- *Corresponding Author:
- Xiaobin Lin
Department of Orthopedics, Second Affiliated Hospital of Fujian Medical University, Quanzhou, Fujian Province 362000, China
E-mail: proflxb@163.com
| Date of Received | 25 February 2023 |
| Date of Revision | 17 November 2023 |
| Date of Acceptance | 04 May 2024 |
| Indian J Pharm Sci 2024;86(3):825-831 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effects of radix Ranunculus ternatus saponins on sarcoma osteogenic-2 cell malignancy and its possible mechanism. Sarcoma osteogenic-2 cells were treated with different doses (50, 100, 200 mg/l) of radix Ranunculus ternatus saponins for 24 h. Effect of radix Ranunculus ternatus saponins on sarcoma osteogenic-2 cell malignant behaviors were detected by cell counting, colony formation, wound-healing, and transwell invasion assays. Protein levels were detected by Western blotting. Treatment with radix Ranunculus ternatus saponins repressed the proliferative, migratory, and invading capacities of sarcoma osteogenic-2 cells in a dose-dependent manner. Radix Ranunculus ternatus saponins reduced neural-cadherin protein levels and elevated epithelial-cadherin protein levels. MicroRNA-1305 was downregulated in osteosarcoma samples, while microRNA-1305 was elevated in microRNA-1305 treated sarcoma osteogenic-2 cells. Upregulation of microRNA-1305 restrained sarcoma osteogenic-2 cell proliferation, metastasis, and invasion, while microRNA-1305 knockdown radix Ranunculus ternatus saponins-mediated inhibiting effects on sarcoma osteogenic-2 cell malignant behaviors. Radix Ranunculus ternatus saponins might inhibit sarcoma osteogenic-2 cell malignant behaviors by up-regulating miR-1305, which has a potential value for osteosarcoma treatment.
Keywords
Radix Ranunculus ternatus saponins, osteosarcoma, microRNA-1305, cisplatin, doxorubicin
Osteosarcoma (OS) is a malignant tumor originating from mesenchymal tissue, with a high degree of malignancy, poor prognosis, easy to transfer, posing a serious threat to human health[1,2]. At present, treatment drugs for OS (such as doxorubicin and cisplatin) often have significant side effects and can easily lead to drug resistance in OS cells, leading to poor treatment efficacy[3,4]. Therefore, exploring effective drugs that inhibit the malignant behavior of OS cells is urgently needed.
The traditional Chinese medicine Radix Ranunculus ternatus (RRT), is a dry root tuber of the Ranunculaceae plant, which has the effects of clearing heat, detoxifying, and relieving cough and phlegm[5]. RRT Saponins (RRTS) is one of the main active ingredients of RRT, which has a significant inhibitory effect on the malignant behavior of ovarian cancer and gastric cancer, showing a certain anti-tumor effect. However, it is still unknown whether RRTS can exert an inhibitory effect on OS.
microRNAs (miRs) play a critical role in the post- transcriptional regulation of gene expression[6]. Variations in the expression of miRs are associated with various malignancies[7], including OS[8]. miR- 1305 is a type of miR that is downregulated in diverse tumors and exerts an anti-tumor role, such as bladder[9], colorectal[10], and cervical[11] cancers. However, whether miR-1305 regulates OS cell malignancy is still unknown.
Here, we mainly explored the effects of RRTS on cell malignant behaviors in Sarcoma osteogenic (Saos)-2 cells and whether RRTS plays a role by regulating miR-1305, providing a certain experimental basis for OS treatment.
Materials and Methods
Clinical data:
43 cases of OS patients (22 females and 21 males) who underwent surgical treatment in our hospital from June 2018 to June 2020 were recruited in the research, and their OS samples and matching adjacent normal tissues were collected and stored in liquid nitrogen. The average age of all patients was 18.26±5.37. Patient was initially diagnosed and had not received any treatment prior to surgery were included in this study. Patients with important organ dysfunction, such as heart and kidney were excluded from this study. The research conformed to the principles of the Declaration of Helsinki.
Production of RRTS:
Preparation of RRTS was carried out according to the reported method[12]. Simply, 500 g of dried RRT was accurately weighed, crushed, followed by the addition of 6-fold the volume of 85 % ethanol, with heating reflux extraction for 3 times, each time for 3 h. Subsequently, the ethanol was recovered and extracted three times with petroleum ether and N-butanol in turn. N-butanol was recovered and concentrated into an extract, followed by vacuum drying to obtain RRTS. When using RRTS, it was dissolved with Roswell Park Memorial Institute (RPMI)-1640 medium, followed by filtration and sterilization, and the RRTS solution was stored in a 4° refrigerator.
Cell transfection and RRTS treatment:
The Saos-2 cell line (Cell Bank of Chinese Academy of Sciences, Shanghai, China) was cultured in Roswell Park Memorial Institute-1640 Medium (Soraibao, Beijing, China) containing 10 % Fetal Bovine Serum (FBS) and maintained at 37° with 95 % relative humidity and 5 % carbon dioxide. When the density reached 90 %, the cells were trypsinized and passaged.
For transfection, miR-1305 mimics, anti-miR-1305, miR-NC, or anti-miR-NC (Sangon Biotech, Shanghai, China) was transfected into Saos-2 cells using the LipofectamineTM 2000 reagent (Invitrogen, United States of America (USA)) according to the manufacturer’s instructions when the confluence reached 30 %-40 %. For RRTS treatment, the cells were divided into 4 groups and incubated with a complete medium containing 0 mg/l (control), 50 mg/l (RRTS-Low (L)), 100 mg/l (RRTS-Medium (M)), and 200 mg/l (RRTS-High (H)) of RRTS.
Detection of cell proliferation:
200 µl of Saos-2 cell suspension (5.0×104 cells/ml) was seeded. 4 h later, the cells were divided into 4 groups and incubated with a complete medium containing RRTS. 24 h later, 10 μl of Cell Counting Kit-8 (CCK-8) solution (Soraibao) was added and incubated for 2 h, followed by measurement of the Absorbance (A) using a microplate reader (wavelength 450 nm).
Inhibitory rate (%)=(Control group-experimental group)/control group×100 %
Colony formation assay:
Saos-2 cell suspension (2.5 ml, 5.0×104 cells/ml) was cultured for 4 h, followed by treatment with different concentrations of RRTS for 14 d. After stabilization with 4 % Paraformaldehyde (PFA) (Soraibao), and coloration with crystal violet (Beyotime, Shanghai, China), the colonies were determined using an optical microscope.
Wound-healing assay:
Saos-2 cell suspension (2.5 ml, 5.0×104 cells/ ml) was cultured for 4 h. The cell monolayer was scratched by a sterilized pipette tip (10 µl), followed by washing with Phosphate Buffer Solution (PBS). A complete medium with different concentrations of RRTS was used for cell culture. A microscope was used to photograph the scratched areas at 0 h and 24 h.
Wound healing rate (%)= (0 h scratch width-24 h scratch width)/0 h scratch width×100 %
Transwell invasion assay:
100 µl of Saos-2 cell suspension (serum-free) (5.0×105 cells/ml) added to the upper chambers coated with Matrigel. A complete medium containing different concentrations of RRTS was added to the lower chambers. After 24 h, the invaded cells were stabilized with 4 % PFA and then dyed with 0.1 % crystal violet. After washing, the observation of the invasive cells was performed using an inverted microscope.
Western blotting:
The collected Saos-2 cells were lysed in cold Radioimmunoprecipitation Assay (RIPA)-strong lysis buffer (Beyotime). The supernatants obtained by centrifugation (12 500 rpm, 4°, 30 min) was quantified using a Bicinchoninic Acid (BCA) assay (Soraibao). Subsequently, the diluted supernatants were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Proteins were transferred to a polyvinylidene fluoride membrane (Bio-Rad, Hercules, California, US) after electrophoresis. The membranes were incubated with primary antibodies following blocking with 5 % skimmed milk, including Epithelial (E)-cadherin (1:500, Abcam, USA), Neural (N)-cadherin (1:500, Abcam), and Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) (1:1000, Abcam). After washing, the membranes were hybridized with horseradish peroxidase-conjugated secondary antibodies. Protein expression was visualized by an enhanced chemiluminescence substrate (Millipore, Billerica, Massachusetts, USA).
Reverse Transcription-quantitative Polymerase Chain Reaction (RT-qPCR):
Extraction of total Ribonucleic Acid (RNA) in cells and tissues was made using miRNA extraction kits (Takara, Dalian, China). Preparation of complementary Deoxyribonucleic Acid (cDNA) was done with the extracted RNA using the reverse transcription kit (Takara). QPCR was carried out by SYBR real-time PCR kit (Takara). The following were the sequences of primers; U6 (forward 5’-ACGCTTCACGATTTGCGT-3’; reverse 5’-CTCGCTTCTTCGGCAGCACA-3’); miR-1305 (forward 5’-ACAGGCCGGACAAGTGCAAATA-3’ and reverse 5’- GCTGCAACGATACTACT ACTACTACTACTAC GCTACGTAACG-3’). Calculation of miR-1305 expression was carried out using 2-∆∆CT method, with U6 as an internal control.
Statistical analysis:
Quantitative data were recorded as mean value±standard deviation with Statistical Package for the Social Sciences (SPSS) 22.0 software (SPSS 22.0 software, Illinois, USA). Independent t-test was conducted for comparison between the two groups. Multiple group comparisons were conducted using one-way Analysis of Variance (ANOVA) followed by a post hoc Least Significant Difference (LSD) test (equal variance). Statistical significance was defined as p<0.05.
Results and Discussion
We first analyzed the effect of RRTS on Saos-2 cell proliferation. Treatment with RRTS resulted in an increase in the inhibitory rate of Saos-2 cell proliferation and a reduction in the number of clones, as verified by CCK-8 and colony formation assays. RRTS inhibited Saos-2 cell proliferation in a dose-dependent manner (Table 1).
| Group | Inhibitory rate (%) (n=9) | Number of cell clones (n=9) |
|---|---|---|
| Control | 0.00±0.00 | 116.96±11.01 |
| RRTS-L | 25.21±2.23* | 90.64±7.43* |
| RRTS-M | 42.98±4.17*# | 71.35±5.13*# |
| RRTS-H | 64.11±5.65*#& | 53.54±4.52*#& |
| F | 490.107 | 119.112 |
| p | 0.000 | 0.000 |
Note: *p<0.05, in comparison with control; #p<0.05, in comparison with RRTS-L and &p<0.05, in comparison with RRTS-M
Table 1: Effect of RRTS on SAOS-2 Cell Proliferation
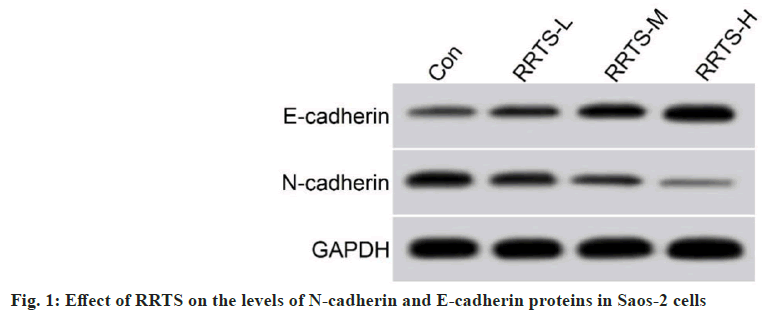
Further experiments were done to determine the toxicity of RRTS on Saos-2 cells. RRTS treatment significantly impaired Saos-2 cell metastasis in wound-healing assay. RRTS treatment also caused a decrease in the invasive ability of Saos-2 cells. In Saos-2 cells, RRTS treatment resulted in reduced N-cadherin protein levels and elevated E-cadherin protein levels. Furthermore, RRTS-mediated effects on the migratory and invasive capacity of Saos-2 cells, as well as N-cadherin and E-cadherin protein levels, were enhanced with the increase of its concentration (fig. 1 and Table 2).
| Group | Wound healing rate (%) (n=9) | Number of invasive cells (n=9) | E-cadherin (n=9) | N-cadherin (n=9) |
|---|---|---|---|---|
| Control | 65.23±4.50 | 131.39±12.72 | 0.23±0.02 | 0.54±0.04 |
| RRTS-L | 51.99±4.88* | 104.27±9.89* | 0.36±0.03* | 0.42±0.04* |
| RRTS-M | 39.12±3.49*# | 82.01±7.73*# | 0.51±0.05*# | 0.28±0.02*# |
| RRTS-H | 24.39±2.34*#& | 64.23±5.79*#& | 0.66±0.05*#& | 0.14±0.02*#& |
| F | 178.352 | 95.477 | 197.714 | 269.7 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05, in comparison with control; #p<0.05, in comparison with RRTS-L and &p<0.05, in comparison with RRTS-M
Table 2: Effect of RRTS on the Migratory and Invasive Capacity of SAOS-2 Cells, as well as the Levels of N-Cadherin and E-Cadherin Proteins
As indicated in the introduction, miR-1305 exerts an anti-tumor role in many cancers. We detected miR-1305 expression in OS samples. Interestingly, miR-1305 expression in OS samples was 0.45±0.04, which was significantly lower than that in adjacent non-tumor samples (1.00±0.11) (t=30.813, p<0.05), proclaiming that miR-1305 downregulation may be associated with the occurrence of OS. Therefore, we next determined the effects of RRTS treatment on miR-1305 expression. RRTS resulted in an overt elevation in miR-1305 expression in a dose-dependent manner, suggesting that RRTS may exert an inhibitory effect through mediating miR-1305 expression (Table 3).
| Group | miR-1305 (n=9) |
|---|---|
| Control | 1.00±0.00 |
| RRTS-L | 1.59±0.14* |
| RRTS-M | 2.22±0.24*# |
| RRTS-H | 3.12±0.26*#& |
| F | 204.669 |
| p | 0.000 |
Note: *p<0.05, in comparison with control; #p<0.05, in comparison with RRTS-L and &p<0.05, in comparison with RRTS-M
Table 3: Effect of RRTS on miR-1305 Expression in SAOS-2 Cells
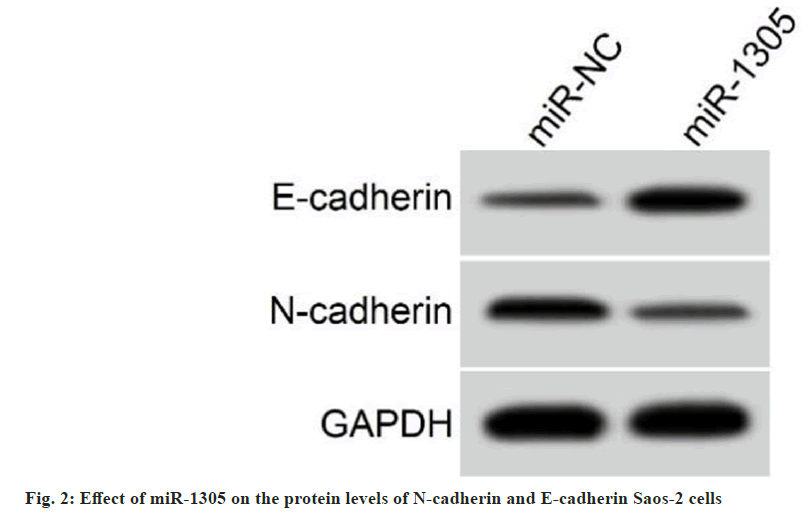
Transfection efficiency of miR-1305 mimic was detected, and the results exhibited that miR- 1305 levels in cells transfected with miR-1305 mimic was increased by 3.05±0.27 fold compared with the miR-NC group (1.00±0.00) (t=22.778, p<0.05). Functionally, miR-1305 upregulation repressed Saos-2 cell proliferation, migration, and invasion in comparison with the miR-NC group. In addition, exogenous expression of miR-1305 elevated E-cadherin protein levels and decreased N-cadherin protein levels (Table 4 and fig. 2). All results manifested the inhibiting effect of miR-1305 on Saos-2 cell malignant behaviors.
| Group | Inhibitory rate (%) (n=9) | Number of cell clones (n=9) | Wound healing rate (%) (n=9) | Number of invasive cells (n=9) | E-cadherin (n=9) | N-cadherin (n=9) |
|---|---|---|---|---|---|---|
| miR-NC | 5.84±0.52 | 113.71±11.82 | 68.14±5.54 | 134.34±11.18 | 0.22±0.02 | 0.56±0.04 |
| miR-1305 | 52.85±4.06* | 61.02±5.58* | 31.58±3.15* | 68.87±5.48* | 0.61±0.05* | 0.22±0.02* |
| t | 34.455 | 12.093 | 17.210 | 15.775 | 21.726 | 22.808 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 compared with miR-NC
Table 4: Effect of miR-1305 on SAOS-2 Cell Proliferation, Metastasis, and Invasion
Transfection of miR-1305 inhibitor into Saos-2 cells was executed to knock down miR-1305. We observed that miR-1305 levels in cells transfected with miR-1305 inhibitor were markedly reduced by 0.29±0.03 fold compared with the anti- miR-NC group (1.00±0.00) (t=71.000, p<0.05).
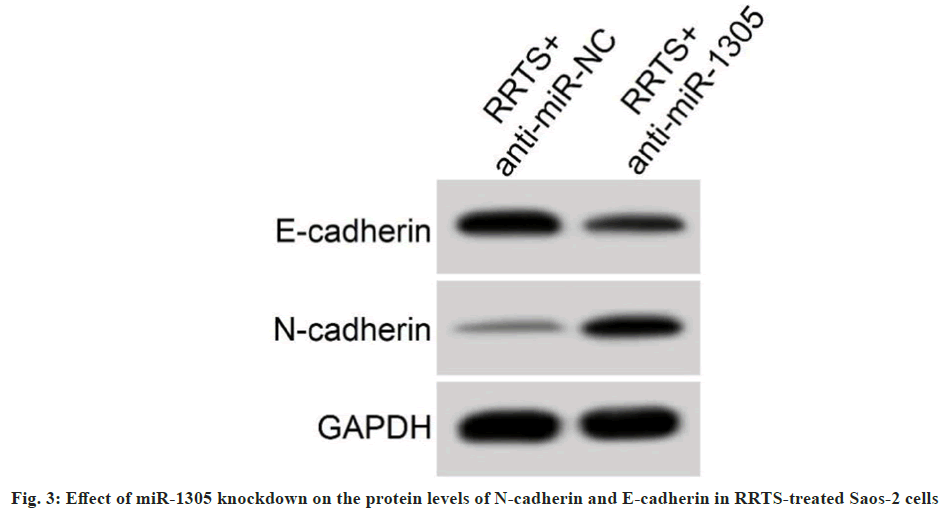
Furthermore, RRTS treatment mediated inhibitory effects on Saos-2 cell malignant behaviors were impaired after miR-1305 knockdown. Changes in N-cadherin and E-cadherin protein levels urged by RRTS treatment were partly overtuned by downregulating miR-1305 (Table 5 and fig. 3). Together, these data suggested that RRTS repressed Saos-2 cell malignant behaviors by elevating miR- 1305 expression.
| Group | miR-1305(n=9) | Inhibitory rate (%) (n=9) | Number of cell clones (n=9) | Wound healing rate (%) (n=9) | Number of invasive cells (n=9) | E-cadherin (n=9) | N-cadherin (n=9) |
|---|---|---|---|---|---|---|---|
| RRTS+anti-miR-NC | 1.00±0.00 | 65.08±5.63 | 51.37±4.78 | 22.86±2.12 | 61.62±5.15 | 0.68±0.05 | 0.13±0.02 |
| RRTS+anti-miR-1305 | 0.29±0.03* | 28.23±2.48* | 95.71±7.05* | 57.55±5.26* | 110.38±11.45* | 0.32±0.03* | 0.46±0.04* |
| t | 71.000 | 17.970 | 15.617 | 18.351 | 11.651 | 18.522 | 22.137 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 compared with RRTS+anti-miR-NC
Table 5: Effect of miR-1305 Silencing on SAOS-2 Cell Proliferation, Metastasis and Invasion under RRTS Treatment
Saponins are a class of glycosides with triterpenes or spirostanes as aglycones and are widely present in higher-plants[13]. A sequence of studies have shown that various natural plant saponins have anti-tumor effects[14]. Wang et al.[15] showed that total saponins of Tupistra chinensis baker could trigger AGS cell apoptosis via the p53- mediated pathway and reduced tumor growth in mouse models. Chang et al.[16] showed that total saponins of Rhizoma Panacis Majoris could inhibit colorectal cancer cell proliferation by regulating c-Jun N-terminal kinase (JNK) and p38 Mitogen- Activated Protein kinase (MAPK) signaling. Han et al. showed that total saponins of Solanum nigrum L. could overcome drug resistance by inducing autophagy through the mammalian Target of Rapamycin (mTOR) signaling[17].
The total saponins of RRT are the main active ingredients of traditional Chinese medicine RRT, which also have anti-tumor effects[18]. The report of Niu et al.[19] have shown that the saponins and polysaccharides from RRT repressed BGC823 cell proliferation. You et al.[20] reported that RRTS suppressed ovarian cancer cell aggressiveness by upregulating miR-let-7b. In this study, 50, 100, and 200 mg/l of RRTS were applied to treat Saos-2 cells for 24 h. RRTS increased the inhibitory rate and reduced the colony formation, metastasis, and invasion of Saos-2 cells with the elevation of RRTS, suggesting the inhibiting effect of RRTS on OS cell malignancy. Epithelial-to-Mesenchymal Transition (EMT) is associated with tumor cell metastasis and invasion[21]. After undergoing EMT, tumor cells experience a decrease in intercellular adhesion and are more prone to metastasis and invasion[22]. Here, RRTS elevated E-cadherin protein levels (epithelial marker) and decreased N-cadherin protein levels (interstitial marker) in Saos-2 cells, indicating that it may hinder OS cell metastasis and invasion by inhibiting EMT.
miRs participate in regulating the malignant behavior of tumor cells, providing potential molecular targets for tumor treatment[23]. A range of reports have demonstrated that multiple miRNAs are downregulated in OS and play an inhibiting function by serving as tumor suppressors, such as miR-557[24], miR-377-3p[25], and miR-877[26]. miR-1305 takes part in cancer cell growth in various tumors[13,27,28]. The downregulation of miR-1305 had been detected in colorectal cancer cells, and its upregulation hindered cancer cell malignant behaviors by targeting Transforming Growth Factor Beta 2 (TGFβ2)/Small Mothers Against Decapentaplegic 3 (SMAD3) pathway[12]. There was a marked reduction in miR-1305 expression in esophageal cancer, and overexpression of miR- 1305 weakened esophageal cancer cell malignant behaviors by directly targeting and inhibiting TXNRD1 expression[27].
Our data showed that miR-1305 was low- expressed in OS samples, and upregulation of miR-1305 had a significant inhibitory effect on Saos-2 cell malignant behaviors, indicating that miR-1305 also plays an anti-tumor role in OS. In order to investigate whether RRTS can affect OS cell malignant behaviors by regulating miR- 1305 expression, we detected miR-1305 levels in Saos-2 cells administrated with RRTS, and RRTS elevated miR-1305 expression in a dose- dependent manner. Moreover, downregulation of miR-1305 reversed the inhibitory effect of RRTS on Saos-2 cell proliferation, metastasis, and invasion, suggesting that RRTS may inhibit OS cell malignant behaviors by upregulating miR- 1305, but the target genes regulated by miR-1305 still need further exploration in the future.
In summary, RRTS exerted an inhibiting effect on Saos-2 cell malignant behaviors, and its mediated mechanism may be related to miR-1305. This study supports the potential value of RRTS in the treatment of OS, but its effect needs to be further verified by nude mouse xenograft experiments in vivo.
Conflict of interests:
The authors declared no conflict of interests.
References
- Pilavaki P, Ardakani GA, Gikas P, Constantinidou A. Osteosarcoma: Current concepts and evolutions in management principles. J Clin Med 2023;12(8):2785.
[Crossref] [Google Scholar] [PubMed]
- Beird HC, Bielack SS, Flanagan AM, Gill J, Heymann D, Janeway KA, et al. Osteosarcoma. Nat Rev Dis Primers 2022;8(1):77.
- Panez-Toro I, Munoz-García J, Vargas-Franco JW, Renodon-Cornière A, Heymann MF, Lezot F, et al. Advances in osteosarcoma. Curr Osteoporos Rep 2023;21(4):330-43.
[Crossref] [Google Scholar] [PubMed]
- Urlic I, Jovicic MS, Ostojić K, Ivkovic A. Cellular and genetic background of osteosarcoma. Curr Issues Mol Biol 2023;45(5):4344-58.
[Crossref] [Google Scholar] [PubMed]
- Wang C, Wang J, Gao M, Gao P, Gao D, Zhang H, et al. Radix Ranunculus ternatus: Review of its chemical constituents, pharmacology, quality control and clinical applications. J Pharm Pharmacol 2022;74(7):930-52.
[Crossref] [Google Scholar] [PubMed]
- Ho PT, Clark IM, Le LT. MicroRNA-based diagnosis and therapy. Int J Mol Sci 2022;23(13):7167.
[Crossref] [Google Scholar] [PubMed]
- Hussen BM, Hidayat HJ, Salihi A, Sabir DK, Taheri M, Ghafouri-Fard S. MicroRNA: A signature for cancer progression. Biomed Pharmacother 2021;138:111528.
[Crossref] [Google Scholar] [PubMed]
- Ram RM, Boro A, Fuchs B. Involvement and clinical aspects of microRNA in osteosarcoma. Int J Mol Sci 2016;17(6):877.
- Su Y, Feng W, Shi J, Chen L, Huang J, Lin T. CircRIP2 accelerates bladder cancer progression via miR-1305/Tgf-β2/smad3 pathway. Mol Cancer 2020;19(1):1-3.
[Crossref] [Google Scholar] [PubMed]
- Gao L, Tang X, He Q, Sun G, Wang C, Qu H. Exosome-transmitted circCOG2 promotes colorectal cancer progression via miR-1305/TGF-β2/SMAD3 pathway. Cell Death Discov 2021;7(1):281.
[Crossref] [Google Scholar] [PubMed]
- He C, Liu L. Hsa_circ_0072008 regulates cell proliferation, migration, and invasion in cervical squamous cell carcinoma via miR-1305/helicase, lymphoid specific (HELLS) axis. Bioengineered 2022;13(4):8311-22.
[Crossref] [Google Scholar] [PubMed]
- Tong YL, Yang F, Dai GH, Ren ZM, Wang BB, Huang T. The effect of RRTS on the growth and expression of EGFR and MMP-9 in nude mice transplanted with human non-small cell lung cancer A549 cells. Chin Arch Tradit Chin Med 2015;24:2-9.
- Sharma P, Tyagi A, Bhansali P, Pareek S, Singh V, Ilyas A, et al. Saponins: Extraction, bio-medicinal properties and way forward to anti-viral representatives. Food Chem Toxicol 2021;150:112075.
[Crossref] [Google Scholar] [PubMed]
- Bachran C, Bachran S, Sutherland M, Bachran D, Fuchs H. Saponins in tumor therapy. Mini Rev Med Chem 2008;8(6):575-84.
- Wang Z, Xu J, Wang Y, Xiang L, He X. Total saponins from Tupistra chinensis Baker inhibits growth of human gastric cancer cells in vitro and in vivo. J Ethnopharmacol 2021;278:114323.
[Crossref] [Google Scholar] [PubMed]
- Chang L, Zhou R, He Y, Meng M, Hu J, Liu Y, et al. Total saponins from Rhizoma Panacis Majoris inhibit proliferation, induce cell cycle arrest and apoptosis and influence MAPK signalling pathways on the colorectal cancer cell. Mol Med Rep 2021;24(2):3009-24.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Wang S, Xu J, Wang Y, Xiang L, He X. Total steroidal saponins from black nightshade (Solanum nigrum L.) overcome tumor multidrug resistance by inducing autophagy‐mediated cell death in vivo and in vitro. Phytotherapy Res 2023;37(7):3009-24.
[Crossref] [Google Scholar] [PubMed]
- Huang X, Zhao Y, Jin X. Structural characterization of a polysaccharide from radix Ranunculus ternatus. Iran J Pharm Res 2014;13(4):1403.
[Google Scholar] [PubMed]
- Niu L, Zhou Y, Sun B, Hu J, Kong L, Duan S. Inhibitory effect of saponins and polysaccharides from radix Ranunculus ternatus on human gastric cancer BGC823 cells. Afr J Tradit Complement Altern Med 2013;10(3):561-6.
[Crossref] [Google Scholar] [PubMed]
- You K, Liu Y, Chen L, Ye H, Lin W. Radix Ranunculus ternatus saponins sensitizes ovarian cancer to Taxol via upregulation of miR‑let‑7b. Exp Ther Med 2022;23(5):315.
[Crossref] [Google Scholar] [PubMed]
- Hinton K, Kirk A, Paul P, Persad S. Regulation of the epithelial to mesenchymal transition in osteosarcoma. Biomolecules 2023;13(2):398.
[Crossref] [Google Scholar] [PubMed]
- Yeung KT, Yang J. Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol 2017;11(1):28-39.
[Crossref] [Google Scholar] [PubMed]
- Hill M, Tran N. miRNA interplay: Mechanisms and consequences in cancer. Dis Model Mech 2021;14(4):047662.
[Crossref] [Google Scholar] [PubMed]
- Qiao Z, Li J, Kou H, Chen X, Bao D, Shang G, et al. Hsa-miR-557 inhibits osteosarcoma growth through targeting KRAS. Front Genet 2022;12:789823.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Chen T, Zhang Y, Zhang N, Li C, Li Y, et al. Long noncoding RNA Linc00339 promotes triple‐negative breast cancer progression through miR‐377‐3p/HOXC6 signaling pathway. J Cell Physiol 2019;234(8):13303-17.
[Crossref] [Google Scholar] [PubMed]
- Chen M, Li Z, Cao L, Fang C, Gao R, Liu C. miR-877-3p inhibits tumor growth and angiogenesis of osteosarcoma through fibroblast growth factor 2 signaling. Bioengineered 2022;13(4):8174-86.
[Crossref] [Google Scholar] [PubMed]
- Li X, Song L, Wang B, Tao C, Shi L, Xu M. Circ0120816 acts as an oncogene of esophageal squamous cell carcinoma by inhibiting miR-1305 and releasing TXNRD1. Cancer Cell Int 2020;20(1):1-20.
[Crossref] [Google Scholar] [PubMed]
- Liu W, Zhuang R, Feng S, Bai X, Jia Z, Kapora E, et al. Long non-coding RNA ASB16-AS1 enhances cell proliferation, migration and invasion via functioning as a ceRNA through miR-1305/Wnt/β-catenin axis in cervical cancer. Biomed Pharmacother 2020;125:109965.
[Crossref] [Google Scholar] [PubMed]