- *Corresponding Author:
- S. Saravandevi
Environmental Health Division, CSIR-National Environmental Engineering Research Institute (CSIR-NEERI), Nehru Marg, Nagpur-440 020, India
E-mail: ss_devi@neeri.res.in
| Date of Submission | 16 January 2017 |
| Date of Revision | 02 December 2017 |
| Date of Acceptance | 28 June 2018 |
| Indian J Pharm Sci 2018;80(4):719-726 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present communication dealt with cytotoxic and free radical scavenging potential of tertiary and quaternary alkaloid extracts of Tribulus terrestris fruits. Tertiary and quaternary alkaloid extracts were found to be cytotoxic to leukemic cells (Jurkat E6-1) with LC50 values of 100 and 42 µg/ml, respectively. Compared to the control, reactive oxygen species and reactive nitrogen species were significantly reduced in the cells treated with lower concentrations of tertiary and quaternary alkaloid extracts. Cells treated with tertiary alkaloid extract demonstrated significantly elevated levels of peroxidise, catalase and superoxide dismutase-like activities, whereas cells treated with quaternary alkaloid extracts showed insignificant elevations of the tested enzyme activities, compared to the control cells. The antioxidant activity of tertiary alkaloid (100 µg/ml) extract was 46.78 and 14.92 µg/ml of ascorbic acid equivalent as estimated in ferric reducing antioxidant potential and total antioxidant assays. Quaternary alkaloid extract displayed an IC50 value of 159 µg/ml in the nitric oxide mitigation assay. Cells treated with quaternary alkaloid extracts showed 1.4-fold increase in superoxide dismutase-like activity compared to the control cells. These results suggest that tertiary and quaternary alkaloid extracts possessed cytotoxic and free radical scavenging potential against leukemic cells.
Keywords
Tribulus terrestris, cytotoxicity, antioxidant, reactive oxygen/nitrogen species, free radicals
Aerobic organisms efficiently amplify energy production from foodstuff through oxidative pathways in a membrane bound organelle, known as mitochondria [1]. This life flourishing oxygen might be poisonous in the form of reactive oxygen species (ROS), which are often generated as side products through cell metabolism. Excess ROS and reactive nitrogen species (RNS) generate oxidative stress, which is defined by the formation of disproportion among production and elimination of free radicals and further damage construction to macromolecules [2]. Excess ROS and RNS generate oxidative stress and which results in the formation of disproportion among production and elimination of free radicals. Reactive oxygen/nitrogen species can be generated in cells through different mechanisms such as i) acquisition of electrons from electron transport chain (ETC), synthesis of NAD(P)H oxidase in phagocytic cell, ii) ionizing radiation induced splitting of hydroxyl bond of water (OH-), iii) nitric oxide (NO) synthesis by vascular endothelial cells, phagocytes and other cell types, iv) hydrogen peroxide (H2O2) synthesized by several oxidative enzymes and in most important case, v) uncontrolled generation occurs in diseased state, like cancer. The mitochondrial oxidative stress (formed in cancer, diabetes mellitus) or inflammatory oxidative stress (formed in atherosclerosis and chronic inflammation) generates favourable circumstances for the progression of the diseases.
Natural compounds due to their chemical diversity and structural complexity are likely to possess enough efficacy to be considered as potential precursors for therapeutic drugs in the treatment of oxidative damage and related disorders [3]. Tribulus terrestris is an annual herb of Zygopyllaceae family, traditionally used as therapeutics for cancer, urinary tract disinfection, kidney stones dissolution, water pills, hypertension and sexual disability [3]. Pharmacological investigation of this plant fruit demonstrated the presence of alkaloids, flavonoids, glycosides and steroidal saponins like diosgenin and protodioscin [4]. The alkaloids group of secondary metabolites have pronounced physiological action on animals and therefore have therapeutic and biological importance. The alkaloid class of this plant fruits have not been tested for cytotoxic activity in perspective with antioxidant activity. In present study, we evaluated cytotoxicity of tertiary and quaternary alkaloids fractions isolated from T. terrestris fruits on leukemic cell line along with its effect on cell’s antioxidant machinery.
Materials and Methods
The fruits of the plant T. terrestris were purchased from local traditional medicinal shop of Nagpur city, Maharashtra, India and were identified in the Department of Botany, University Campus, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra (Voucher Specimen No. 9978 dated on 25th May 2016).
Preparation of extraction
Alkaloids were isolated by the method of Maldoni (1991), a brief description of which is as follows [5]. Fruits were dried in an incubator below 50°, finely pulverized and used for extraction. The fruit powder was then defatted with hexane in a Soxhlet apparatus to remove lipid and carotenoid contents. Later, it was again Soxhleted (at 50°) for obtaining ethanol extract at three repeated cycles of 8 h and combined decoctions (approximately 1 l) were concentrated under reduced pressure at 45° in a rotary evaporator. Presence of alkaloids in the extract was confirmed by Mayer’s reagent (1.36 % HgCl2 and 5 % KI in water) and, acid treatment with 0.5 N HCl (2:1 v/v) to extract was given in an ice bath with continuous stirring for 3 h and finally kept in refrigerator. Next day, it was filtered and washed with 0.5 N HCl for removing gummy material and acid washed fractions were combined to the filtrate. Acid solution was then basified to pH 10 by adding 15 % NaOH drop-wise and the liberated bases were partitioned with chloroform, which was evaporated under vacuum to represent the tertiary alkaloids of T. terrestris fruits (TTA). For obtaining the T. terrestris quaternary alkaloids (TQA), the residual aqueous basic solution was again acidified with 2 N HCl to pH 2-2.5 and precipitated with Reinecke's salt. The precipitate was kept in a refrigerator for overnight, filtered using a Buchner funnel and was washed with cold water to make it pH neutral. The formed reineckate salt was dissolved in acetone and later with methanol (1:1 v/v) according to the ratio 1:50 weight/volume. This solution is then passed through anion exchanger resin column (chloride form, 100-200 mesh size) and final extract was regained by washing the column with acetone:methanol (1:1).
Cell culture
Acute T cell leukemic (Jurkat E6-1) cell lines was purchased from National Centre of Cell Science, Pune and cultured in Roswell Park Memorial Institute- 1640 (RPMI) medium with 10 % foetal bovine serum provided with 100 U/ml penicillin and 50 μg/ml streptomycin. The cells were incubated at 37° in a humidified atmosphere of 5 % CO2.
Cell viability assay
The cell viability of Jurkat E6-1 were carried out by measuring purple formazan formed after 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction by live cell mitochondrial reductase. The cells were platted at a density of 5×104/ml in a flat transparent 96-well plate and incubated at 37° with required atmospheric humidifier concentration of CO2 (5 %). Each well was then dispensed with different concentration of tertiary and quaternary alkaloids, such as 10, 20, 30, 40, 50, 60, 70, 80, 90 and 100 μg/ml of the medium. Staurosporine (5 mM) and dimethyl sulfoxide (DMSO; 0.1 %) were taken as positive and vehicle control, respectively. After the period of 24 h, 20 μl of MTT dye (5 μg/ml of phosphate buffer) was added in each well and the plate was again kept for 4 h. The formed formazan crystals were dissolved in 200 μl DMSO, after the centrifugation of plate at 650 g. Finally absorbance was read at 565 nm against blank sample, which contain an equal amount of solvent as test compounds.
In vitro RONS scavenging activity
T. terrestris alkaloid extracts induced RONS scavenging inside Jurkat E6-1 cell line was estimated by using ROS/RNS assay kit (Oxyselect™). The cells were seeded at a density of 1.25×106/ml of RPMI and alkaloids were exposed in medium for 1 h incubation. The cells were pre-exposed to 100 mM H2O2 for 1 h incubation and afterwards the medium were replaced by fresh RPMI containing test samples. The cells were centrifuged to discard the medium and later washed with phosphate buffer (pH 7.2) and centrifuged. Finally cell pallets were suspended in 250 μl of buffer and cell membranes were disrupted by using Sonics Vibra cell at 15 s on/off cycle at 85 % amplitude for 2 min. The 50 μl of sample were used to test activity in 50 μl of catalyst in a flat black 96-well plate. Both the solutions were mixed well and incubated for 5 min at room temperature. Dichlorodihydrofluorescein (DCFHDiOxyQ) solution, 100 μl added to every well and plate was incubated in dark for 45 min. The fluorescence was read at 480 nm excitation and 530 emission and the fluorescent 2’7’-dichlorodihydrofluorescein (DCF) generated was compared against control and blank samples.
Peroxidase and catalase activity assay
In vitro effects on peroxidase and catalase activity were estimated in control and test samples upon the exposure (6 h) of alkaloids extracts to Jurkat E6-1 cell line. Enzyme extract was prepared as described above and 50 μg of protein from each sample were loaded for activity assay. Peroxidase was estimated [6] in assay mixture consists of 0.5 ml of 1 % H2O2 and 3 ml of 0.05 M pyrogallol-phoshpate buffer (pH 6.5) with 0.05 ml of test sample. The absorbencies were recorded at 430 nm at 30 s interval for up to 3 min. The slope values were used to estimate the enzyme activity as follows, peroxidase (U/ml) = (slop value×3.52)/ enzyme extract (ml).
The catalase activity was evaluated by the method of Jing et al. [7]. Ten percent H2O2 in 50 mM phosphate buffer, pH 7.0 gives an absorbance 0.45 at 240 nm. The time Δt were noted for decrease in absorbance from 0.45 to 0.4, after addition of sample. The specific activity of catalase (U ml-1) was calculated as time required for decreasing in absorbance divided by a constant value, 17.
Ferric reducing antioxidant potential (FRAP)
The antioxidant potentials of TTA and TQA were estimated by formation of Perl's Prussian blue complex (Fe2+) from ferricyanide (Fe3+) reduction [8]. Hundred microgram of each sample were prepared in 0.2 M phosphate buffer (pH 6.5) and incubated at 50° with 0.1 % potassium ferricyanide (1:1 v/v) for 20 min. The reaction was terminated by mixing one volume of 10 % trichloroacetic acid and later centrifuged at 650 g for 10 min. The supernatant was then diluted to equal volume and addition of 0.5 ml of ferric chloride (1:1 v/v) produced a coloured ferric ferrous complex. The absorbance of the chromophore was measured at 700 nm and was directly proportional to reduction potential. The controls were kept without test compounds as well as reference plot prepared using ascorbic acid as a standard antioxidative agent.
Total antioxidant activity
Estimation of total antioxidant activity was also performed for both the alkaloids extracts [9]. Each sample of concentration 100 μg in 0.1 ml was mixed with 1 ml reaction reagents (composite of 0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The reaction tubes were incubated at 95° for 90 min and later cooled at room temperature. Blank and standard ascorbic acid (as positive control) were also taken and absorbance of each was noted at 695 nm.
Total phenolic content
The standardized Folin-Ciocalteu (F-C) assay method was employed for quantification of phenolics contents in TTA and TQA [10]. Assay was performed in 2-ml microtubes containing 100 μg of sample with 200 μl of F-C reagent (1 N). Standard phenol was used for relative measurement of phenolic contents in extracts and blank tube without phenol also prepared. Subsequently 800 μl of 700 mM Na2CO3 was added in each tube and incubated at room temperature for 2 h. A 200 μl solution from each tube were transferred to a transparent, flat bottom, 96-well microplate and light absorbance was recorded at 765 nm. Standard curve calculated from standard phenol graph and total phenolics were represented in phenol equivalents using regression equation.
Superoxide dismutase (SOD) activity
The SOD activity assay was performed according to the method of Kumar et al. [11]. SOD induced inhibition of photo-reduction of superoxide anions to form blue colour formazan, after nitro blue tetrazolium (NBT) reduction was assayed. Reaction mixture of 200 μl was prepared for control samples containing 50 mM phosphate buffer (pH 7.8), 5.7×10−5 M NBT, 9.9×10−3 M methionine, 1.17×10−6 M riboflavin and 0.025 % Triton X-100. The test samples were prepared in 100 μg ml-1 and kept in same reaction mixture against control. For evaluation of in vitro SOD activity, 50 μg of protein from each sample added to the final volume (200 μl) of reaction mixture. The reactions were illuminated for 40 min under white light at 25° and the absorbance’s were examined at 560 nm.
NO scavenging activity
The NO mitigation activity of test alkaloids were determined by negative estimation of produced nitrite ions by Greiss reagent [12]. In this reaction, NO generated from sodium nitroprusside was further allowed to scavenge/react with test compounds or oxygen respectively, and in later case nitrite ions were generated. Hundred micrograms of alkaloids extracts were incubated with 5 mM sodium nitroprusside in 0.025 M phosphate buffer (pH 7.4) and reactions were incubated at 25° for 5 h. After that 0.5 ml solution was mixed with 0.5 ml a composite of 1 % sulphanilamide, 2 % o-phosphoric acid and 0.1 % 1-(1-naphthyl)-1,2- ethanediamine dichloride (Griess’s reagent). The pink colour formed during nitrite-sulphanilamide diazonium salt coupling with naphthylethylene diamine dichloride, was measured at 546 nm and percent inhibition was calculated.
Statistical analysis
The data of all the experiments were expressed as mean±standard deviations of number of experiments (n=3). Statistical calculations and graphs for various experiments were prepared using Microsoft Excel and GraphPad Prism 5.0® software.
Results and Discussion
The medicinal herb of the family Zygopyllaceae specifically, T. terrestris was described in the Ayurveda for presence of constituents, therapeutic property and important formulations. Different parts of this plant and phytochemicals were found to be beneficial with a variety of characteristics ranging from diuretic, immunomodulatory, antidiabetic, aphrodisiac and anticancer activity [4]. The fruits of plant were well reported for the presence and characterization of saponins and steroidal glycosides [13,14]. The TTA and TQA fractions from fruits of the plants were isolated by using their combine properties of being soluble in organic solvents against opposite solubility of their salts. The percent yield of TTA and TQA were 1.15 % (6.9 g) and 0.49 % (2.94 g) to that of dry weight of plant fruit powder. Wu et al., reported isolation and structural elucidation of alkaloids namely, terrestribisamide, tribulusterine, terristriamide, trans-N-p-coumaroyl tyramine, aurantiamide acetate and xanthosine from fruits of the same plant [15]. In present communication, we further tried to figure out cytotoxicity of these alkaloids and its effect on antioxidant mechanism in Jurkat E6-1 cells.
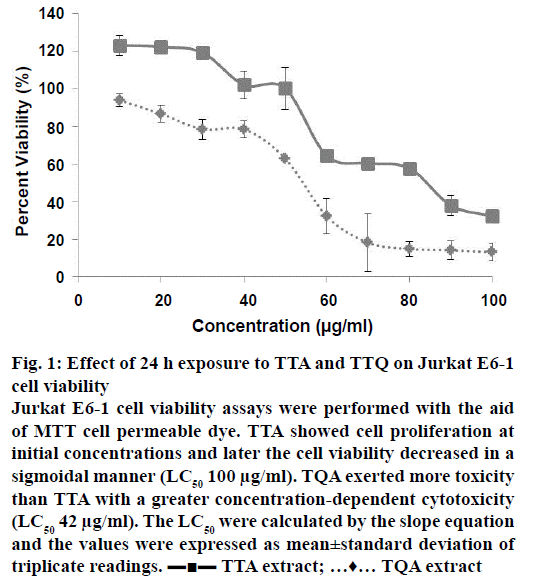
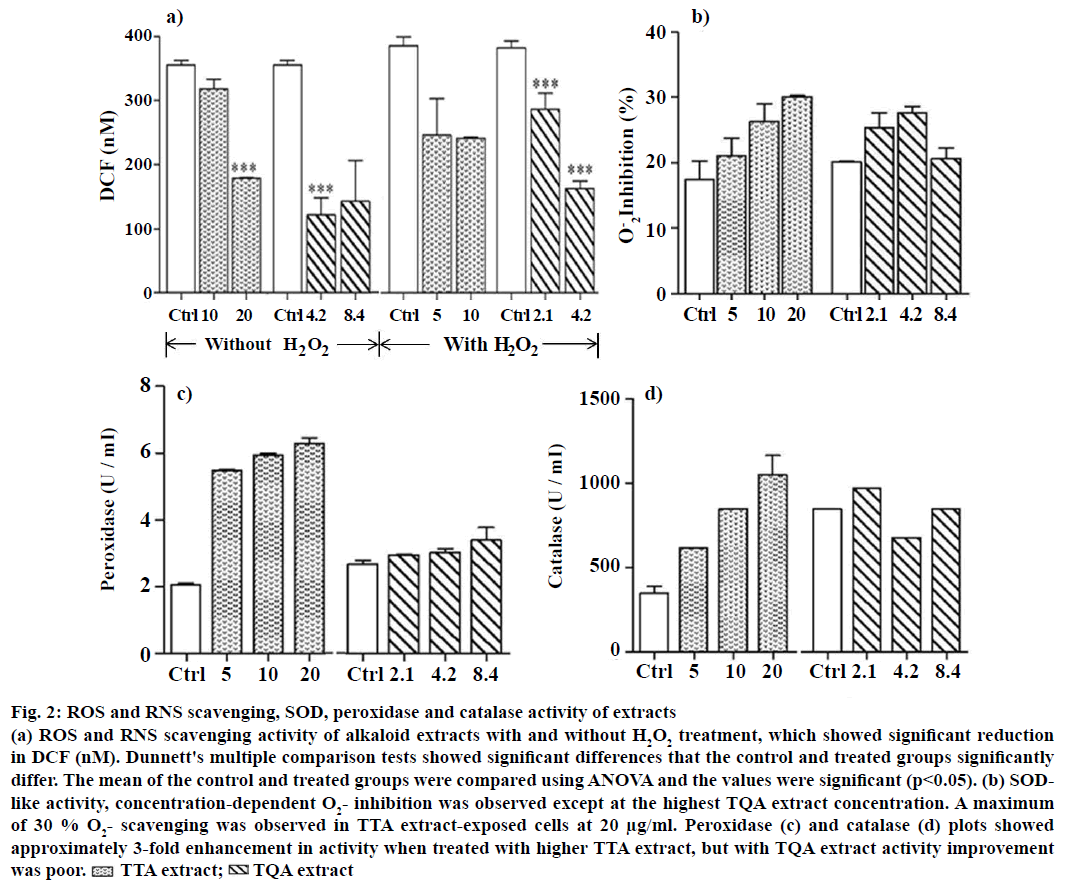
The in vitro cytotoxic effect of present alkaloids was assessed by MTT assays for 24 h duration. The Jurkat E6-1 cell viability was decreased upon exposure of TTA and TQA at a given concentration in sigmoidal manner (Figure 1). The LC50 values calculated by using slope equation and it was 100 μg ml-1 for TTA and 42 μg ml-1 for TQA. The 1/5th, 1/10th and 1/20th value of LC50 were selected to induce stress in Jurkat cells to determine the effect on antioxidant enzymatic profile within cell. ROS and RNS levels upon exposure to TTA and TQA were measured using DCFH-DiOxyQ. Prior to a quench removing agent, it is stabilize in highly reactive DCFH form, and the later it is oxidized to fluorescent DCF, where the intensity is directly proportional to total ROS and RNS. In Figure 2a, there were shown less formation of DCF at concentrations of alkaloids extracts in association with control samples. The ROS and RNS mitigation activity at any given dose of alkaloids extracts have found to be increased. Quaternary alkaloids have strong scavenging potential for total ROS and RNS than tertiary, although there were quite decrease scavenging activities occurred at increase concentrations (8.4 μg ml-1). There were 1.11 and 1.76-fold decreased DCF fluorescent estimated in cells exposed to TTA at 5 and 10 μg/ml concentrations, respectively. In later experiments, we used H2O2 as an inducer of oxidative stress in Jurkat cells and studied the effect of alkaloid extracts. Quaternary alkaloids at 4.2 μg/ml have significant ROS and RNS inhibiting activity (140 nM DCF), which showed its 2.35-fold lessening radical contents as compare to 330 nM DFC in control cells samples (Figure 2a). The cellular wear and tear by oxidative stress is taken care by antioxidant biomolecules, like vitamin C and some antioxidant enzymes. Out of the antioxidant enzymes in human cells the SOD neutralise superoxide radicals into either oxygen molecule or H2O2. The superoxide radical scavenging by both the alkaloids extracts were checked by in vitro cell line as combined activities of both the SOD isozymes (cytosolic Cu, Zn-SOD and mitochondrial Mn-SOD, respectively) in the cell lysates. As shown in Figure 2b, TTA (20 μg/ml) exposed cells (30 % O–2 inhibition) has approximately 2-fold increased enzyme activity than control cell lines (17 % inhibition) while for TQA’s, it’s not much improved at concentration of 4.2 μg/ml. The set of experimental data have generally shown Mn-SOD as a tumour suppressor protein and in certain cancers it was reported to be under active than healthy cells, where its stimulated expression gives malignant suppression phenotypes [16]. Peroxidase family of enzymes (EC 1.11.1.7) and catalase are beneficial antioxidant enzymes that eukaryotic cells have to cope with highly ROS, in addition to SOD [17]. The glutathione peroxidase family of isoenzymes reduces H2O2 and organic hydroperoxides to water or corresponding alcohols using reduced glutathione as an electron donor [18]. Here (Figure 2c), about 2.5 to 3-fold increased peroxidase activity (5-6 U/ml) was observed for each concentrations of TTA tested against vehicle control (2 U/ml). The cumulative decanted H2O2 generated from various enzymatic reactions like SOD, xanthine oxidase and monoamine oxidase were removed by catalase (EC 1.11.1.6), which catalysed removal of two H2O2 molecules by forming two H2O and an O2 molecule under high substrate concentrations [19]. In our experiment, catalase activity were boosted (3-fold) from 351 to 1052 U/ml at consecutively increase of TTA concentrations (5 to 20 μg/ml; Figure 2d). The TQA’s, where, sum of efficacy to induce scavenging by in vitro antioxidant enzymes is less significant compared to TTA. The exposure of a therapeutic compound, increasing SOD enzyme activity should be with concomitant increase in catalase and peroxidase activities, as the SOD generated product (H2O2) were further neutralising by mentioned enzymes.
Figure 1: Effect of 24 h exposure to TTA and TTQ on Jurkat E6-1 cell viability
Jurkat E6-1 cell viability assays were performed with the aid of MTT cell permeable dye. TTA showed cell proliferation at initial concentrations and later the cell viability decreased in a sigmoidal manner (LC50 100 μg/ml). TQA exerted more toxicity than TTA with a greater concentration-dependent cytotoxicity (LC50 42 μg/ml). The LC50 were calculated by the slope equation and the values were expressed as mean±standard deviation of triplicate readings. ??? TTA extract; …♦… TQA extract
Figure 2: ROS and RNS scavenging, SOD, peroxidase and catalase activity of extracts
(a) ROS and RNS scavenging activity of alkaloid extracts with and without H2O2 treatment, which showed significant reduction in DCF (nM). Dunnett's multiple comparison tests showed significant differences that the control and treated groups significantly differ. The mean of the control and treated groups were compared using ANOVA and the values were significant (p<0.05). (b) SODlike activity, concentration-dependent O2-inhibition was observed except at the highest TQA extract concentration. A maximum of 30 % O2- scavenging was observed in TTA extract-exposed cells at 20 μg/ml. Peroxidase (c) and catalase (d) plots showed approximately 3-fold enhancement in activity when treated with higher TTA extract, but with TQA extract activity improvement was poor.  TTA extract;
TTA extract;  TQA extract
TQA extract
The superoxide anion (O–2), are important ROS entities frequently generated primarily from leakage of ETC, auto-oxidation and by various enzymatic systems [20]. The direct O–2 radical scavenging potential has also checked for present alkaloids extracts in SOD activity assays. SOD-like activity assay also showed significant percent inhibition by TTA. The TTA vigorously scavenges O–2 radicals with IC50 of 112.62 μg/ml (Table 1) and possibly expressing themselves as potential antioxidants as likely as SOD enzyme in the biological systems. The O–2 radical scavenging in relation to TQA was negligible for highest concentration (250 μg/ml). In all the mentioned antioxidative assays, estimation of TTA were worth than quaternary alkaloids extract, may be due to the existence of phenolic groups and distinctive structureactivity relationship companionable to act them as free radical scavengers. The superoxide radicals engenders highly reactive peroxynitrite (OnOO–) radicals whilst it cohabited with NO– [21]. The peroxynitrite species modifies a great variety of biomolecules and in nitrosativa stress (excessive production of RNS) it executes loss of cell functioning. In contrast to its usual potentials observed in all antioxidant assays, the TQA have shown significant NO inhibition activity with IC50 (159 μg/ml) values than TTA extract (IC50 392 μg/ml) comparatively (Table 1). Hence, TQA had better NO scavengers than TTA with 2.46-fold greater potential. The characteristic of O2 - scavenging activity in concomitance with NO mitigation potential might makes both the alkaloids extracts of T. terrestris as superior antiinflammatory drug entities, as like β-carboline alkaloids from Stellaria dichotoma [22]. Although the functioning of NO in inflammation concerns are quite obscure [23].
| Assays | Tertiary alkaloids extract (µg) | Quaternary alkaloids extract (µg) |
|---|---|---|
| Ferric ions (Fe3+) reducing antioxidant power (FRAP)a | 46.78 (±0.72) | ND |
| Total antioxidant (TA)a | 14.92 (±0.34) | 2.03 (±0.23) |
| Phenolic contentsb | 97.51 (±1.48) | 47.25 (±1.18) |
| SOD-like activityc | 112.70 (±1.63) | ND |
| NO Scavenging activityc | 457.71 (±8.29) | 166.51 (±5.33) |
| Cytotoxicity on Jurkat E6-1 Celld | 100.43 (±2.54) | 41.85 (±3.02) |
Table 1: Antioxidant activity and Cytotoxicity of T. terrestris fruit alkaloid extracts
The antioxidant potentials of present alkaloid extracts have also been tested using various chemical assays in order to understand correlation with in vitro assays. The free radical scavenging activities of alkaloid extracts were estimated as calculus of their Fe3+ reducing capacity in FRAP assay and formation of green phosphate/Mo5+complex by Mo4+ reduction in total antioxidant activity assay. In couple of assays, concentrations were directly proportional to antioxidant potentials of both alkaloids extracts, but however the activity was negligible in case of TQA (Table 1). The TTA had significant reducing potential at higher concentrations as such 100 μg of sample was corresponds to 46.78 and 14.92 μg/ml of ascorbic acid equivalence in both the assays, respectively. There come better insights on antioxidant prospective of chemical entities owing to the examination in different antioxidant assays, which aids reducing accessibility to a variety of compounds. This is actually mandatory to evaluate true free radical scavenging potential and also for manifestation of their mechanism of actions with structure-activity relationships [11]. The iron ion in Fenton reaction acts as a catalyst to produce highly reactive hydroxyl radical from H2O2 precursor and there is circumstantial evidence towards its role in free radical pathology [24]. The antioxidant activity imparted maximums by TTA in FRAP shows that they may act as a safeguard against DNA damage in normal cells by lessening the hydroxyl ion production through Fe3+ reduction. Alkaloids from leaves and stem bark of quinine tree (Rauvolfia caffra) accessed on DPPH scavenging assay demonstrated competitively strong free radical inhibitory activity than likelihood positive control quercetin [25].
The presence of number of phenolic moiety in a compound directly prospers better antioxidant activity, due to characteristics ROS chain reaction interruption by donation of their hydrogen atoms. Total phenolics were found to be increased with spontaneous increase at each concentration with a regression coefficient (R2) of 0.988 and 0.997 for TTA and TQA, respectively (Table 1). The observed absorbance compared with standard phenolic graph plot and the phenolic contents determined were 97.51 μg/ml for tertiary and 47.25 μg/ml for quaternary types of alkaloids (for 100 μg/ml), respectively. Therefore, it may be concluded that antioxidative belongings resulted are due to qualitative and quantitative presence of phenolic groups in their structures. The alkaloid asimilobine isolated from bark of Annona salzmannii has showed two and half fold more oxygen radical absorbance capacity than trolox, due to the phenolic hydroxyl group [26].
Current studies demonstrate that TQA scavenge more ROS and RNS compared to TTA in in vitro cell line models. While in chemical assays, TTA had fair antioxidant scavenging activities in different chemical assays compared to TQA. Hence both extract revelled different state of antioxidant potential depending on assay employed. It might became possible that there may be somewhat pre-disposed state of free radicals in cell microenvironment and hence xenobiotic exposure had some distorted and equilibrated results than that observed in various chemical assays. The primary ROS and RNS species have supposed to have their role in cell signalling and even at high concentration of a single free radical, the deleterious effect do not appear, while its secondary ROS and RNS species, who serves as toxicants to the cell microcosm [24]. In transformed cell lines, antioxidant elevated thiols induces apoptosis through tumour protein 53 guided regulation of redox state at cellular level [27]. Contrarily, antioxidant supress apoptosis through neutralising ROS, which play important role in activation of cell suicidal event [28]. The TTA has revealed substantial antioxidant in chemical assays and antioxidant enzyme (SOD, catalase and peroxidase) fortifying activity compared to TQA and both had shown cytotoxicity increase with concentrations. Harmaline and harmalol are the quaternary alkaloids were reported in the callus culture of T. terrestris [29]. In pure form, these alkaloids individually demonstrated to have protective effect against dopamine- or 6-hydroxydopamine-induced oxidative damage and rescue viability in PC12 cells by scavenging ROS and inhibition of thiol oxidation [30].
An antioxidant should be a potential candidate in regulating oxidative stress according to the disease microenvironment and normal physiology, for example in neurodegenerative diseases an antioxidant must have to cross blood-brain barrier but most of the antioxidant cannot, as like carotenoids [31]. Beyond involvement of ROS in disease and their development, interestingly increased level of free radicals can inhibit tumor cell growth [32]. The therapeutic application of pro-oxidant, which induces oxidative stress to a cytotoxic level in cancer cell can selectively kill them. Fascinatingly, the antioxidant and pro-oxidant capabilities can be achieved by a single agent depending upon the concentration used, curcumin, a chemotherapeutic agent, for an instance. Hence, the screening for the new and potential natural product as an effective oxidative stress manager at various physiological circumstances continues to give the best application of resourceful natural compounds.
Alkaloids of T. terrestris fruits act as an antioxidant at lower concentrations and cytotoxic at higher doses. Quaternary alkaloids extract induced potent cell death at higher concentrations (LC50 42 μg ml-1), which explicit its cytotoxic effects to leukemic cells. Contrarily, tertiary alkaloids have significant antioxidant enzyme fortifying activities along with Jurkat cell toxicity (LC50 100 μg ml-1). Future studies may be targeted on identifying the bioactive individual compounds in the alkaloid extracts along with their antioxidant/cytotoxic properties and mechanism of actions.
Acknowledgements
The authors are grateful to the Council of Scientific and Industrial Research (CSIR) for providing necessary facilities (KRC No.: KRC\2016\AUG\EHD\1) and Mr. Shriniwas S. Basaiyye is grateful to the Department of Science and Technology (DST) for providing a fellowship.
Conflicts of interest
There are no conflicts of interest.
References
- Davies JA. Oxidative stress, antioxidant defences, and damage removal, repair, and replacement systems. IUBMB Life. 2000;50(4-5):279-89.
- Halliwell B. Oxidative stress in cell culture: an under-appreciated problem? FEBS Lett 2003;540(1-3):3-6.
- Butler MS. The role of natural product chemistry in drug discovery. J Nat Prod 2004;67(12):2141-53.
- Chhatre S, Nesari T, Somani G, Kanchan D, Sathaye S. Phytopharmacological overview of Tribulus terrestris. Pharmacogn Rev 2014;8:45-51.
- Maldoni B. Alkaloids: Isolation and Purification. J Chem Educ 1991;68:700-03.
- Cavalcanti M, Rodrigues E, Okada K, Campos-takaki G, Elesbao A. Copper-induced adaptation, oxidative stress and its tolerance in Aspergillus niger UCP1261. Electron J Biotechnol 015;18:418-27.
- Jing Y, Dai J, Chalmers-Redman R, Tatton W, Waxman S. Arsenic Trioxide Selectively Induces Acute Promyelocytic Leukemia Cell Apoptosis Via a Hydrogen Peroxide-Dependent Pathway. Blood 1999;94:2102-11.
- Oyaizu M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr Diet 1986;44:307-15.
- Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med 2012;12:221.
- Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc 2007;2:875-7.
- Kumar A, Kaachra A, Bhardwaj S, Kumar S. Copper, zinc superoxide dismutase of Curcuma aromatica is a kinetically stable protein. Process Biochem 2014;49;1288-96.
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 1982;126:131-38.
- Liu T, Chen G, Yi GQ, Xu JK, Zhang TL, Hua HM, et al. New pregnane and steroidal glycosides from Tribulus terrestris L. J Asian Nat Prod Res 2010;12:209-14.
- Liu T, Lu X, Wu B, Chen G, Hua HM, Pei YH. Two new steroidal saponins from Tribulus terrestris L. J Asian Nat Prod Res 2010;12:30-5.
- Wu TS, Shi SL, Kuo SC. Alkaloids and other constituents from Tribulus terrestris. Phytochemistry 1999;50:1411-15.
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007;39:44-84.
- Michiels C, Raes M, Toussaint O, Remacle J. Importance of se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic Biol Med 1994;17:235-48.
- Margis R, Dunand C, Teixeira FK, Margis-Pinheiro M. Glutathione peroxidase family - An evolutionary overview. FEBS J 2008;275:3959-70.
- Scibior D, Czeczot H. Catalase: structure, properties, functions. Postepy Hig Med Dosw 2006;60:170-80.
- Aruoma OI. Free Radicals, Oxidative Stress, and Antioxidants in Human Health and Disease. J Am Oil Chem Soc 1998;75:199-212.
- Chirino YI, Orozco-Ibarra M, Pedraza-Chaverri J. Role of peroxynitrite anion in different diseases. Rev Invest Clin 2006;58:350-8.
- Chen YF, Kuo PC, Chan HH, Kuo IJe, Lin FW, Su CR, et al. β-carboline alkaloids from Stellaria dichotoma var. lanceolata and their anti-inflammatory activity. J Nat Prod 2010;73:1993-98.
- Korhonen R, Lahti A, Kankaanranta H, Moilanen E. Nitric Oxide Production and Signaling in Inflammation. Curr Drug Targets Inflamm Allery 2005;4:471-79.
- Weidinger A, Kozlov AV. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015;5:472-84.
- Milugo TK, Omosa LK, Ochanda JO, Owuor BO, Wamunyokoli FA, Oyugi JO, et al. Antagonistic effect of alkaloids and saponins on bioactivity in the quinine tree (Rauvolfia caffra sond.): further evidence to support biotechnology in traditional medicinal plants. BMC Complement Altern Med 2013;13:285-90.
- Costa EV, da Cruz PE, de Lourenço CC, de Souza Moraes VR, de Lima Nogueira PC, Salvador MJ. Antioxidant and antimicrobial activities of aporphinoids and other alkaloids from the bark of Annona salzmannii A. DC. (Annonaceae). Nat Prod Res 2013;27:1002-6.
- Liu M, Pelling JC, Ju J, Chu E, Brash DE. Antioxidant Action via p53-mediated Apoptosis1. Cancer Res 1998;158:1723-9.
- Seifried HE, Anderson DE, Sorkin BC, Costello RB. Free Radicals: The Pros and Cons of Antioxidants. Executive summary report. J Nutr 2004;134:3134S-63S.
- Nikam TD, Ebrahimi MA, Patil VA. Embryogenic callus culture of Tribulus terrestris L. a potential source of harmaline, harmine and diosgenin. Plant Biotechnol Rep 2009;3(3):243-50.
- Kim DH, Jang YY, Han ES, Lee CS. Protective effect of harmaline and harmalol against dopamine- and 6-hydroxydopamine-induced oxidative damage of brain mitochondria and synaptosomes, and viability loss of PC12 cells. Eur J Neurosci 2001;13:1861-72.
- Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 2001;18(9):685-716.
- Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol 2012;2012:137289.