] [PubMed]
- Gris D, Hamilton EF, Weaver LC. The systemic inflammatory response after spinal cord injury damages lungs and kidneys. Exp Neurol 2008;211(1):259-70.
[Crossref] [Google Scholar] [PubMed]
- David S, Kroner A, Greenhalgh AD, Zarruk JG, Lopez-Vales R. Myeloid cell responses after spinal cord injury. J Neuroimmunol 2018;321:97-108.
[Crossref] [Google Scholar] [PubMed]
- Riegger T, Conrad S, Liu K, Schluesener HJ, Adibzahdeh M, Schwab JM. Spinal cord injury-induced immune depression syndrome (SCI-IDS). Eur J Neurosci 2007;25(6):1743-7.
[Crossref] [Google Scholar] [PubMed]
- Failli V, Kopp MA, Gericke C, Martus P, Klingbeil S, Brommer B, et al. Functional neurological recovery after spinal cord injury is impaired in patients with infections. Brain 2012;135(11):3238-50.
[Crossref] [Google Scholar] [PubMed]
- Pruss H, Tedeschi A, Thiriot A, Lynch L, Loughhead SM, Stutte S, et al. Spinal cord injury-induced immunodeficiency is mediated by a sympathetic-neuroendocrine adrenal reflex. Nat Neurosci 2017;20(11):1549-59.
[Crossref] [Google Scholar] [PubMed]
- Campagnolo DI, Bartlett JA, Keller SE, Sanchez W, Oza R. Impaired phagocytosis of Staphylococcus aureus in complete tetraplegics. Am J Phys Med Rehabil 1997;76(4):276-80.
[Crossref] [Google Scholar] [PubMed]
- Cruse JM, Lewis RE, Bishop GR, Kliesch WF, Gaitan E. Neuroendocrine-immune interactions associated with loss and restoration of immune system function in spinal cord injury and stroke patients. Immunol Res 1992;11:104-16.
[Crossref] [Google Scholar] [PubMed]
- Iversen PO, Hjeltnes N, Holm B, Flatebo T, Strom-Gundersen I, Ronning W, et al. Depressed immunity and impaired proliferation of hematopoietic progenitor cells in patients with complete spinal cord injury. Blood J Am Soc Hematol 2000;96(6):2081-3.
[Crossref] [Google Scholar] [PubMed]
- Jogia T, Lubstorf T, Jacobson E, Scriven E, Atresh S, Nguyen QH, et al. Prognostic value of early leukocyte fluctuations for recovery from traumatic spinal cord injury. Clin Transl Med 2021;11(1):e272.
[Crossref] [Google Scholar] [PubMed]
- Wu B, Matic D, Djogo N, Szpotowicz E, Schachner M, Jakovcevski I. Improved regeneration after spinal cord injury in mice lacking functional T-and B-lymphocytes. Exp Neurol 2012;237(2):274-85.
[Crossref] [Google Scholar] [PubMed]
- Riegger T, Conrad S, Schluesener HJ, Kaps HP, Badke A, Baron C, et al. Immune depression syndrome following human spinal cord injury (SCI): A pilot study. Neuroscience 2009;158(3):1194-9.
[Crossref] [Google Scholar] [PubMed]