- *Corresponding Author:
- R. Kumar
Department of Pharmaceutical Chemistry, Noida Institute of Engineering and Technology (Pharmacy Institute), Greater Noida, Uttar Pradesh 201310, India
E-mail: mpharm.rajnish@gmail.com
| Date of Received | 1 February 2023 |
| Date of Revision | 09 October 2023 |
| Date of Accepted | 06 August 2024 |
| Indian J Pharm Sci 2024;86(4):1187-1198 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
On basis of various research reports, pyridine was found to possess a wide spectrum of pharmacological activities along with many other industrial applications. Because of its diverse applications, pyridine moiety is the centre of attraction for researchers and a large number of patents have been granted focusing on it. Several synthetic protocols such as cyclo-condensation, cyclization, cycloaddition, electrolysis, etc., were used by researchers to synthesize pyridine and analogs. Each synthetic protocol has its merits and demerits and required several types of reagents, catalysts and reaction conditions. So, there is always a need for careful analysis of reported synthetic protocols whenever researchers like to initiate research consisting of the synthesis of pyridine and its analogs.
Keywords
Pyridine, pharmacological activities, patents, synthetic protocols
Nitrogen comprising heterocyclic moieties has high pharmacologically active molecules[1]. Pyridine (C5H5N) is a six-membered heterocyclic compound that exists as a colourless liquid at room temperature, is water-soluble and has an acrid smell[2]. It was discovered by Scottish chemist Thomas Anderson in 1849. Arthur Hantzch afterward synthesized pyridine compounds in 1881 through a multi-component reaction, consisting of β-ketoester, an aldehyde and ammonia[3]. Like benzene, all pyridine ring atoms are sp2 hybridized involving π electron resonance. The N atom is highly electronegative and its lone pair in an aromatic environment makes pyridine distinctive in chemistry[3]. The presence of an electronegative nitrogen atom in the ring prevents equal distribution of electron density over the ring because of its negative inductive effect causing weaker resonance stabilization[2]. Pyridine is also used as a chemical solvent and reagent[4]. Pyridine is found in many natural products like vitamins such as niacin, pyridoxal phosphate, alkaloids like nicotine and many drugs[5]. Based on numerous research reports pyridine was found to be effective as an anti-cancer[6], anticonvulsant[7], anti-microbial[8], anti-tubercular[9], anti-viral[10], anti-depressant[11], anti-inflammatory[12], antidiabetic[ 13], anti-Alzheimer[14], analgesic agent[15]. Pyridine also has many other industrial applications, such as optics[16] and agrochemicals[17]. The targets for pyridine and its derivatives are diverse such as enzymes, proteins and deoxyribonucleic acid[18,19]. This pyridine ring is a biologically active core (pharmacophore) in a large number of pharmaceutically available drugs (Table 1)[20-27]. Due to the wide range of pharmacological and industrial applications of pyridine, it has always been the focus of researchers. Several patents have been granted on the synthetic and pharmacological works related to pyridine and its derivatives. The recently granted patents in 2022 are highlighted in Table 2[28-39].
| S No. | Drug | Company | Description |
|---|---|---|---|
| 1 | Prevacid | Cipla | Proton-pump inhibitor |
| (Lansoprazole)[20] | |||
| 2 | Clarinex | Dr. Reddy | NSAID for allergic rhinitis and urticaria |
| (Desloratadine)[21] | |||
| 3 | Xalkori | Pfizer | Anticancer |
| (Crizotinib)[22] | |||
| 4 | Solonex | Macleods | Tuberculosis |
| (Isoniazid)[23] | |||
| 5 | Torsemide[24] | Cipla | Anti-hypertensive |
| 6 | Phenazopyridin[25] | A. Menarini | Lower urinary tract infections |
| 7 | Tedizolid[26] | Cubist | Antibiotic |
| 8 | Alpelisib[27] | Novartis | Anticancer |
Table 1: Marketed Drugs Bearing Pyridine Ring
| Patent Date | Patent No. | Description |
|---|---|---|
| 10-Nov-22 | US20220359090A1[28] | Predisposition Determination of Health conditions |
| 27-Oct-22 | US20220340874A1[29] | Enhanced Expansion of Tumor-Infiltrating Lymphocytes |
| 27-Oct-22 | US20220339154A1[30] | Generating inner ear hair cells for the treatment of hearing loss |
| 20-Oct-22 | US20220331334A1[31] | Prodrug comprising a Drug-Linker Conjugate |
| 20-Oct-22 | US20220331282A1[32] | Neutral Endopeptidase inhibitor (NEPi) |
| 13-Oct-22 | US20220324872A1[33] | CDK2/4/6 Inhibitors |
| 13-Oct-22 | US20220325360A1[34] | Methods for computer processing sequence reads to detect molecular residual disease |
| 22-Sep-22 | US20220296496A1[35] | Topical skin care formulations comprising plant extracts |
| 22-Sep-22 | US20220296575A1[36] | Pharmaceutical compositions |
| 22-Sep-22 | US20220296863A1[37] | Drug-releasing coatings for medical devices |
| 08-Sep-22 | US20220281854A1[38] | Combination therapy for treating cancer |
| 15-Sep-22 | US20220288229A1[39] | Targeted conjugates encapsulated in particles and formulations |
Table 2: List of Patents Bearing Pyridine Ring
Synthetic Approaches
The synthesis of pyridine involves several methods like Chichibabin synthesis, Bonnemann cyclization, Krohnke pyridine synthesis, Gattermann-Skita synthesis and several other methods. In Chichibabin pyridine synthesis firstly, acrolein is formed via Knoevenagel condensation from acetaldehyde and formaldehyde, then acrolein condenses with acetaldehyde and ammonia to give aminopyridine[40]. In Bonnemann cyclization, the trimerization of one part of a nitrile molecule and two parts of acetylene gives pyridine[41]. In Krohnke pyridine synthesis, the reaction of pyridine with bromomethyl ketones gives related pyridinium salt[42]. In Gattermann-Skita synthesis, malonate ester was made to react with dichloro methylamine[43].
Green synthesis:
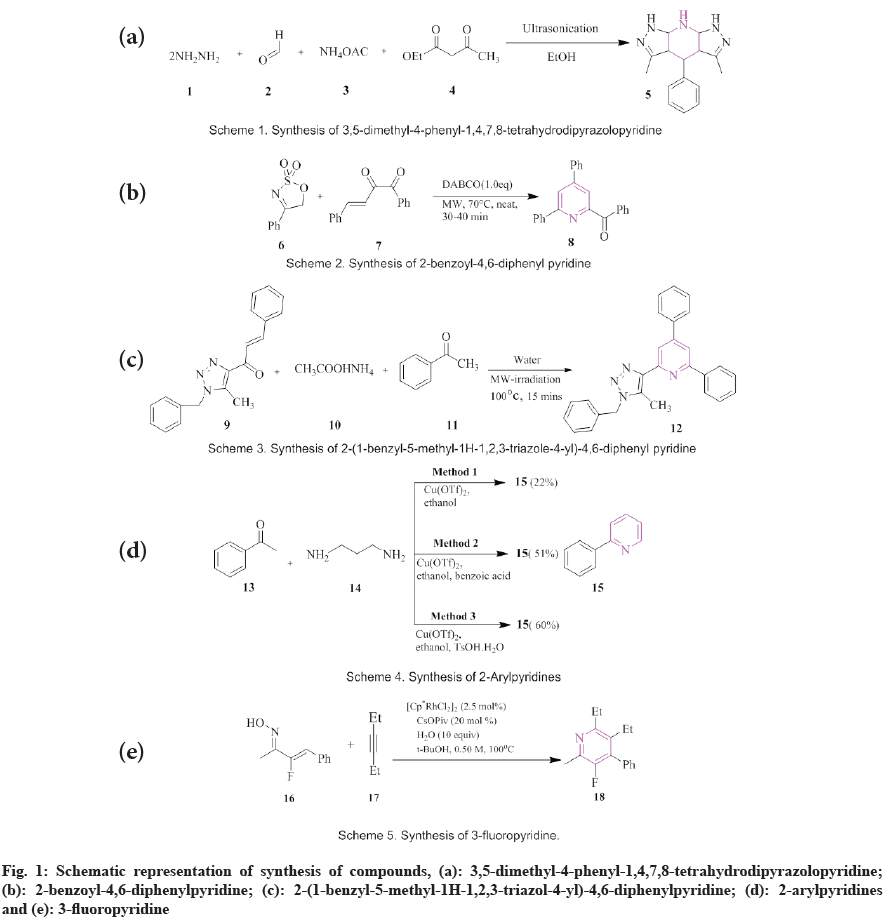
Dohare et al.[44] introduced ultrasound-induced synthesis of 3,5-dimethyl-4-phenyl-1,4,7,8- tetrahydrodipyrazolopyridine (5). The reaction takes place between hydrazine hydrate (1), β-dicarbonyl compound (2), substituted aldehydes (3) and ammonium acetate (4) using ethanol as a catalyst. The whole reaction takes 30- 40 min to complete (fig. 1a). Biswas et al., synthesized 2-benzoyl-4,6-diphenylpyridine (8) from cyclic sulfamidate imines (6) and β,γ- unsaturated α-keto carbonyl (7) in the presence of 1,4-diazabicyclo[2.2.2]octane. The reaction concoction was exposed to microwave irradiation in an open atmosphere at 70° for 30-40 min giving the desired product benzoyl-4,6-diphenylpyridine (fig. 1b)[45]. Raja et al., showed the synthesis of 2-(1-benzyl-5-methyl-1H-1,2,3-triazol-4- yl)-4,6-diphenylpyridine (12) by microwaveassisted reaction. The product was synthesized by a reaction of 1-benzyl-5-methyl-1,2,3-triazol- 4-yl-3-arylprop-2-en-1-ones (9), ammonium acetate (10) and ketone (11) in water. The product was obtained with a yield of 90 % (fig. 1c)[46].
Metal-catalyzed reaction:
Copper (Cu)-catalyzed synthesis: Xi et al., stated Cu-catalyzed aerobic reaction for the synthesis of 2-arylpyridines (18). On heating acetophenone (13), with 1,3-diamino propane (14) in the presence of Copper(II) triflate (Cu(OTf)2) in ethanol for 80° in an oxygen environment for 72 h giving 2-arylpyridine with 22 % yield (fig. 1d); The second method consists of a reaction of 13 with 14 in the presence of Cu(OTf)2 in ethanol and benzoic acid giving 2-arylpyridine with 51 % yield (fig. 1d). The third method consists of acetophenone with 1,3-diamino propane in the presence of Cu(OTf)2 in ethanol and p-Toluene Sulphonic Acid (PTSA) giving 2-arylpyridine with 60 % yield (fig. 1e)[47].
Rhodium (Rh) (III)-catalyzed synthesis: Chen et al., synthesized a one-step method for the preparation of 3-fluoropyridine (18) from α-fluoro- α,β-unsaturated oximes (16) with terminal alkyne (17) by Rh(III)-catalyzed C-H functionalization (fig. 1)[48].
Cyclization:
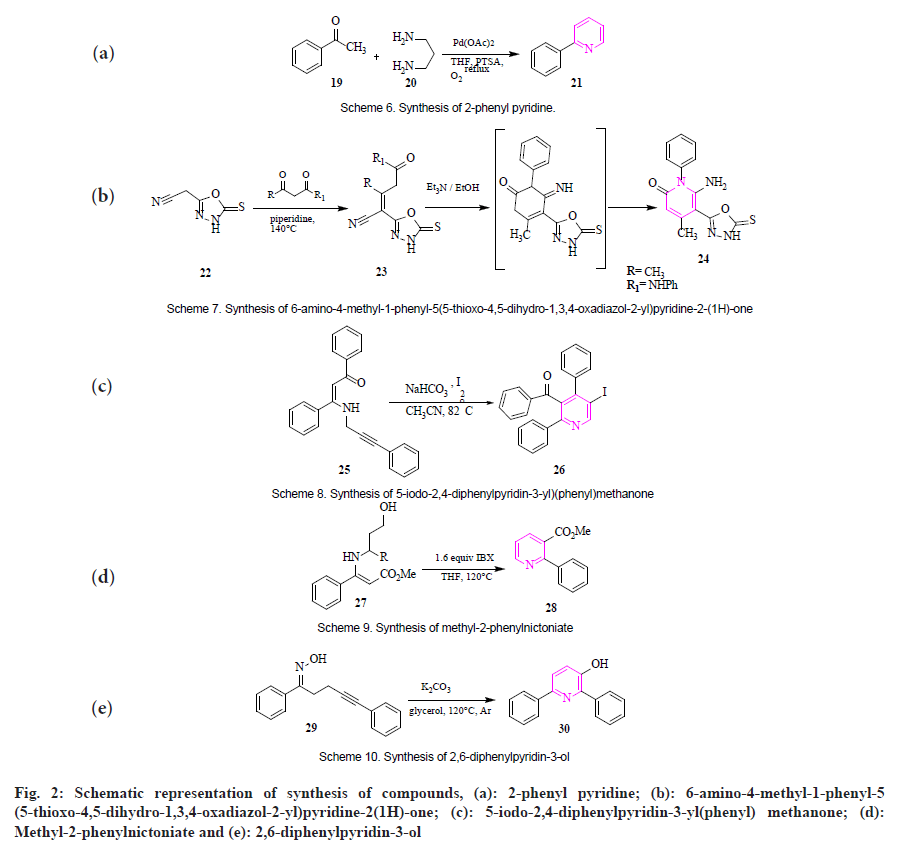
In presence of PTSA: Ghodse et al., showed the synthesis of 2-phenyl pyridine (21) by acetophenone (19) and 1,3-diaminopropane (20) in presence of palladium acetate and PTSA in tetrahydrofuran as solvent at reflux temperature for 10 h in presence of oxygen. The product was obtained with a good yield (fig. 2a)[49].
In the presence of triethylamine: Albratti et al., synthesized oxadiazole-based pyridine derivative 6-amino-4-methyl-1-phenyl-5(5- t h i o x o - 4 , 5 - d i h y d r o - 1 , 3 , 4 - o x a d i a z o l - 2 - y l ) pyridine-2(1H)-one (24). When 2-(5-thioxo-4,5- dihydro-1,3,4-oxadiazole-2-yl) acetonitrile (22) reacts with acetylacetone or acetoacetanilide to form 4-cyano-3-methyl-N-phenyl-4(5- thioxo-4,5-dihydro-1,3,4-oxadiazole-2-yl) but-3-enamid (23). 1,5-diphenylpent-4-yn- 1-one oxime goes through cyclization to afford 2,6-diphenylpyridin-3-ol (fig. 2b)[50].
By electrophilic cyclization: Karadeniz et al., showed a facile electrophilic cyclization for the synthesis of 5-iodo-2,4-diphenylpyridin-3- yl (phenyl)methanone (26) from N-propargylic β-enaminones (25) in the presence of CH3CN, NaHCO3 and iodine at 82°. The product was obtained with an 80 % yield (fig. 2c)[51].
2-Iodoxybenzoic acid-mediated selected oxidative cyclization: Gao et al., reported the synthesis of methyl-2-phenylnictoniate (28) by reaction of enaminoesters (27) bearing hydroxypropyl derivatives, which were made to react with 1.6 equivalent of 2-iodoxybenzoic acid in tetrahydrofuran as solvent gave desired product with 82 % yield (fig. 2d)[52].
By Potassium carbonate (K2CO3)-mediated cyclization: Wang et al., designed K2CO3-mediated cyclization and rearrangement of γ,δ-alkynyl oximes for the synthesis of 2,6-diphenylpyridin- 3-ol (30). Compound (E)-1,5-diphenylpent-4-yn- 1-one oxime (29) in presence of K2CO3 as base and glycerol as solvent at 120° for 12 h giving 2,6-diphenylpyridin-3-ol in 74 % yield (fig. 2e)[53].
Fig. 2: Schematic representation of synthesis of compounds, (a): 2-phenyl pyridine; (b): 6-amino-4-methyl-1-phenyl-5 (5-thioxo-4,5-dihydro-1,3,4-oxadiazol-2-yl)pyridine-2(1H)-one; (c): 5-iodo-2,4- diphenylpyridin-3-yl(phenyl) methanone; (d): Methyl-2-phenylnictoniate and (e): 2,6-diphenylpyridin-3-ol
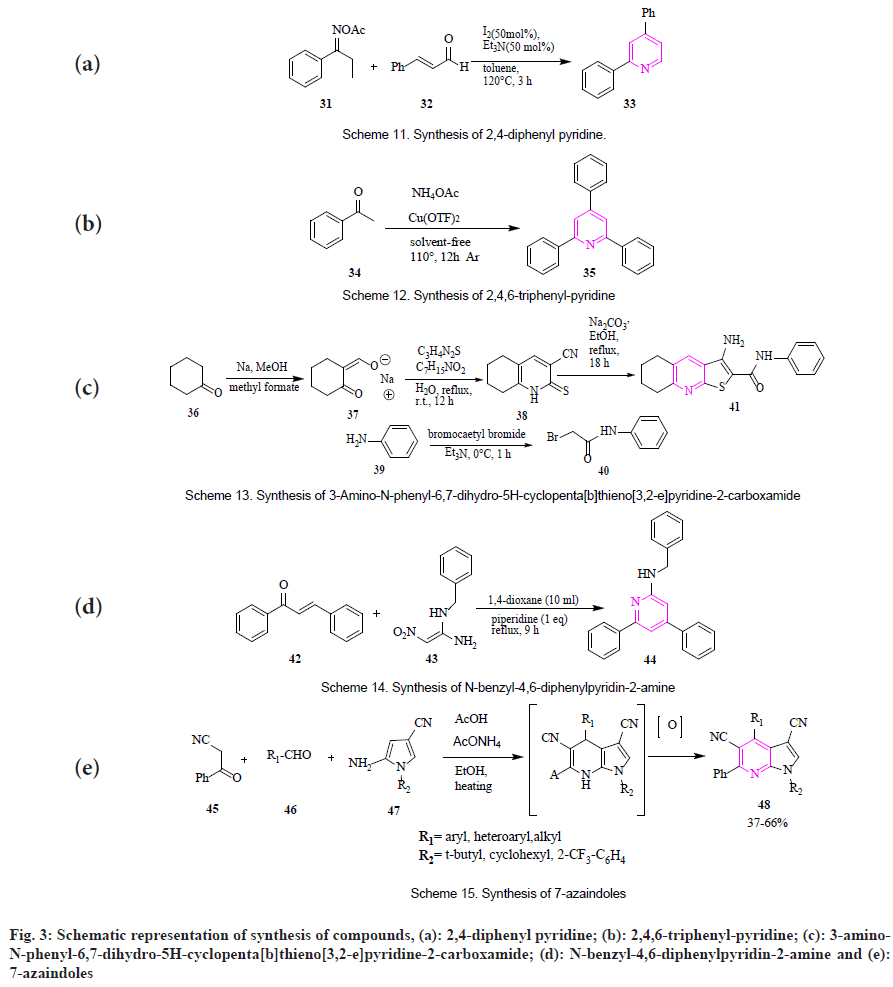
By metal-free cyclization: Huang et al., reported synthesis for 2,4-diphenyl pyridine (33) by reaction of o-acetyl ketoxime (31) and α,β- unsaturated aldehydes (32) in presence of iodine, triethylamine and toluene as solvent (fig. 3a)[54].
By Cu-catalyzed cyclization: Zhang et al., synthesized 2,4,6-triphenyl-pyridine (35) in presence of acetophenone (34) and NH4AOc, using Cu(OTf)2 as a catalyst in the solventfree environment releasing CH4. The product was obtained with a yield of 71 % (fig. 3b)[55].
By concomitant cyclization: Arabhshahi et al., showed the synthesis of 3-amino-N-phenyl-6,7- dihydro-5H-cyclopenta[b]thieno[3,2-e]pyridine- 2-carboxamide (41) in a three-step process. Initially, sodium (E) and (Z)-(2-oxocyclopentylidene) methanolate (37) was produced from the reaction of cyclopentanone (36) with freshly-prepared sodium methoxide and methyl formate. Secondly, (Z)-(2-oxocyclopentylidene) methanolate was made to react with cyanothioacetamide and piperidinium acetate followed by acidification with acetic acid giving 2-thioxo-2,5,6,7-tetrahydro- 1H-cyclopenta[b] pyridine-3-carbonitrile (38). Further, 2-bromo-N-phenylacetamide (40) was synthesized from the reaction between aniline (39) and bromoacetyl bromide in presence of triethylamine. Lastly, 2-bromo-N-phenylacetamide was mixed with 2-thioxo-2,5,6,7-tetrahydro-1Hcyclopenta[ b] pyridine-3-carbonitrile in presence of anhydrous sodium carbonate in absolute ethanol giving desired product in 55 % yield (fig. 3c)[56].
By regioselective cyclization: Luo et al., reported the synthesis of N-benzyl-4,6-diphenylpyridin- 2-amine (44) by regioselective Michael reaction, cyclization and loss of one molecule of NO2. In this, when α, β-unsaturated ketones (42) and reductive aminases (43) in presence of 1,4-dioxane as solvent was made to react with 1,4-dioxane. Further piperidine was added to the mixture and the solution was stirred at heating conditions (fig. 3d)[57].
Condensation reaction:
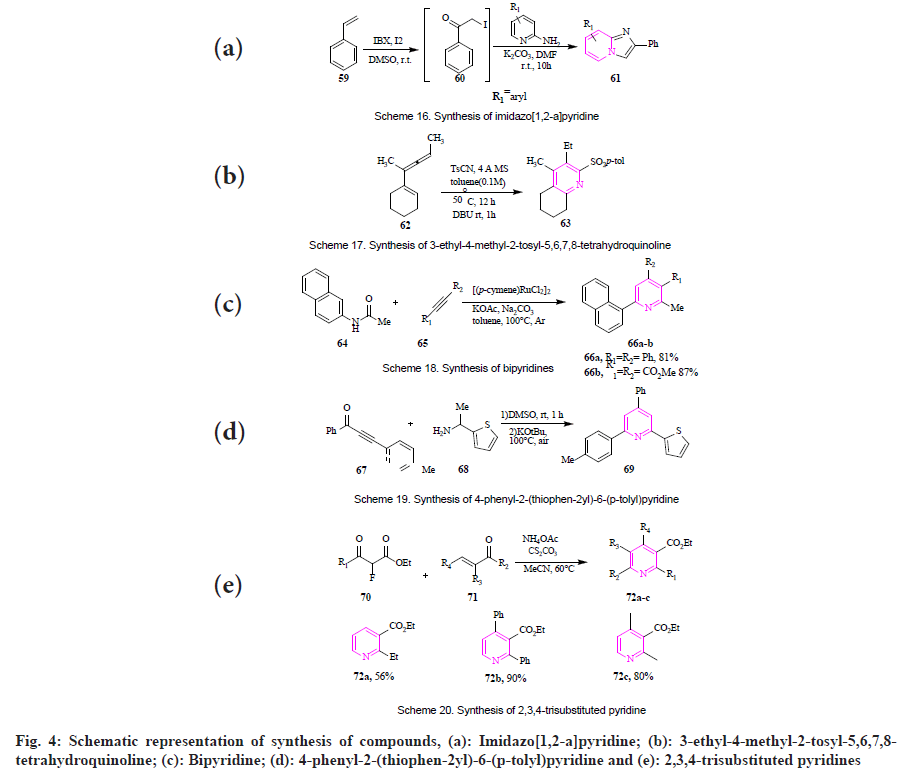
Motati et al., demonstrated that 7-azaindoles (48) were synthesized through a multicomponent condensation reaction. The reaction occurs between 2-amino-4-cyano pyrrole (47) with compounds having active methylene group (45) and different aldehydes (46) followed by oxidation using AcOH and AcONH4 as catalysts (fig. 3e)[58]. Peicherla et al., established the synthesis of imidazo[1,2-a]pyridine (51) by using cyclocondensation. The reaction forms an intermediate of α-halo carbonyl compound (50) by the reaction of alkenes (49) in the presence of 2-iodoxy-benzoic acid/iodine/dimethyl sulfoxide. Further, the α-iodo ketones (50) were mixed with 2-aminopyridine in presence of K2CO3 and dimethylformamide to give imidazo[1,2-a]pyridine. The product was obtained with a yield of 55 %-71 % (fig. 4a)[59].
Addition reaction:
By ammonium acetate+β-dicarbonyl compound cycloaddition: Bartko et al., designed the synthesis of 3-ethyl-4-methyl-2-tosyl-5,6,7,8- tetrahydroquinoline (63) in presence of 1-(cyclohexyl-1-en-yl)-5-phenylpent-1-yn-3-ol (62) in presence of toluenesulfonyl cyanide and toluene as solvent (fig. 4b)[60]. Wu et al., reported the synthesis of bipyridines (66) by reaction of N-vinyl amide (64) and alkyne (65) in the presence of [(p-cymene RuCl2]m, Na2CO3, KOAc and toluene at 100° under argon atmosphere at 56 h gave highly substituted bipyridines (fig. 4c)[61].
By Michael addition: Shen et al., reported the synthesis of 4-phenyl-2-(thiophen-2yl)-6-(p-tolyl) pyridine (69) from ynones (67) and 1-arylethamine (68) through intramolecular Michael addition reaction in presence of dimethyl sulfoxide and potassium tert-butoxide at 100° under an air atmosphere. The product was obtained with a 68 % yield (fig. 4d)[62]. Song et al., reported the synthesis of 2,3,4-trisubstituted pyridines (72a-c) by reaction of α-fluoro-β-ketoester (70) reacted with α, β-unsaturated aldehydes (71) as Michael acceptors in presence of Cs2CO3 and MeCN at 60° (fig. 4e)[63].
Electrolysis:
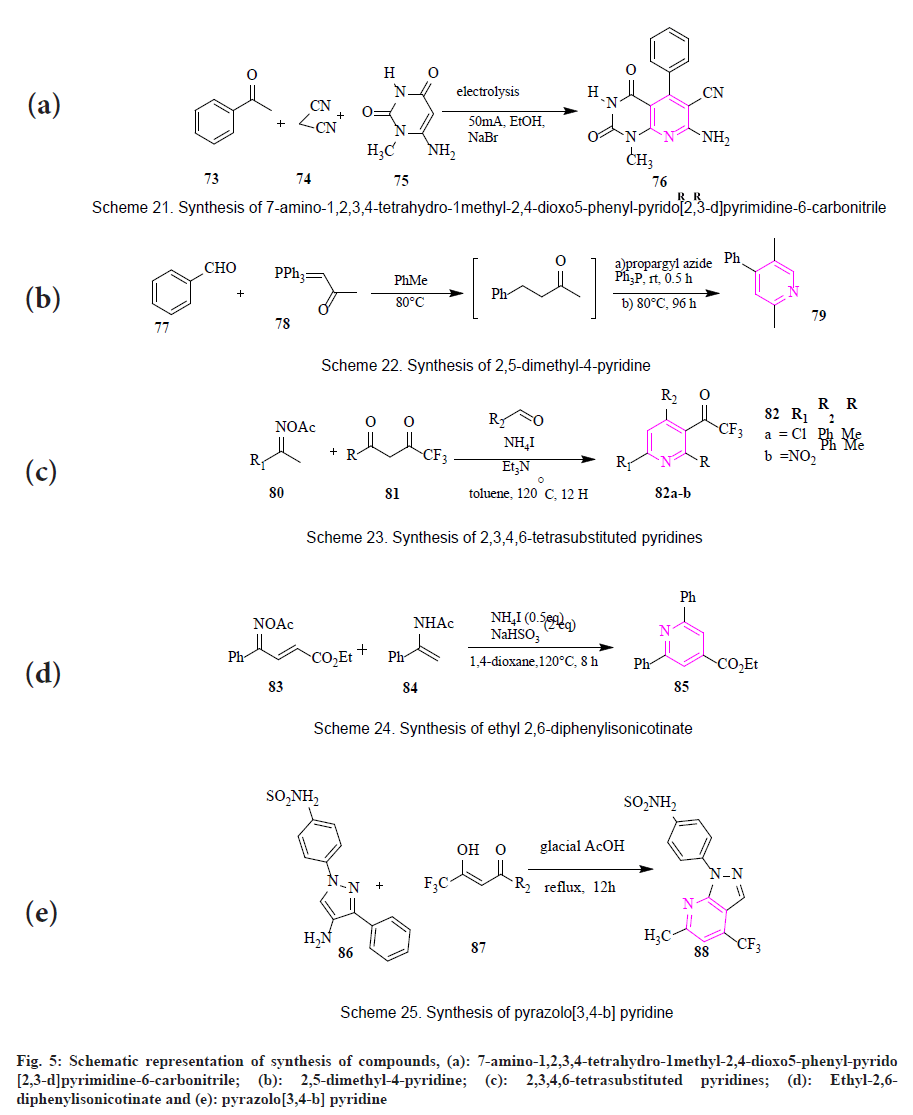
Upadhyay et al., synthesized 7-amino-1,2,3,4- tetrahydro-1methyl-2,4-dioxo5-phenyl-pyrido [2,3-d] pyrimidine-6-carbonitrile (76) by an electrochemical induced transformation of aryl aldehydes (73), malononitrile (74) and 6-aminouracil (75) in presence of NaBr in ethanol giving 7-amino-1,2,3,4-tetrahydro- 1methyl-2,4-dioxo5-phenyl-pyrido [2,3- d] pyrimidine-6-carbonitrile (fig. 5a)[64].
Wittig reaction:
Wei et al., gave an efficient strategy for the synthesis of 2,5-dimethyl-4-pyridine (79). Wittig reaction of benzaldehyde (77) and phosphorus ylide (78) was conducted at a temperature of 90° for 5 h in the presence of PhMe.
The mixture was then cooled until it reached room temperature. When it reached room temperature propargyl azide was added along with triphenylphosphine. The product was obtained with a 79 % yield (fig. 5b)[65].
Annulation type reaction:
By Hantzch-type annulation: Huang et al., displayed the synthesis of 2,3,4,6-tetrasubstituted pyridines (82a-b) by a three-component reaction of oximes (80) with trifluoromethyl-diketones (81) and aldehydes in presence of NH4I and triethylamine giving 2,3,4,6-tetrasubstituted pyridines in moderate yields (fig. 5c)[66].
By Ammonium iodide (NH4I)-triggered (ammonium acetate+β-dicarbonyl compound) annulation: Duan et al., designed and synthesized ethyl-2,6-diphenylisonicotinate (85). The reaction between ketoxime-enoates (83) and N-acetyl enamide (84) in presence of NH4I and sodium bisulfate, the product was obtained when 1,4-dioxane was added and the mixture was stirred for 8 h at 120° with 83 % yield (fig. 5d)[67].
Miscellaneous:
By using glacial acetic acid: Maria et al., synthesized pyrazolo[3,4-b] pyridine (88) by reacting 3-substituted-(5-amino-1Hpyrazol- 1-yl) benzenesulfonamide (86) with trifluoromethyl-β-diketone (87) (fig. 5e)[3].
Fig. 5: Schematic representation of synthesis of compounds, (a): 7-amino-1,2,3,4-tetrahydro-1methyl-2,4-dioxo5-phenyl-pyrido [2,3-d]pyrimidine-6-carbonitrile; (b): 2,5-dimethyl-4-pyridine; (c): 2,3,4,6-tetrasubstituted pyridines; (d): Ethyl-2,6- diphenylisonicotinate and (e): pyrazolo[3,4-b] pyridine
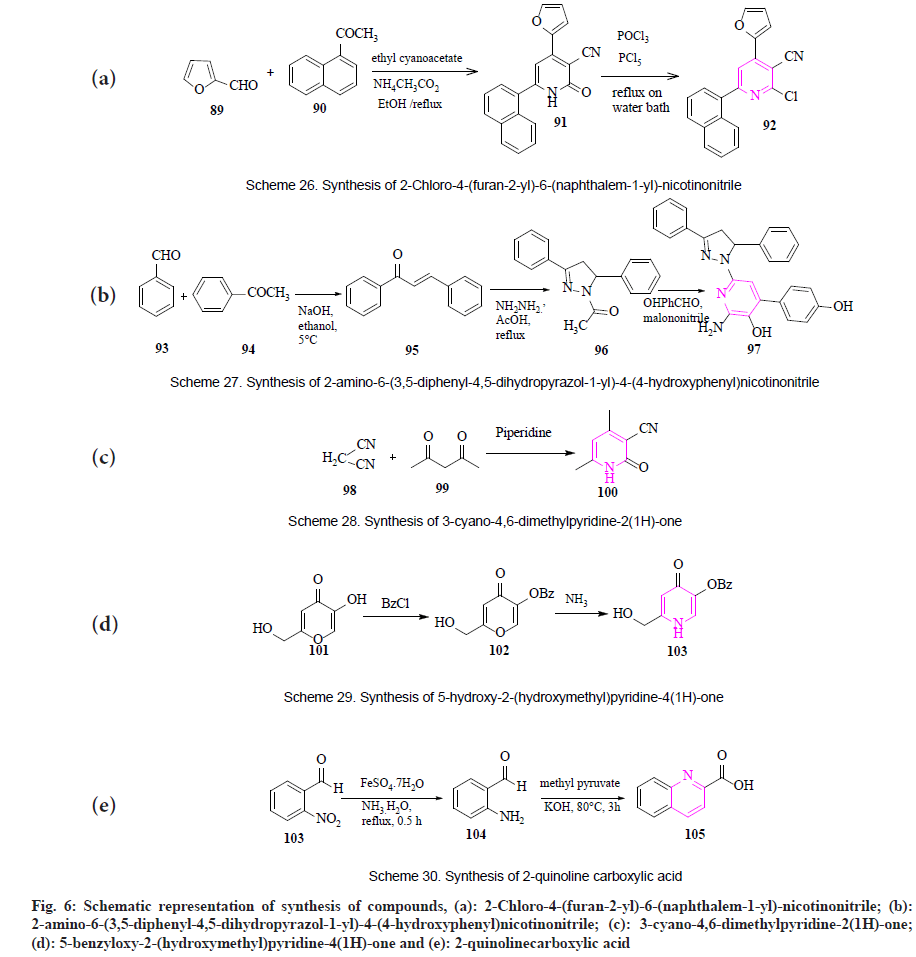
By using Phosphoryl chloride (POCl3): Salem et al., synthesized 2-chloro-4-(furan- 2 - y l ) - 6 - ( n a p h t h a l e m - 1 - y l ) - n i c o t i n o n i t r i l e (92) by the four-component reaction. Firstly, the one-pot reaction between 1-acetylnaphthalene (89), furfural (90), ethyl cyanoacetate and ammonium acetate in absolute ethanol giving 4-(furan-2-yl)-6-(naphthalen-1- yl)-2-oxo-1,2-dihydropyridine-3-carbonitrile (91). Secondly, the chlorination of enaminoesters in a mixture of phosphorus oxychloride and phosphorus pentachloride was heated giving off 2-chloro-4-(furan-2-yl)-6- (naphthalem-1-yl)-nicotinonitrile (fig. 6a)[68].
By using malononitrile: Siddiqui et al., designed and synthesized 2-amino-6-(3,5- d i p h e n y l - 4 , 5 - d i h y d r o p y r a z o l - 1 - y l ) - 4 - ( 4 - hydroxyphenyl) nicotinonitrile (97). The reaction takes place among substituted acetophenone (93) and benzaldehyde (94) giving 1-(hydroxy phenyl)-3-phenylpropenones (95). 1-(hydroxy phenyl)-3-phenylpropenones on reaction with hydrazine hydrate gave 1-(3-hydroxy p h e n y l - 5 - p h e n y l - 4 , 5 - d i h y d r o p y r a z o l - 1 - yl) ethanones (96), was further treated with malononitrile and ammonium acetate and refluxed for 10 h giving 2-amino-6-(3,5-diphenyl-4,5- dihydropyrazol-1-yl)-4-(4-hydroxyphenyl) nicotinonitrile in good yield (fig. 6b)[69].
By using piperidine: Kamal et al., synthesized some new heterocyclic pyridine derivative 3-cyano- 4,6-dimethylpyridine-2(1H)-one (100) from the reaction of malononitrile (98) with acetylacetone (99) to give 3-cyano-4,6-dimethylpyridine-2(1H)- one (fig. 6c)[70].
By using aqueous ammonia: Lachowicz et al., presented a synthesis of 5-benzyloxy-2- (hydroxymethyl)pyridine-4(1H)-one (103) obtained by using kojic acid (101). Firstly, it comprises the protection of the 5-hydroxyl group of kojic acid with a benzyl group, by a reaction of benzyl chloride leading to the formation of 5-benzyloxy- 2-(hydroxymethyl)-4H-pyran-4-one (102). It was further converted into 5-benzyloxy- 2 - ( h y d r o x y m e t h y l ) p y r i d i n e - 4 ( 1 H ) - one by reaction with aqueous ammonia under reflux conditions (fig. 6d)[71].
By using methyl pyruvate: Sun et al., synthesized 2-quinolinecarboxylic acid (105) by treating 2-nitrobenzaldehyde (104) with ferrous sulphate and ammonia in the presence of N, N-dimethylformamide giving 2-aminobenzaldehyde (104). Further, 2-aminobenzaldehyde was treated with methyl pyruvate in alkaline conditions giving 2-quinolinecarboxylic acid (fig. 6e)[72]. In conclusion, this article mainly highlights newly stated synthetic procedures for pyridine-containing compounds accompanied by pharmacological activity and structure-activity relationship. In this, different approaches for the synthesis of pyridine derivatives like green synthesis, metal-catalyzed reaction, condensation, cyclization, addition, annulation-type reaction, etc., are reported. Out of these, green synthesis was reported as most easy and time-saving method, as they need easily available solvents (water, ethanol) and operate at an optimum temperature (70°-100°) requiring lesser time (30-40 min) as compared to conventional methods.
Fig. 6: Schematic representation of synthesis of compounds, (a): 2-Chloro-4-(furan-2-yl)-6-(naphthalem-1-yl)-nicotinonitrile; (b): 2-amino-6-(3,5-diphenyl-4,5-dihydropyrazol-1-yl)-4-(4-hydroxyphenyl)nicotinonitrile; (c): 3-cyano-4,6-dimethylpyridine-2(1H)-one; (d): 5-benzyloxy-2-(hydroxymethyl)pyridine-4(1H)-one and (e): 2-quinolinecarboxylic acid
Metal-catalyzed reactions were found not so favourable for small scale preparations as they required expensive solvents (i-PrOH, 2,2,6,6-tetramethylpiperidine 1-oxyl radical, tert-butyl alcohol), yields were also found to be moderate but reagents required were easily available (cyclohexanone, acetophenone, propiophenone). Further, cyclization reactions showed good yield of derivatives (55 %-85 %) and mostly easily available reagents were used (ketones). In addition reaction, optimum temperature was used (50°- 110°) and mostly least expensive solvent was used (toluene). Lastly, an annulation-type reaction was carried out at 120° for approximately 8-12 h showing moderate to good yields using easily available solvent (toluene, 1.4-dioxane). In the structure-activity relationship section, a link was recognized between various pyridine-containing derivatives and functional groups.
Pyridine is a pharmacologically active moiety exhibiting anti-cancer, anti-viral, anti-depressant, anti-convulsant, anti-diabetic, anti-inflammatory, anti-tubercular, and anti-microbial. We hope that this article provides much-needed recent information to researchers who are engaged with pyridine in any way.
Conflict of interests:
The authors declare no conflict of interest.
References
- El Azab IH, Bakr RB, Elkanzi NA. Facile one-pot multicomponent synthesis of pyrazolo-thiazole substituted pyridines with potential anti-proliferative activity: Synthesis, in vitro and in silico studies. Molecules 2021;26(11):3103.
- Pal S. Pyridine: A useful ligand in transition metal complexes. Pyridine 2018;57:57-74.
- Marinescu M, Popa CV. Pyridine compounds with antimicrobial and antiviral activities. Int J Mol Sci 2022;23(10):5659.
[Crossref] [Google Scholar] [PubMed]
- Mahmood RM, Aljamali NM. Synthesis, spectral investigation, and microbial studying of pyridine-heterocyclic compounds. Eur J Mol Clin Med 2020;7(11):4444-53.
- Shankaraiah N, Sharma P, Pedapati S, Nekkanti S, Srinivasulu V, Praveen Kumar N, et al. Synthesis of novel C3-linked β-carboline-pyridine derivatives employing khronke reaction: DNA-binding ability and molecular modeling studies. Lett Drug Des Discov 2016;13(4):335-42.
- Eissa IH, El-Naggar AM, El-Hashash MA. Design, synthesis, molecular modeling and biological evaluation of novel 1H-pyrazolo [3, 4-b] pyridine derivatives as potential anticancer agents. Bioorg Chem 2016;67:43-56.
[Crossref] [Google Scholar] [PubMed]
- Grover G, Nath R, Bhatia R, Akhtar MJ. Synthetic and therapeutic perspectives of nitrogen containing heterocycles as anti-convulsants. Bioorg Med Chem 2020;28(15):115585.
[Crossref] [Google Scholar] [PubMed]
- Malani A, Makwana A, Monapara J, Ahmad I, Patel H, Desai N. Synthesis, molecular docking, DFT study, and in vitro antimicrobial activity of some 4‐(biphenyl‐4‐yl)‐1, 4‐dihydropyridine and 4‐(biphenyl‐4‐yl) pyridine derivatives. J Biochem Mol Toxicol 2021;35(11):e22903.
[Crossref] [Google Scholar] [PubMed]
- Jain S, Sen DJ. Synthesis, characterization, drug likeliness and biological activity of imidazopyridine derivatives as anti-tubercular agents. Indian J Chem 2022;61(3):305-12.
- Pawar SD, Gosavi DH, Pawar SD. Isoquinoline derivatives and its medicinal activity: A. 2021.
- Jwaid M. Design, synthesis, molecular docking and antifungal evaluation of mixed heterocyclic moieties containing pyridine, 1,3,4-oxadiazole and 1,2,3-triazol rings. Int J Drug Deliv Technol 2022;12(2):705-10.
- Ali EM, Abdel-Maksoud MS, Hassan RM, Mersal KI, Ammar UM, Se-In C, et al. Design, synthesis and anti-inflammatory activity of imidazol-5-yl pyridine derivatives as p38α/MAPK14 inhibitor. Bioorg Med Chem 2021;31:115969.
[Crossref] [Google Scholar] [PubMed]
- Sadawarte G, Jagatap S, Patil M, Jagrut V, Rajput JD. Synthesis of substituted pyridine based sulphonamides as an antidiabetic agent. Eur J Chem 2021;12(3):279-83.
- Abdpour S, Jalili-Baleh L, Nadri H, Forootanfar H, Bukhari SN, Ramazani A, et al. Chromone derivatives bearing pyridinium moiety as multi-target-directed ligands against Alzheimer’s disease. Bioorg Chem 2021;110:104750.
[Crossref] [Google Scholar] [PubMed]
- Pham HT, Martin JP, Le TG. Organocatalyzed synthesis and biological activity evaluation of hybrid compounds 4 H-pyrano [2, 3-b] pyridine derivatives. Synthetic Commun 2020;50(12):1845-53.
- Tashrifi Z, Mohammadi‐Khanaposhtani M, Larijani B, Mahdavi M. C3‐Functionalization of Imidazo [1, 2‐a] pyridines. Eur J Org Chem 2020;2020(3):269-84.
- Higasio YS, Shoji T. Heterocyclic compounds such as pyrroles, pyridines, pyrollidins, piperdines, indoles, imidazol and pyrazins. Appl Catal 2001;221(1-2):197-207.
- Altaf AA, Shahzad A, Gul Z, Rasool N, Badshah A, Lal B, et al. A review on the medicinal importance of pyridine derivatives. J Drug Des Med Chem 2015;1(1):1-11.
- Patil P, Sethy SP, Sameena T, Shailaja K. Pyridine and its biological activity: A review. Asian J Res Chem 2013;6(10):888-99.
- Agranat I, Marom H. In defense of secondary pharmaceutical patents in drug discovery and development. ACS Med Chem Lett 2020;11(2):91-8.
[Crossref] [Google Scholar] [PubMed]
- Baumann M, Baxendale IR. An overview of the synthetic routes to the best selling drugs containing 6-membered heterocycles. Beilstein J Org Chem 2013;9(1):2265-319.
[Crossref] [Google Scholar] [PubMed]
- Goetz AE, Garg NK. Regioselective reactions of 3, 4-pyridynes enabled by the aryne distortion model. Nat Chem 2013;5(1):54-60.
[Crossref] [Google Scholar] [PubMed]
- Ling Y, Hao ZY, Liang D, Zhang CL, Liu YF, Wang Y. The expanding role of pyridine and dihydropyridine scaffolds in drug design. Drug Des Devel Ther 2021:4289-338.
[Crossref] [Google Scholar] [PubMed]
- Lopez B, González A, Hermida N, Laviades C, Díez J. Myocardial fibrosis in chronic kidney disease: Potential benefits of torasemide: New strategies to prevent cardiovascular risk in chronic kidney disease. Kidney Int Suppl 2008;74:S19-23.
[Crossref] [Google Scholar] [PubMed]
- Shore SN, Britnell SR, Brown JN. Safety analysis of long-term phenazopyridine use for radiation cystitis. J Oncol Pharm Pract 2020;26(2):306-11.
[Crossref] [Google Scholar] [PubMed]
- Roger C, Roberts JA, Muller L. Clinical pharmacokinetics and pharmacodynamics of oxazolidinones. Clin Pharmacokinet 2018;57:559-75.
[Crossref] [Google Scholar] [PubMed]
- Konstantinopoulos PA, Barry WT, Birrer M, Westin SN, Cadoo KA, Shapiro GI, et al. Olaparib and α-specific PI3K inhibitor alpelisib for patients with epithelial ovarian cancer: A dose-escalation and dose-expansion phase 1b trial. Lancet Oncol 2019;20(4):570-80.
[Crossref] [Google Scholar] [PubMed]
- Andrew AK, Charles AE. Attribute combination discovery for predisposition determination of health conditions. United States of America Patent 0359090; 2022.
- Sarnik AA, Weber JS, Thomas SP, Radvanyi LG, Chacon JA, Mule JJ, et al. Enhanced expansion of tumor-infiltrating lympocytes for adoptive cell therapy. United States of America Patent 0340874;2022.
- Karp JM, Langer RS, Yin X, Joshi N. Compositions, systems and methods for generating inner ear hair cells for treatment of HEARing loss. United States of America Patents 0339154;2022.
- Cleeman F, Hersel U, Kaden S, Rau H, Wegge T. Prodrug comprising a drug-linker conjugate. United States of America Patent 0331334;2022.
- Feng L, Godtfredsen E, Sutton PA, Prashad M, Girgis MJ, Hu B, et al. Amorphous solid form of compounds containing S.-N. Valeryl -N-{[2′-1H -Tetrazole-5-yl)-biphenyl-4-yl]-methyl}-valine and (2R, 4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl pentanoic acid ethyl ester moieties and sodium cations. United States of America Patent 0331282;2022.
- Behenna DC, Chen P, Freeman-Cook KD, Hoffman RL, Jalaie M, Nagata A, et al. CDK2/4/6 inhibitors. United States of America Patent 0324872;2022.
- Mortimer SAW, Talasaz AA, Chudova D, Eltoukhy H. Methods for computer processing sequence reads to detect molecular residual disease. United States of America Patent 0325360;2022.
- Florence T, Gan D, Hines M. Topical skin care formulations comprising plant extracts. United States of America Patent 0296496;2022.
- Brisander M, Demirbuker M, Jesson G, Malmsten M, Derand H. Pharmaceutical compositions. United States of America Patent 0296575;2022.
- Wang L. Drug releasing coatings for medical devices. United States of America Patent 0296863;2022.
- Keilhack H, Knutson SK, Kuntz KW. Combination therapy for treating Cancer. United States of America Patent 0281854;2022.
- Bilodeau MT, Kadiyala S, Shinde RR, White BH, Wooster R, Moreau B. Targeted conjugates encapsulated in particles and formulations thereof. United States of America Patent 0288229;2022. [Crossref] [Google Scholar] [PubMed]
- Shimizu S, Watanabe N, Kataoka T, Shoji T, Abe N, Morishita S, Ichimura H. Pyridine and pyridine derivatives. Ullmann's Encyclopedia of Industrial Chemistry; 2000.
- Raja VA, Tenti G, Perumal S, Menéndez JC. A heavy metal-and oxidant-free, one-pot synthesis of pyridines and fused pyridines based on a Lewis acid-catalyzed multicomponent reaction. Chem Commun 2014;50(82):12270-2.
- Adib M, Tahermansouri H, Koloogani SA, Mohammadi B, Bijanzadeh HR. Kröhnke pyridines: an efficient solvent-free synthesis of 2, 4, 6-triarylpyridines. Tetrahedron Lett 2006;47(33):5957-60.
- Siddiqui N, Ahsan W, Alam MS, Azad B, Akhtar MJ. Newer biologically active pyridines: A potential review. Res J Pharm Technol 2011;4(12):1918-32.
- Dohare P, Quraishi MA, Verma C, Lgaz H, Salghi R, Ebenso EE. Ultrasound induced green synthesis of pyrazolo-pyridines as novel corrosion inhibitors useful for industrial pickling process: Experimental and theoretical approach. Result Phys 2019;13:102344.
- Biswas S, Majee D, Guin S, Samanta S. Metal-and solvent-free approach to diversely substituted picolinates via domino reaction of cyclic sulfamidate imines with β, γ-Unsaturated α-ketocarbonyls. J Org Chem 2017;82(20):10928-38.
[Crossref] [Google Scholar] [PubMed]
- Raja R, Sivasubramaniyan A, Murugan D, Subbaiah N, George J, Poovan S, et al. A green synthesis of 1, 2, 3-triazolyl-pyridine hybrids and evaluation of their antibacterial activity. Res Chem Intermed 2016;42:8005-21.
- Xi LY, Zhang RY, Liang S, Chen SY, Yu XQ. Copper-catalyzed aerobic synthesis of 2-arylpyridines from acetophenones and 1, 3-diaminopropane. Org Lett 2014;16(20):5269-71.
[Crossref] [Google Scholar] [PubMed]
- Chen S, Bergman RG, Ellman JA. Facile Rh (III)-catalyzed synthesis of fluorinated pyridines. Org Lett 2015;17(11):2567-9.
[Crossref] [Google Scholar] [PubMed]
- Ghodse SM, Telvekar VN. Synthesis of 2-phenyl pyridine derivatives from aryl ketones and 1, 3-diaminopropane using palladium acetate as a catalyst. Tetrahedron Lett 2017;58(6):524-6.
- Albratty M, El-Sharkawy KA, Alhazmi HA. Synthesis and evaluation of some new 1, 3, 4-oxadiazoles bearing thiophene, thiazole, coumarin, pyridine and pyridazine derivatives as antiviral agents. Acta Pharm 2019;69(2):261-76.
[Crossref] [Google Scholar] [PubMed]
- Karadeniz E, Zora M, Kılıçaslan NZ. Facile synthesis of aryl-substituted pyridines via Suzuki–Miyaura approach. Tetrahedron 2015;71(47):8943-52.
- Gao P, Chen HJ, Bai ZJ, Zhao MN, Yang D, Wang J, et al. IBX-promoted oxidative cyclization of N-hydroxyalkyl enamines: A metal-free approach toward 2, 3-disubstituted pyrroles and pyridines. J Org Chem 2020;85(12):7939-51.
- Cheng G, Xue L, Weng Y, Cui X. Transition-metal-free cascade approach toward 2-Alkoxy/2-Sulfenylpyridines and Dihydrofuro [2, 3-b] pyridines by trapping in situ generated 1, 4-Oxazepine. J Org Chem 2017;82(18):9515-24.
[Crossref] [Google Scholar] [PubMed]
- Huang H, Cai J, Tang L, Wang Z, Li F, Deng GJ. Metal-free assembly of polysubstituted pyridines from oximes and acroleins. J Org Chem 2016;81(4):1499-505.
[Crossref] [Google Scholar] [PubMed]
- Zhang X, Wang P, Yuan X, Qin M, Chen B. Synthesis of Pyridine Derivatives from Acetophenone and Ammonium Acetate by Releasing CH4. Asian J Org Chem 2019;8(8):1332-5.
- Arabshahi HJ, van Rensburg M, Pilkington LI, Jeon CY, Song M, Gridel LM, et al. A synthesis, in silico, in vitro and in vivo study of thieno [2, 3-b] pyridine anticancer analogues. Med Chem Commun 2015;6(11):1987-97.
- Luo Q, Huang R, Xiao Q, Yao Y, Lin J, Yan SJ. Highly selective synthesis of 2-amino-4, 6-diarylpyridine derivatives by the cascade reaction of 1, 1-enediamines with α, β-unsaturated ketones. J Org Chem 2019;84(4):1999-2011.
- Motati DR, Amaradhi R, Ganesh T. Recent developments in the synthesis of azaindoles from pyridine and pyrrole building blocks. Org Chem Front 2021;8(3):466-513.
- Pericherla K, Kaswan P, Pandey K, Kumar A. Recent developments in the synthesis of imidazo [1, 2-a] pyridines. Synthesis 2015;47(07):887-912.
- Bartko SG, Hamzik PJ, Espindola L, Gomez C, Danheiser RL. Synthesis of highly substituted pyridines via [4+ 2] cycloadditions of vinylallenes and sulfonyl cyanides. J Org Chem 2019;85(2):548-63.
- Wu J, Xu W, Yu ZX, Wang J. Ruthenium-catalyzed formal dehydrative [4+ 2] cycloaddition of enamides and alkynes for the synthesis of highly substituted pyridines: Reaction development and mechanistic study. J Am Chem Soc 2015;137(29):9489-96.
[Crossref] [Google Scholar] [PubMed]
- Shen J, Cai D, Kuai C, Liu Y, Wei ME, Cheng G, et al. Base-promoted β-C (sp3)–H functionalization of enaminones: An approach to polysubstituted pyridines. J Org Chem 2015;80(13):6584-9.
[Crossref] [Google Scholar] [PubMed]
- Song Z, Huang X, Yi W, Zhang W. One-pot reactions for modular synthesis of polysubstituted and fused pyridines. Org Lett 2016;18(21):5640-3.
[Crossref] [Google Scholar] [PubMed]
- Upadhyay A, Sharma LK, Singh VK, Singh RK. An efficient one pot three component synthesis of fused pyridines via electrochemical approach. Tetrahedron Lett 2016;57(50):5599-604.
- Wei H, Li Y, Xiao K, Cheng B, Wang H, Hu L, et al. Synthesis of polysubstituted pyridines via a one-pot metal-free strategy. Org Lett 2015;17(24):5974-7.
- Huang H, Cai J, Xie H, Tan J, Li F, Deng GJ. Transition-metal-free N–O reduction of oximes: A modular synthesis of fluorinated pyridines. Org Lett 2017;19(14):3743-6.
- Duan J, Zhang L, Xu G, Chen H, Ding X, Mao Y, et al. NH4I-triggered [4+ 2] annulation of α, β-unsaturated ketoxime acetates with N-acetyl enamides for the synthesis of pyridines. J Org Chem 2020;85(12):8157-65.
[Crossref] [Google Scholar] [PubMed]
- Salem MS, Sakr SI, El‐Senousy WM, Madkour HM. Synthesis, antibacterial, and antiviral evaluation of new heterocycles containing the pyridine moiety. Arch Pharm 2013;346(10):766-73.
[Crossref] [Google Scholar] [PubMed]
- Siddiqui N, Ahsan W, Alam MS, Ali R, Srivastava K, Ahmed S. Anticonvulsant activity of a combined pharmacophore of pyrazolo-pyridines with lesser toxicity in mice. Bull Korean Chem Soc 2011;32(2):576-82.
- El-Dean AM, Abd-Ella AA, Hassanien R, El-Sayed ME, A. Abdel-Raheem SA. Design, synthesis, characterization, and insecticidal bioefficacy screening of some new pyridine derivatives. ACS Omega 2019;4(5):8406-12.
[Crossref] [Google Scholar] [PubMed]
- Lachowicz JI, Nurchi VM, Crisponi G, Jaraquemada-Pelaez MG, Arca M, Pintus A, et al. Hydroxypyridinones with enhanced iron chelating properties. Synthesis, characterization and in vivo tests of 5-hydroxy-2-(hydroxymethyl) pyridine-4 (1 H)-one. Dalton Trans 2016;45(15):6517-28.
- Sun B, Dong Y, Lei K, Wang J, Zhao L, Liu M. Design, synthesis and biological evaluation of amide-pyridine derivatives as novel dual-target (SE, CYP51) antifungal inhibitors. Bioorg Med Chem 2019;27(12):2427-37.
[Crossref] [Google Scholar] [PubMed]