- *Corresponding Author:
- K. Tirumala Rao
Analytical Research, Product and Technology Development, ecoLogic Technologies Limited, Hyderabad-500 072, India
E-mail: tirumalwithu@gmail.com
| Date of Submission | 15 Apr 2015 |
| Date of Revision | 08 Mar 2016 |
| Date of Acceptance | 20 Apr 2016 |
| Indian J Pharm Sci, 2016;78(2):252-258 |
Abstract
A green, novel gradient stability-indicating reverse phase rapid resolution liquid chromatographic method was developed and validated for simultaneous estimation of irbesartan and along with six related impurities in active pharmaceutical ingredient samples. The chromatographic separation was achieved on Kromasil C8 (3.5 µm, 150×4.6 mm) short column with 0.1% v/v ortho-phosphoric acid and acetonitrile as mobile phase using gradient elution. The developed method showed good resolution between irbesartan and its six related impurities and were eluted within 15 min. run time of LC chromatogram. Regression analyses indicate correlation coefficient value greater than 0.999 for irbesartan and its six related impurities. The limit of detection for irbesartan and the known related impurities were observed at a level below 0.004% (0.019 µg/ml and the method is showing better recoveries for irbesartan (99.6–100.7%) and also for its six known impurities (88.5–98.9%). The test solution and related substances were found to be stable in the diluents for 24 h. The developed stability-indicating method is found to be rapid, accurate, precise, linear, specific, sensitive, rugged, robust, and stability-indicating. The application of developed method was also verified by an assay of irbesartan and related substances in commercial API bulk drug samples and more essentially, the method is economic and environment friendly than the other published methods.
Keywords
Irbesartan, RRLC, stability-indicating, related substances, validation
Irbesartan (IRB) is chemically described as 2-butyl- 3- [p-(o-1H-tetrazol-5-ylphenyl)benzyl]-1,3- diazaspiro [4.4]non-l-en-4-one. Its empirical formula is C25H28N6O, and molecular weight is 428.5 amu. IRB is an active non-peptide specific angiotensin II receptor antagonist (AT1 subtype) used as anti-hypertensive agent. Hypertension is the most prevalent cardiovascular disease in the developed as well as developing countries, affecting as many as one quarter of the adult population.
Furthermore, hypertension is an independent risk factor for cardiovascular diseases and associated with an increased incidence of stroke and coronary heart disease. Angiotensin II antagonists are major drugs used in hypertension management in the recent decade. Their lower side effect profile and specificity in the action provided a good condition for patient compliance as well as effectiveness. Therefore, these drugs are used as first line treatment for hypertension [1-4]. Stability testing of new drug substances and drug products requires a stress testing, which should be carried out to elucidate the inherent stability characteristics of the active substance. It suggests that the degradation products, which were formed under variety of conditions, should be identified and degradation pathways are to be established [5]. The literature survey reveals that several methods [6-13] were reported for the determination of IRB and hydrochlorothiazide. The methods [14-17] for IRB in combination with other drugs in plasma and serum were done by high-performance liquid chromatography (HPLC) and the few analytical methods have been reported on stability-indicating assay by HPLC method [18,19]. However, there is no stability-indicating fast LC (RRLC) method for simultaneous estimation of IRB and its related impurities in IRB bulk drug samples. The present research work is focused to develop the simple and rapid analytical procedure, which could serve as stability indicating assay method for simultaneous estimation of IRB and its pharmacopoeia specified impurities, along with process related, intermediate and degradation impurities. The present method can reduce the analysis time, manpower and instrument occupancy, effluent load and also significantly reduce analysis cost in routine analysis.
The developed method was validated with respect to International Conference on Harmonisation (ICH) requirements. The present validated stability indicating method can be used as an alternative for routine quality control analysis and stability study of API test samples.
Materials and Methods
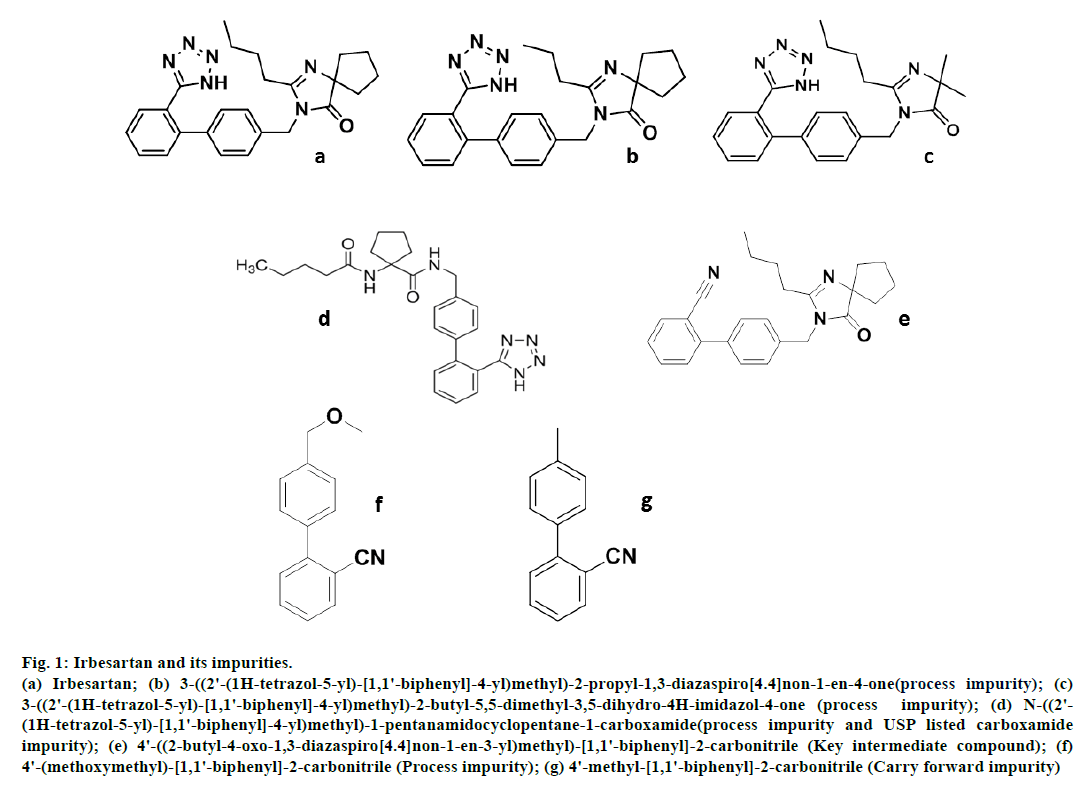
IRB and six impurity standards and API test samples were obtained from ecoLogic Technologies Limited, Hyderabad, India. Chemical structure of IRB and known impurities are shown in fig. 1 and these are confirmed by 1HNMR, mass spectroscopy data. Acetonitrile and ortho-phosphoric acid (HPLC grade), sodium hydroxide (NaOH), hydrochloric acid (HCl) and hydrogen peroxide (H2O2) GR grade chemicals were purchased from Merck Fine Chemicals, Mumbai, India. Milli Q water is obtained from Millipore direct 8 l/h system.
Fig 1: Irbesartan and its impurities.
(a) Irbesartan; (b) 3-((2'-(1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-2-propyl-1,3-diazaspiro[4.4]non-1-en-4-one(process impurity); (c)
3-((2'-(1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-2-butyl-5,5-dimethyl-3,5-dihydro-4H-imidazol-4-one (process impurity); (d) N-((2'-
(1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-1-pentanamidocyclopentane-1-carboxamide(process impurity and USP listed carboxamide
impurity); (e) 4'-((2-butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl)-[1,1'-biphenyl]-2-carbonitrile (Key intermediate compound); (f)
4'-(methoxymethyl)-[1,1'-biphenyl]-2-carbonitrile (Process impurity); (g) 4'-methyl-[1,1'-biphenyl]-2-carbonitrile (Carry forward impurity)
Agilent infinity series RRLC system consisting quaternary solvent delivery pump, a degasser, an auto injector, column thermostat and photo diode array detector with open lab CDS chemstation and EZ-Chrom software (Agilent Technologies, Clara, US) was used for method development and subsequent validation study.
Optimization of chromatographic conditions
The main target of the chromatographic method is to get the separation of above said known impurities and degradation products generated during stress studies from the analyte peak. Initially, USP method was attempted to separate the process related impurities along with USP specified impurities. During the analysis, impurity-A and B co-elution was observed by performing USP method condition. It is necessary to know the amount of other process related impurities in bulk drug sample during manufacturing of IRB commercial process. Indeed, the method should be optimized to monitor the impurities during manufacturing of IRB process samples. The present method is developed for the above uncertainty results of impurity-A and B and similarly late eluted other known, unknown impurities in given samples. Various buffer pH, gradient conditions and columns were chosen for method development and optimization. Finally, succeeded on Kromasil C8 3.5 μm, 150×4.6 mm (Kromasil, Brewster, NY) HPLC short column with mobile phase consisting A: ortho phosphoric acid 0.05% v/v and B: acetonitrile, using gradient elution program T (min)/% B: 0/30, 6/55, 9/65, 12/80, 15/80, 16/30, 20/30. Column flow rate was operated at the rate of 1.0 ml/min, injection volume was 5.0 μl and detector was set at 220 nm. The column thermostat temperature was maintained at 35°. Acetonitrile was used as diluent for standard and sample preparations.
Preparation of standard and test sample solutions
Standard and test solution of IRB were prepared at concentration of 500 μg/ml using diluent for assay determination and the same solution was used for purity determination. Standard stock solutions of impurities were prepared at concentration of 100 μg/ ml and further diluted to 0.5 μg/ml level and spiked in test sample for system suitability evaluation. The same impurity stock solutions were used for related substances method validation study to determine the known impurities with respect to IRB API test sample solutions. A composite sample of IRB API test sample was taken for the entire study.
Results and Discussion
The specificity of the developed LC method for IRB was carried out in the presence of its six impurities. Stress studies were performed at an initial concentration of 500 μg/ml of IRB API test sample, to provide an indication of stability indicating property and specificity of the proposed method. Acidic and basic stress were performed in 1N HCl and 1N NaOH at 60° for 12 h, respectively. Oxidation study was carried out at 60º in 3% hydrogen peroxide for 12 h. Photo degradation studies were carried out according to Option 2 of Q1B in International Conference on Harmonisation of Technical Requirements for registration of pharmaceuticals for human use guidelines. The drug sample was exposed to light and overall illumination of 1.2 million lux h and an integrated near ultraviolet energy of 200 W.h/ m2. The drug sample was exposed to dry heat at 80° for 10 days to evaluate the ability of the proposed method to separate IRB from its degradation products. Photodiode array detector was employed to ensure the homogeneity and purity of IRB peak in the entire stressed sample solutions. Assessment of mass balance in the degraded samples was carried out to confirm the amount of impurities detected in stressed samples and matched with the amount present before the stress. The mass balance (% assay+% sum of all impurities+% sum of all degradation products) was tabulated in Table 1. According to stress study data (Table 1), product degradation is very less for the duration of stress study performed. The peak purity for IRB peak was passing in all the stressed samples and also there was no interference from degradation products from the analyte peak. Quantitative determination of IRB was carried out for all the stressed samples against qualified working standard.
| Stress condition | Time | Assay (%w/w) | Total impurities (%area) | Mass balance (%w/w) |
|---|---|---|---|---|
| Acid hydrolysis using 1N HCl (at 60°) | 12h | 99.8 | 0.35 | 100.2 |
| Base hydrolysis using 1N NaOH (at 60°) | 12 h | 99.8 | 0.34 | 100.1 |
| Oxidative degradation using 3% H2O2 | 12 h | 99.7 | 0.48 | 100.2 |
| Photolytic degradation-controlled | 11 days | 99.5 | 0.34 | 99.8 |
| Photolytic degradation-uncontrolled | 11 days | 99.1 | 0.34 | 99.4 |
| Thermal degradation at 80° | 10 days | 99.3 | 0.34 | 99.6 |
Table 1: Results Of Stress Studies
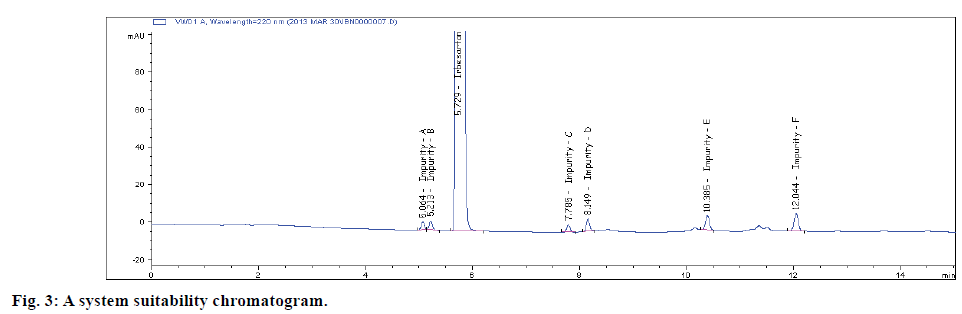
The selectivity of the method was established from the resolution of IRB from the nearest peak and also among all the other peaks. System suitability results are depicted in Table 2. Typical blank and selectivity chromatograms are shown in figs. 2A and 2B.. All the impurities were separated well and from analyte as well with a resolution greater than 1.8. Hence, the method was proved selective. There is no interference was observed from the blank peaks. Peak homogeneity test result is satisfying the requirement during peak purity measurement (Table 2).
| Name | Retention time (tR) in min. | Resolution(Rs)by Tangent | USP TheoreticalPlates | Tailingfactor (T) |
|---|---|---|---|---|
| Impurity-A | 5.20 | - | 36116 | 0.96 |
| Impurity-B | 5.37 | 1.8 | 38955 | 1.10 |
| IRB | 5.88 | 3.7 | 19295 | 1.37 |
| Impurity-C | 7.80 | 13.5 | 75837 | 0.98 |
| Impurity-D | 8.38 | 5.3 | 101288 | 1.01 |
| Impurity-E | 10.42 | 16.3 | 84300 | 0.98 |
| Impurity-F | 12.08 | 11.6 | 111955 | 1.00 |
Table 2: System Suitability Results
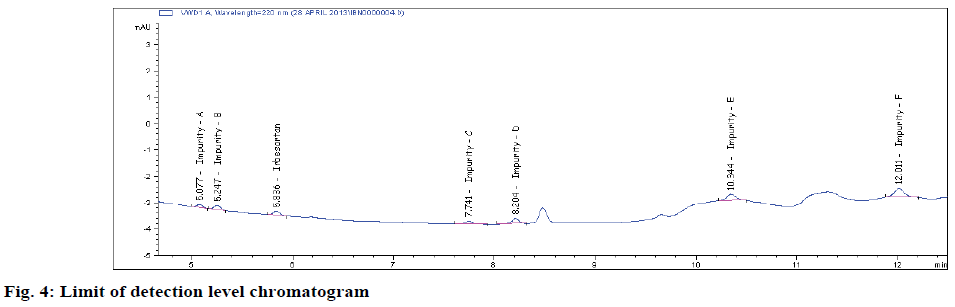
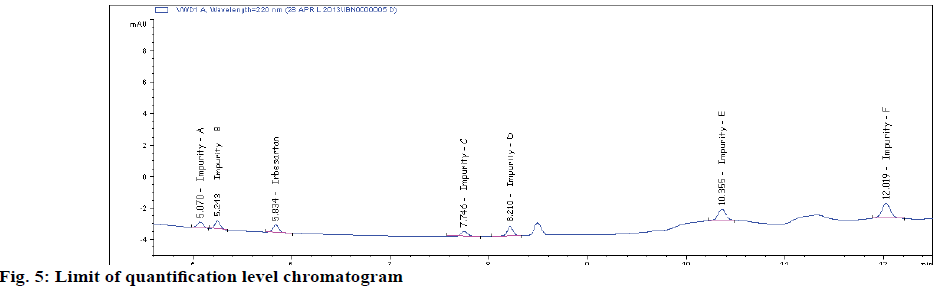
Typical method sensitivity LOD and LOQ chromatograms are shown in figs 2C and 2D. The LOD and LOQ for IRB and its impurities were determined at a signal to noise ratio of 3:1 and 10.1, respectively. By injecting a series of dilute solutions with known concentrations. The obtained LOQ concentration is < 0.06 μg/ml. Precision study was also carried out at the LOQ level by injecting six (n=6) individual preparations and calculating the RSD percentage of the area (Table 3). The observed %RSD value is below 2.6 in method precision study and below 2.1 in intermediate precision study (Table 3).
| Parameter | IRB | Imp-A | Imp-B | Imp-C | Imp-D | Imp-E | Imp-F |
|---|---|---|---|---|---|---|---|
| LOD (µg/ml) | 0.019 | 0.018 | 0.018 | 0.017 | 0.017 | 0.016 | 0.018 |
| LOQ (µg/ml) | 0.064 | 0.059 | 0.058 | 0.056 | 0.056 | 0.054 | 0.06 |
| Regression equation | |||||||
| Slope (m) | 33973.5 | 30589.7 | 42613.7 | 30708 | 50500.6 | 75940.7 | 88045.2 |
| Intercept (c) | -486.7 | -1738.2 | -2090.5 | -1595 | -2276.6 | -3271.6 | - 3337.4 |
| Correlation coefficient | 0.9997 | 0.9998 | 0.9998 | 0.9992 | 0.9996 | 0.9993 | 0.9995 |
| Y-intercept at 100% level | -1.2% | -5.4% | -4.6% | -5.5% | -4.5% | -4.8% | -3.4% |
| Method precisiona | 0.28% | 0.28% | 0.33% | 0.31% | 2.2% | 2.6% | 0.35% |
| Intermediate precisiona | 0.54% | 0.82% | 0.35% | 0.98% | 1.9% | 2.1% | 0.45% |
aSix determinations of specified level impurities with respect to analyte concentration (500 µg/ml) 100 µg/ml for assay of IRB.
Table 3: Lod, Loq, Regression And Precision Data
Assay method precision, intermediate precision and similarly related substances method precisions were shown in Table 3. Linearity test solutions for the assay method were performed from 125 to 1000 μg/ml (i.e., 125, 250, 375, 500, 750 and 1000 μg/ ml). The responses were measured as peak areas and plotted against concentration. Assay method precision was carried out using six independent test solutions and a standard preparation. The intermediate precision of assay method was also evaluated using different instruments on different days. Similarly, related substances method precision and intermediate precision was also carried out using six independent test solutions containing 0.2% level of known impurities with respect to the test sample concentration (i.e., 500 μg/ml) (Table 3).
Similarly, linearity test solutions for the related substances (RS) method were performed from LOQ to 2 μg/ml of impurity level (i.e., LOQ to 0.4% impurity level with respect to the test conc. 500 μg/ ml). The calibration curve was drawn by plotting the each impurity peak area versus its corresponding concentration. Both the methods (RS and Assay) are showing good correlation coefficient >0.999 and it indicates existence of an excellent correlation between the peak area and concentration of IRB and six impurities. The obtained linearity experiment results are given in Table 3.
The accuracy of the assay method was evaluated in triplicate using three concentration levels such as 250, 500 and 750 μg /ml (i.e., 50, 100 and 150% level of assay test concentration) and the percentage of recoveries of IRB were calculated at each level and the % recovery is 99.6 to 100.7%. Related substance method accuracy was also carried out in triplicate using three concentration levels of 0.5, 1 and 1.5 μg/ ml (i.e., 0.1, 0.2 and 0.3% levels of impurities with respect to the test concentration of 500 μg/ml) and the % recovery is 88.5 to 98.9% (Table 4).
| Name | Level ( %) | Amount added in µg/ml | Amount recovered in µg/ml | %Recovery |
|---|---|---|---|---|
| Impurity-A | 50 100 150 |
0.564 1.128 1.691 |
0.544 1.080 1.590 |
96.5 95.7 94.0 |
| Impurity-B | 50 100 150 |
0.564 1.128 1.692 |
0.535 1.035 1.591 |
94.9 91.8 94.0 |
| IRBa | 50 100 150 |
0.627 1.255 1.882 |
0.569 1.178 1.665 |
90.7 93.9 88.5 |
| Impurity-C | 50 100 150 |
0.503 1.007 1.510 |
0.474 0.972 1.385 |
94.2 96.5 91.7 |
| Impurity-D | 50 100 150 |
0.534 1.068 1.601 |
0.515 1.006 1.498 |
96.4 94.2 93.6 |
| Impurity-E | 50 100 150 |
0.553 1.105 1.658 |
0.535 1.093 1.597 |
96.7 98.9 96.3 |
| Impurity-F | 50 100 150 |
0.589 1.179 1.658 |
0.577 1.141 1.622 |
98.0 96.8 97.8 |
| IRBb | 50 100 150 |
258.96 507.81 753.86 |
257.96 508.11 759.24 |
99.6 100.1 100.7 |
aUnknown impurity level of IRB with respect to analyte concentration (500 µg/ml),
bAssay of IRB concentration (500 µg/ml).
Table 4: Results Of Accuracy For Related Substance And Assay
Accuracy parameter is performed to determine the closeness of test results with that of the true value which is expressed as %recovery. The results of accuracy were depicted in Table 4. Robustness of the method was determined as a measure of the analytical method capability to be unaffected by small variations in method parameters (Table 5). The robustness was determined by the variation of flow rate by ± 0.2 ml/min, column temperature by ±5°, composition of mobile phase by ±10% (in terms of organic component) and slight variation in wavelength by ±2 nm. At these changed conditions, the system suitability was evaluated at each condition. In all the conditions, the resolution between critical pair was greater than 1.7 and tailing factor of IRB peak was found be less than or equal to 1.5 (Table 5).
| Chromatographic changes | Resolutiona | Tailing factor | Theoretical plate count |
|---|---|---|---|
| Flow rate (ml/min) 0.8 1.2 | 1.8 1.5 |
1.21 1.41 |
22733 16146 |
| Temperature (°) 30 40 | 1.6 1.7 |
1.40 1.37 |
17611 19846 |
| Wavelength(nm) 218 222 | 1.6 1.6 |
1.43 1.36 |
17988 19690 |
| Mobile phase composition (%) 67:33 27:73 | 1.7 1.6 |
1.51 1.18 |
13389 23165 |
aResolution measured between Impurities-A and B peaks
Table 5: Results Of Robustness Evaluation
The %RSD of assay of IRB during solution stability and mobile phase stability experiments is less than 1.0%. No significant changes were observed in the content of impurity-1, impurity-2, impurity-3, impurity-4, impurity-5 and impurity-6 during solution stability and mobile phase stability experiments. The solution stability and mobile phase stability experiments data confirms that the sample solutions, mobile phase used for assay and related substances determination are stable up to the study period of 48 h.
The current stability-indicating RRLC method was found to be suitable for the determination of assay of IRB and its related impurities. The developed method is simple, specific and rapid, and the method was fully validated as per regulatory requirements i.e., ICH and USP. The present method can be successfully used for the quality determination of IRB in commercial manufacturing batches and also it for stability monitoring (accelerated, long term stability studies) in quality control laboratories. Most importantly, the established method is greener than other published methods in terms of analysis cost, time and effluent load at laboratories.
Acknowledgements
Authors are thankful to ecoLogic Technologies Limited, Hyderabad, India for supporting all the facilities to carry out this work. We would also thank our colleagues, process and analytical research departments of ecoLogic Technologies Limited for their support.
Financial Assistance
None.
Conflict of Interests
None declared.
References
- The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med 1997;157:2413-46.
- Guidelines Subcommittee. 1999 World Health Organization‐International Society of Hypertension Guidelines for the Management of Hypertension. J Hypertens 1999;17:151-83.
- Effects morbidity of treatment on in hypertension: II. results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA 1970;213:1143-52.
- Messerli FH, Grossman E, Goldbourt U. Are β-blockers efficacious as first-line therapy for hypertension in the elderly? A systematic review. JAMA 1998;279:1903-07.
- ICH, Q1A (R2), Harmonised Tripartite Guideline, Stability testing of new drug substances and products. February, 2003.
- Al-Momani IF. Determination of hydrochlorothiazide and enalapril maleate in tablet formulations by reversed-phase HPLC. Turk J Chem 2001;25:49-54.
- Sasa SI, Jalal IM, Khalil HS. Determination of atenolol combination with hydrochlorothiazide and chlorthalidone in tablet formulations by reverse-phase HPLC. J Liq Chromatogr 1988;11:1673-96.
- Kirschbaum J, Perlman S. Analysis of captopril and hydrochlorothiazide combination tablet formulations by liquid chromatography. J Pharm Sci 1984;73:686-87.
- Belal F, Al-Zaagi IA, Gadkariem EA, Abounassif MA. A stability-indicating LC method for the simultaneous determination of ramipril and hydrochlorothiazide in dosage forms. J Pharm Biomed Anal 2001;24:335-42.
- Ertürk S, Çetin SM, Atmaca S. Simultaneous determination of moexipril hydrochloride and hydrochlorothiazide in tablets by derivative spectrophotometric and high-performance liquid chromatographic methods. J Pharm Biomed Anal 2003;33:505-11.
- Panderi IE, Parissi-Poulou M. Simultaneous determination of benazepril hydrochloride and hydrochlorothiazide by micro-bore liquid chromatography. J Pharm Biomed Anal 1999;21:1017-24.
- Carlucci G, Palumbo G, Mazzeo P, Quaglia MG. Simultaneous determination of losartan and hydrochlorothiazide in tablets by high-performance liquid chromatography. J Pharm Biomed Anal 2000;23:185-9.
- Shah NJ, Suhagia BN, Shah RR, Shah PB. Development and validation of a HPLC method for the simultaneous estimation of telmisartan and hydrochlorothiazide in tablet dosage form. Indian J Pharm Sci 2007;69:202-5.
- Chang S-Y, Whigan DB, Vachharajani NN, Patel R. High-performance liquid chromatographic assay for the quantitation of irbesartan (SR 47436/BMS-186295) in human plasma and urine. J Chromatogr B: Biomed Sci Appl 1997;702:149-55.
- Mbah CJ. Kinetics of decomposition of irbesartan in aqueous solutions determined by high performance liquid chromatography. Pharmazie 2004;59:920-22.
- Chando TJ, Everett DW, Kahle AD, Starrett AM, Vachharajani N, Shyu WC, et al. Biotransformation of Irbesartan in man. Drug Metab Dispos 1998;26(5):408-17.
- Rahman N, Siddiqui MR, Azmi SNH. Quantitative analysis of Irbesartan in commercial dosage forms by kinetic spectrophotometry. Chem Pharm Bull 2006;54:626-31.
- Rane VP, Patil KR, Sangshetti JN, Yeole RD, Shinde DB. Stability indicating LC method for simultaneous determination of irbesartan and hydrochlorothiazide in pharmaceutical preparations. J Chromatogr Sci 2010;48:595-600.
- Lakshmi Narasimham YSL, Barhate VD. Development and validation of stability indicating UPLC method for the simultaneous determination of beta-blockers and diuretic drugs in pharmaceutical dosage forms. J Chem Metrol 2010;4:1-20.