- *Corresponding Author:
- Z. Muslim
Department of Pharmacy, Health Polytechnic, Ministry Of Health Bengkulu, Bengkulu 38225, Indonesia
E-mail: zamhariramuslim@yahoo.com
| Date of Submission | 13 June 2016 |
| Date of Revision | 10 September 2016 |
| Date of Acceptance | 30 October 2016 |
| Indian J Pharm Sci 2016;78(6):840-844 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Infectious diseases are one of the biggest health problems not only in Indonesia, but also in the whole world. Prescribing antibiotics in Indonesia are quite high and increased the incidence of resistance. This study is aimed to evaluate the rationality of antibiotic use in children at the hospital qualitatively. This research is descriptive analysis of retrospective antibiotic prescribing data in children use a Gyssens’ criteria and types of antibiotic therapy. Incidence of infection in children under 12 y of age amounted to 93.2%. The analysis results by using the Gyssens’ criteria are obtained category O (rational use) of 32%, V (no indication) of 27.4%, IIA (inappropriate dose) of 0.6%, IIB (inappropriate interval) of 1.7%, IIIA (duration too short) of 2.9%, IIIB (duration too long) of 11.4%, IVA (alternative more effective) of 17.7%, IVB (alternative less toxic) of 2.9%, IVC (alternative less cost) of 2.9% and IVD (alternative narrower spectrum) of 0.6%. The results showed that the grouping of most types of therapy was antimicrobial drugs empiric therapy in 47.4% cases followed successively by antimicrobial drugs unknown therapy 32.6%, antimicrobial drugs documented therapy amounted to 12%, antimicrobial drugs extended empiric therapy amounted to 6.9% and antimicrobial drugs prophylaxis therapy amounted to 1.1%. The use of antibiotics in pediatric patients at the hospital in Bengkulu, Indonesia substantively is inappropriate based on qualitative analysis.
Keywords
Antibiotics, paediatric, Gyssens’ criteria
Infectious diseases in Indonesia are still among the top ten most prevalent diseases. Most infections, especially in children under 5 y old (toddlers) are acute respiratory tract infections amounted to 18% [1]. The prevalence of respiratory infections and pneumonia are highest in the age group 1-4 years in the population of Indonesia. An antibiotic prescribing in Indonesia is quite high, which might contribute to increase in the incidence of resistance. The impact of antibiotic resistance is increasing the morbidity, mortality and health care costs. In the last decade, many microorganisms showed an increase in the incidence of resistance to standard therapy. It is an estimated worldwide phenomenon caused by misuse and overuse of antibiotics.
A study in two major cities in Indonesia (Semarang and Surabaya) found 76% of prescribing antibiotics were intended for groups of paediatric patients. Another report from the city of Denpasar, Indonesia showed that antibiotic prescriptions for children was at a high level of 265 prescriptions (90.4%) out of a total of 293 [2]. In the United States, each year a large number of antibiotic prescriptions are for children and out of those in 50% of the cases, prescribing an antibiotic was not indicated [3]. In Canada, 74% of preschool children were prescribed antibiotics for the treatment of respiratory infections and 85% of these cases got a prescription for an antibiotic, when not needed [4]. Most at risk were young children, teenagers, and those with medical conditions that interfere with their ability to fight disease or make them particularly vulnerable to bacterial infections.
Antibiotics prescribed by a doctor more often were not according to recommended guidelines, but for a variety of reasons such as advertising, pressure from patients and for reasons which were not appropriate, such as for the treatment of viral infections [5]. Rational use of medicines should include a lot of things like proper diagnosis, proper drug selection, appropriate indications, right patient, right dose, right way and duration of administration, right price, precise information, be aware of side effects. Irrational therapy reduced drug benefits and further at risk the possibility of the emergence of side effects and increased costs of health [6]. The number of antibiotic prescriptions allow the isolation and emergence of microorganisms that are resistant to antibiotics [7]. Antibiotic prescriptions in paediatric patients is a major concern in terms of public health, because infections are a major cause of illness in children and the number of antibiotic prescriptions in preschool children is one of the three reasons [8].
The irrational antibiotic prescribing in children has a major impact on the future of the child, because it will increase the potential for the emergence of antibiotic resistance while discovery of newer types of antibiotics that could counter the resistant organisms is in its all-time low. Health agencies around the world need to focus on antibiotic resistance, which is a major threat to public health [9]. Based on these data, it is necessary to evaluate rationality of prescribing antibiotics for children in Bengkulu to support the success of therapy and reduce the emergence of antibiotic-resistant pathogenic organisms. This study was conducted by descriptive analytic research and retrospective methods. Observations documented over 6 months were collected from the retrospective prescription data of antibiotics in children admitted to one of the general hospital of M. Yunus, Bengkulu, Indonesia from January to June 2014. The study was approved by the ethics commission, health polytechnic and Ministry of Health, Bengkulu. Out of the data of 447 hospitalized children, the inclusion criteria were fulfilled in case of 103 medical records and the use of antibiotics, which recorded 175 prescriptions. The data collected from medical records include names of antibiotics, diagnosis, dosage and frequency, duration of administration, route of administration, the number of combinations, demographic data and clinical data. Data obtained was analysed for qualitative antibiotic use using Gyssens’ criteria and by the type of antibiotic therapy [10]. A Gyssens’ criteria and type of antibiotic therapy was analysis based on clinical pathway of general hospital on M. Yunus and also other antibiotic guidance.
Demographic characteristic of this study was recorded in 175 prescriptions, which were predominantly boys (64.1%) compared to girls (35.9%). Based on age groupings, we found the highest incidence of infection at the age of 1-3 y in 28.2% cases and the lowest at the age of >12 years in 6.8% cases (Table 1). The result showed that as the children grew older, the incidence of infections decreased. Previous studies also reported that increasing age reduced the incidence of infection. Children catch infections 3 to 6 times a year, but some develop infections more often, especially during the 2-3 y age and at times through the rest of their lives [11]. Incidence of infections in children was very high because the immune system of children of this age was not fully developed for preventing infections. Disease patterns found in this study were quite varied. The top five diseases recorded in the observations of this study were fever (27.2%), respiratory infections (9.7%), typhoid (7.8%), diarrhoea (5.8%) and malaria (4.9%). This data revealed that these are the 5 major infectious diseases among the study group of children.
| Variable | n (%) |
|---|---|
| Sex | |
| Male | 66 (64.1) |
| Female | 37 (35.9) |
| Age | |
| Age<1 y | 22 (21.4) |
| 1≤Age<3 y | 29 (28.2) |
| 3≤Age<6 y | 21 (20.4) |
| 6≤Age<12 y | 24 (23.3) |
| 12 years≤Age | 7 (6.8) |
Table 1: Demographic characteristics
This study demonstrated that 7 types of antibiotics have been mainly prescribed. The antibiotics used commonly for the paediatric patients in this study were gentamicin (34.9%) and ampicillin (34.3%). Children under hospital care also get a variety of combinations of antibiotics, such as a combination of three different types of antibiotics (10.7%), a combination of two types of antibiotics (48.5%) and a single antibiotic (40.8%) as shown in Table 2. Most frequently used antibiotic combination was ampicillin-gentamicin in 41.75% of cases. The purpose of using a combination of antibiotics was to increase the efficacy of antibiotics in specific infections (synergistic or additive effect) or to address mixed infections that cannot be controlled by one type of antibiotic or in cases of life threatening infections of unknown bacterial cause.
| Variable | n (%) |
|---|---|
| Antibiotic | |
| Gentamicin | 61 (34.9) |
| Ampicillin | 60 (34.3) |
| Cefotaxime | 34 (19.4) |
| Ceftriaxone | 12 (6.9) |
| Chloramphenicol | 6 (3.4) |
| Meropenem | 1 (0.6) |
| Ceftazidine | 1 (0.6) |
| Combination antibiotics | |
| Single | 42 (40.8) |
| Double | 50 (48.5) |
| Triple | 11 (10.7) |
Table 2: Antibiotic usage
Combination therapy of gentamicin and ampicillin was used as the first line treatment for paediatric patients. This was due to the reason that gentamicin in combination with ampicillin produced a strong bactericidal effect, partly due to the increase in drug absorption resulting from inhibition of cell wall synthesis by ampicillin, which changed the structure of the cell wall so as to facilitate the penetration of gentamicin into bacteria [12]. Gentamicin should be used with caution in infants and toddlers. Determination of the gentamicin dose based on body weight and condition of the kidneys, as well as monitoring the auditory function is recommended at an initial dose of 7 mg/kg. Combination of β-lactam antibiotics and aminoglycosides produces synergistic activity against a wide range of Gram-positive and Gram-negative bacteria [13]. The addition of gentamicin and penicillin showed bactericidal activity, while the use of this combination in certain individual cases did not show significant activity [14].
The use of penicillin group is also very high, especially the use of ampicillin. A lot of things should be taken into consideration to use ampicillin and group of antibiotics with broad spectrum activity for initial therapy of infection. In addition to its activity in Gram-positive bacteria, ampicillin is also active against some Gramnegative bacteria such as Haemophilus influenzae, Escherichia coli, and Proteus mirabilis [15]. Ampicillin has low toxicity, lower price with low incidence of the possibility of colonization of resistant organisms as well as low extent of Candida complications. This led ampicillin being chosen as the primary choice of therapy [16].
Research aimed at evaluation of rationality of the use of antibiotics in the children ward of hospital Semarang, Indonesia in 2011 found that the antibiotic most widely used in quantity was ceftriaxone. This 3rd generation cephalosporin antibiotic use was also quite high after the use of ampicillin and gentamicin. The third generation cephalosporin have a wider spectrum activity compared to penicillin in addition to have an activity against Gram-positive and Gram-negative bacterial infections and also have been more effective in resisting Enterobacteriae compared to the second generation cephalosporins. The third generation cephalosporins were also active against penicillin nonsusceptible Staphylococcus pneumoniae, Haemophilus, Neisseria, Moraxella sp. A group of cephalosporins for broad spectrum can be used as empirical therapy of various types of infections and third generation cephalosporins have been in use to replace penicillin as a first line therapy [17].
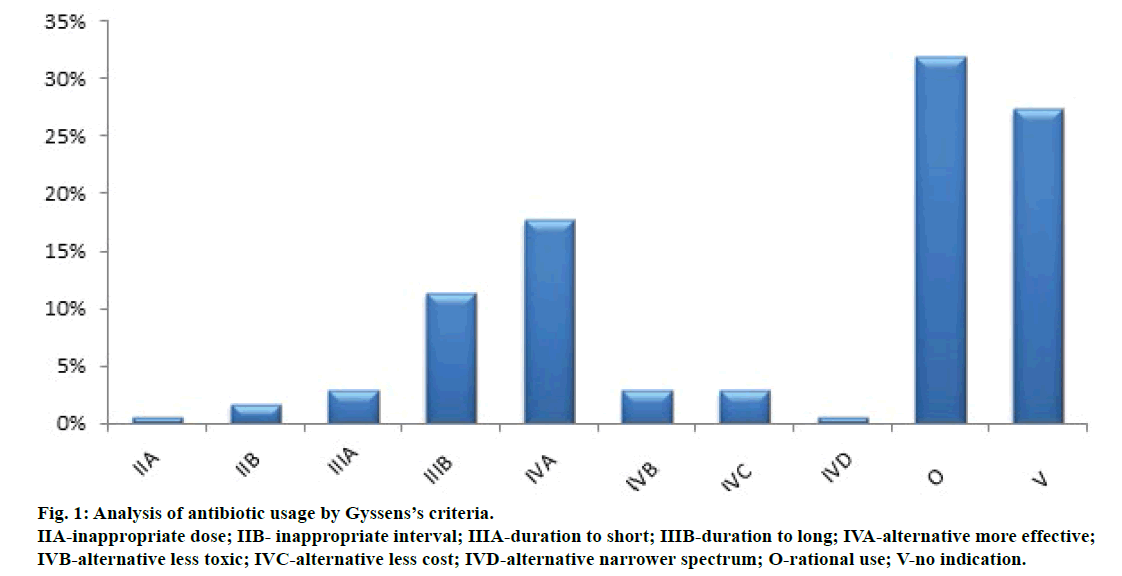
Assessment of rational antibiotic usage was qualitatively performed using the Gyssens’ criteria and by type of antibiotic therapy. The higher results of the analysis using the Gyssens’ criteria obtained category O (rational use) of 32% and followed by category V (no indication) of 27.4% (Figure 1). Adjustment in each category refers to the hospital clinical pathway, antibiotic guidance and discussion with a doctor. Inappropriate selection of antibiotics also seen in a number of cases at 17.7% because there is a choice of antibiotics more effective in the treatment of infection. Another study by a team of antimicrobial resistance in Indonesia (AMRIN), prevalence and prevention, there are 49% up to 97% of paediatric patients hospitalized received a prescription for antibiotics and most of which (46-54%) are not needed and are not appropriate indications [18]. Longer duration of antibiotic administration was also found to be 11.4% of cases. The use of antibiotics in the long term will increase the risk of side effects and toxic effects. In this study, the use of gentamicin is sufficiently longer than 9 to 13 days in children. This can increase the side effects of gentamicin like disorders of the kidneys and the ototoxicity which may occur in children [19]. In Indonesia duration use of antibiotics is grouped based on the study of literature conducted or most infection is for 3-7 days.
Figure 1: Analysis of antibiotic usage by Gyssens’s criteria.
IIA-inappropriate dose; IIB- inappropriate interval; IIIA-duration to short; IIIB-duration to long; IVA-alternative more effective;
IVB-alternative less toxic; IVC-alternative less cost; IVD-alternative narrower spectrum; O-rational use; V-no indication.
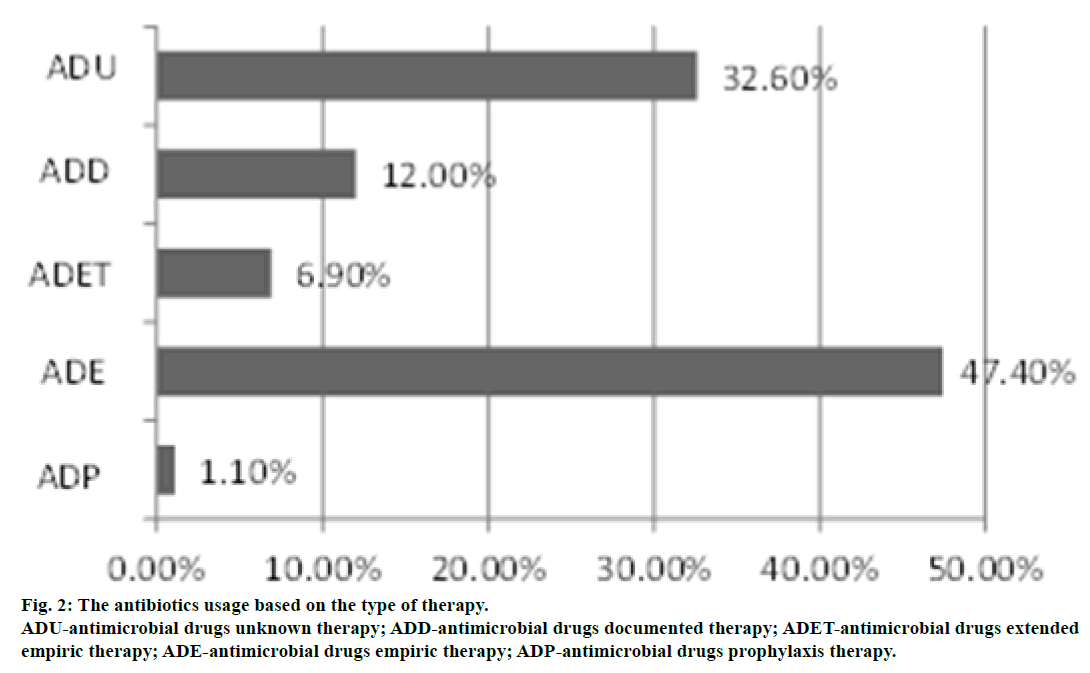
Grouping by type of antibiotic therapy in this study showed the higher antimicrobial drugs empirical therapy (ADE) amounted to 47.4%, followed consecutively by antimicrobial drugs unknown therapy (ADU) of 32.6% (Figure 2). Based on the hospital clinical pathway, empirical antibiotic that was used for paediatric patient by general hospital by M. Yunus Bengkulu, Indonesia is a combination of ampicillin and gentamicin but for adult infection cases a thirdgeneration cephalosporin is used. As the microbiology results cannot be provided within 48 to 72 hours, initial therapy of infection is given empirically based on the hospital clinical pathway and guidance from clinical journals. It is inadequate for the treatment of infectious diseases in critical, patient care bad condition, including morbidity and mortalities that increases the length of time hospitalization [20,21]. Antibiotics can be prescribed prophylactically to prevent the transfer of pathogens in the appropriate locations. Without a clear indication of use, it will increase the level of antibiotic resistance groups, especially β-lactams and aminoglycosides.
Some studies show that there is resistance to penicillin and tetracycline in some areas in Indonesia with percentages close to 100%. This finding makes the penicillin out of date as a first-line therapy; many clinicians are turning to the class of cephalosporin’s that are considered capable of defending against bacteria that cause problems of resistance [22].
Acknowledgements
I would like to express my sincere thanks to the Director of Health Polytechnic, Ministry of Health Bengkulu and Dr. M. Yunus General Local Hospital Bengkulu, Indonesia for their excellent technical assistance and support throughout my study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Mulholland EK, Adegbola RA. Bacterial infections - a major cause of death among children in Africa. N Engl J Med 2005;352:75-7.
- Aman G. Polypharmacy in pediatric practice in Denpasar. MKU 2000;31:159.
- McCaig LF. Trends in antimicrobial drug prescribing among office-based physicians in the United States. JAMA 2015;273:214.
- Wang EE, Einarson TR, Kellner JD, Conly JM. Antibiotic prescribing for Canadian preschool children: evidence of overprescribing for viral respiratory infections. Clin Infect Dis 1999;29:155-60.
- Lusini G, Lapi F, Sara B, Vannacci A, Mugelli A, Kragstrup J, et al. Antibiotic prescribing in paediatric populations: A comparison between Viareggio, Italy and Funen, Denmark. Eur J Public Health 2009;19:434-8.
- Siswati S. Analysis of use of antibiotics irrational in toddlers patients not penumonia in Padang. Sainstek 2009;9:73-80.
- Čižman M. The use and resistance to antibiotics in the community. Int J Antimicrob Agents 2003;21:297-307.
- Thrane N, Olesen C, Schønheyder HC, Sørensen HT. Socioeconomic factors and prescription of antibiotics in 0-2 year old Danish children. J Antimicrob Chemother 2003;51:683-9.
- World Health Organization. Antimicrobial resistance: Global report on surveillance. Geneva, Switzerland; 2014.
- Gyssens IC, Van Den Broek PJ, Kullberg BJ, Hekster Y, Van Der Meer JW. Optimizing antimicrobial therapy: a method for antimicrobial drug use evaluation. J Antimicrob Chemother 2005;30:724-7.
- BNF. BNF for children. London: British National Formulary Publications, Royal Pharmaceutical Society of Great Britain; 2009.
- Katzung BG, Masters SB, Trevor AJ. Basic and Clinical Pharmacology. 12th ed. United States: The McGraw-Hill Companies; 2012. p 1245.
- Drusano GL. Human pharmacodynamics of beta-lactams, aminoglycosides and their combination. Scand J Infect Dis Suppl 1989;74:235-48.
- Leekha S, Terrell CL, Edson RS. General principles of antimicrobial therapy. Mayo Clin Proc 2011;86:156-67.
- Aberg JA, William Alvarez Jr, Armstrong L, Bachmann KA, Baughman VL, Beizer JL, editors. Drug Information Handbook. 17th ed. New York:Lexi-Comp;2009.
- Reese RE, Betts RF, Gumustop B. Handbook of antibiotics. 3rd ed. Philadelphia: Lippincott Williams and Wilkins; 2000.
- Chavez-Bueno S, Stull TL. Antibacterial agents in pediatrics. Infect Dis Clin North Am 2009;23:865-80.
- Duerink O, Wibowo B, Parathon H, Hadi U, Lestari ES, Groot I, et al. Optimizing Surveillance of Surgical Site. In: Surveillance of Surgical Site Infections. Jakarta 2008. p. 58-67.
- Bauer LA. Applied Clinical Pharmacokinetics. 3rd ed. USA: McGraw-Hill Companies; 2008.
- Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000;118:146-55.
- Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections a risk factor for hospital mortality among critically ill patients. Chest 1999;115:462-74.
- Ieven M, Van Looveren M, Sudigdoadi S, Rosana Y, Goossens W, Lammens C, et al. Antimicrobial susceptibilities of Neisseria gonorrhoeae strains isolated in Java, Indonesia. Sex Transm Dis LWW 2003;30:25-9.