- *Corresponding Author:
- Hong Shen
Department of Gastroenterology, Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine, Na njing, Jiangsu 210004, China
E-mail: shenhong999@163.com
| Date of Received | 26 October 2022 |
| Date of Revision | 08 September 2023 |
| Date of Accepted | 02 May 2024 |
| Indian J Pharm Sci 2024;86(3):1091-1101 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Gastric cancer has become the fifth most common cause of cancer-related death worldwide. However, the treatment of gastric cancer remains a challenge. Recently, caveolin-1 has attracted the attention because of its association with cancer-associated fibroblasts that play essential roles in tumor growth. In this study, we established a co-culture model using fibroblasts and gastric cancer cells to mimic tumor microenvironment to investigate the effect of caveolin-1 on the growth of gastric cancer cells. It was found that down-regulation of caveolin-1 in cancer-associated fibroblasts led to cellular autophagy and increased gastric cancer cell growth. This co-culture model promoted the expression of biomarkers of myofibroblasts transformation, including alpha-smooth muscle actin and vimentin. Moreover, quercetin, a natural polyphenolic flavonoid compound which is often used for the treatment of gastric cancer, showed a strong inhibitory effect on gastric cancer cell growth in the co-culture; quercetin unregulated caveolin-1 leading to inhibit myofibroblasts oncogenic transformation both in vitro and in vivo. Quercetin treatment significantly reduced cancer cell migration and invasion, suppressed tumor size and weight. Taken together, our findings have provided new insights on the effect of tumor microenvironment, particularly cancer-associated fibroblast, on tumor cell growth. It has also illustrated the molecular mechanism of quercetin in the treatment of gastric cancer.
Keywords
Caveolin-1, gastric cancer, cancer-associated fibroblast, autophagy, quercetin
Gastric cancer is the 5th most common cause of cancer-related deaths worldwide. Gastric cancer relies on aerobic glycolysis rather than oxidative phosphorylation, namely “Warburg effect”, to support energy and macromolecule synthesis for its growth[1]. Due to its high tendency of invasion and chemo resistance, regular surgical resection, chemotherapy and radiotherapy are not enough for gastric cancer treatment[2]. Recently, a phytochemical quercetin was reported to have pro-apoptotic, antiproliferative and anti-angiogenic ability in gastric cancer[3], however, the mechanism behind these properties needs to be elucidated.
Tumor microenvironment, including many different types of cells such as fibroblasts, immune cells, endothelial cells and inflammatory cells, plays essential roles in governing tumor proliferation and progression[4]. Fibroblasts in tumor stroma have the potential to develop as Cancer-Associated Fibroblasts (CAFs) and myofibroblasts to promote tumor growth[5]. During the transformation from fibroblasts to myofibroblasts, the expression of some genes, such as Alpha-Smooth Muscle Actin (α-SMA) and vimentin increased and these genes could serve as biomarkers for tumor growth[6], because myofibroblasts secret a myriad of vital factors to facilitate growth of neighboring cells[7]. CAFs oncogenic transformation could be considered as a mediator in cancer progression, which is correlated with poor prognosis in various malignancies including breast, colorectal and gastric cancers[8].
Caveolin-1 (Cav-1) is a major scaffolding protein in caveolae that is the main types of lipid rafts at the plasma membrane[9]. A recent study reported the tumor-inhibitory function of Cav-1 in breast and gastric cancers[10]. Besides, overexpression of Cav- 1 could reduce cell proliferation and tumor size in vivo, and down-regulation of Cav-1 in CAFs by tumor cell may lead to fibroblasts transformation to myofibroblasts[11-13]. Emerging evidence has revealed that co-culture of fibroblasts with cancer cells could decrease Cav-1 and increase growth factors[14]. Furthermore, myofibroblast biomarkers such as α-SMA and vimentin were up regulated when fibroblasts co-culture with cancer cells[15]. Recently, Transforming Growth Factor-Beta (TGF-β1) activated Suppressor of mothers against decapentaplegic (Smad) 2/3 nuclear translocation was shown to be associated with α-SMA and vimentin expression in fibroblasts oncogenic transformation[16]. Elevated autophagy caused by Cav-1 may decline and can be as survival strategy for cancer cell upon nutrition deficiency[17].
Quercetin, a flavonoid existed in many edible and medicinal plants, contains 3,3’,4’,5,7-pentahydroxyavone structure with various pharmacological activities such as antioxidant, anti-inflammatory, anti-microbial and anti-allergic characteristics as well as antitumor property[18]. This phytochemical has been commonly used to treat gastric cancer for its proapoptotic effect[19], however, whose mechanism remains ambiguous.
In this study, we established a co-culture model of gastric cancer cells and fibroblasts. We found that cancer cell proliferation was facilitated with down-regulation of Cav-1 in CAFs. In addition, autophagy was increased and myofibroblasts transformation was up regulated. Quercetin had the ability to inhibit the co-culture induced tumor progression by up regulating Cav-1 and declining autophagy thereby inhibiting CAFs development. Our findings provided new insights on the effect of tumor microenvironment, particularly cancerassociated fibroblast and on tumor cell growth. It also illustrated the molecular mechanism of quercetin in the treatment of gastric cancer.
Materials and Methods
Cell culture:
Human skin fibroblasts immortalized with human Telomerase Reverse Transcriptase (hTERT-BJ1) were bought from Hangzhou Qingda Kerui Biotechnology, Co., Ltd., and were cultured in Dulbecco's Modified Eagle Medium (DMEM) with high glucose, 10 % Fetal Bovine Serum (FBS) and penicillin 100 units/ml-streptomycin 100 μg/ ml (Invitrogen) at 37° in a humidified incubator with 5 % Carbon dioxide (CO2). Gastric cancer cell line, SGC-7901 was originally collected from the Shanghai Silaike Experimental Animal Center and was maintained in Roswell Park Memorial Institute (RPMI) 1640 medium with 10 % FBS at 37° with 5 % CO2 in a humidified atmosphere. After 3 d-5 d, when the cultured cells reached 80 %-90 % confluence they were digested with trypsin for 1:3 passage.
Co-culture of gastric cancer cells and fibroblasts:
Fibroblasts and SGC-7901 cells were co-plated on glass coverslips in 12-well plates to detect the immunofluorescent staining. SGC-7901 cells were plated within 2 h of fibroblast plating, and 1×105 cells were plated per well. The cell ratios.5:1 of fibroblast-to SGC-7901 was applied to the observation of the. Monocultures of fibroblasts or SGC-7901 cells were plated in parallel, co-culture model was seeded with the same number of cells for (3-7) d.
Fibroblasts and SGC-7901 cells were co-cultured using polyester Transwell inserts (0.4 μm pore size, BD Biosciences, United States of America (USA)) to detect the expression of protein. 1×106 fibroblasts cells were plated at the bottom of each well of the companion culture plates and allowed to adhere for a minimum of 2 h without apical Transwell inserts. Subsequent to plating, fibroblasts cells were exposed to SGC-7901 cell conditioned media by placing the SGC-7901 Transwell inserts into the wells previously plated with fibroblasts cells. This method allowed the fibroblasts and SGC-7901 cells to grow in the same medium without direct contact between them. Mono-cultures of fibroblasts were plated in the lower chamber of Transwell inserts. Both mono-cultures and co-cultures were maintained at 37° with 5 % CO2 in a humidified atmosphere.
Antibodies and reagents:
The antibodies namely, Anti-Cav-1 (#3267, CST, USA), anti-cytokeratin 8/18 (K8-18, MA5-32118, Invitrogen, USA), anti-smooth muscle actin (#19245, CST, USA), anti-LC3B II (#3868, CST, USA), anti-beclin1 (ab210498, Abcam, (United Kingdom (UK), anti-calmodulin (ab124742, Abcam, UK), anti-vimentin (ab92547, Abcam, UK), anti-p-Smad2 (#18338, CST, USA) and anti-Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) (#5174, CST, USA) were used. 4', 6-Diamidino-2-Phenylindole (DAPI) was produced by Sangon Biotech of China. Similalrly, TGF-β1 content kit was obtained from Abcam of UK (ab100647) and quercetin was obtained from Aladdin of China (Q111273).
Enzyme-Linked Immunosorbent Assay (ELISA):
The quercetin treated cells (Human fibroblasts siCav 1 cells) and control groups were cultured for 72 h subsequent to treatment. The culture supernatants were collected and stored at 80°. The concentrations of growth factors in the supernatants were determined using a TGF-β1, ELISA kit according to the manufacturer’s protocol. Standards and sample proteins were added and incubated at room temperature for 2 h. Subsequent to the washing stage, the conjugate was added to the wells for 2 h at room temperature. The reaction was stopped with a stop solution. The Optical Density (OD) of each well was measured at 450 nm (MULTISKAN MK3 enzyme standard instrument, Thermo) and values were correlated to the standard curve to determine protein concentration.
Western blotting:
Cells were lysed in lysis buffer (25 mM 4-(2-Hydroxyethyl)-1-Piperazineethanesulfonic (HEPES) acid, 2 mM Magnesium chloride (MgCl2), 2 mM Dithiothreitol (DTT), 1 mM Ethylenediaminetetraacetic Acid (EDTA), 1 mM Protein-Sparing Modified Fast (PSMF) and 5 μg/ml leupeptin at 7.4 pH) on ice for 30 min followed by centrifugation at 12 000 rpm for 15 min at 4°. The tissue samples were frozen in the liquid nitrogen and homogenized 2 min followed by lysis on ice. The protein concentration in supernatants was determined by Bicinchoninic Acid (BCA) assay and 10-20 μg protein extracts were separated by 12 % Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) followed by electrophoretically transferring to Polyvinylidene Fluoride (PVDF) membranes. The membranes were blocked with 5 % skim milk for 2 h at room temperature. After being washed three times with Tris-Buffered Saline with 0.1 % Tween® 20 detergent (TBST), membranes were incubated with corresponding primary antibody at 4° overnight. The same washing process was repeated and membranes were incubated with corresponding secondary antibody for 2 h. The detection of target molecule band was performed by enhanced chemiluminescence. These bands were quantified to measure relative protein expression level using quantity one software (Bio-Rad, USA).
Immunofluorescence:
After (3-7) d of culture, cells were fixed for 30 min in 2 % paraformaldehyde at room temperature followed by permeabilization with 0.5 % TritonX-100 for 20 min. Then, primary antibodies were incubated for 1 h at room temperature. After being washed three times with Phosphate Buffered Saline (PBS), cells were incubated with fluorochrome-conjugated secondary antibodies for 30 min at room temperature. After the washing process, samples were incubated with DAPI for 15 min followed by washing and adding anti-fade reagent. Images were collected with a C2+ Nikon confocal microscope.
Immunohistochemistry:
Protein expression of Cav-1, calmodulin, α-SMA, vimentin, LC3B and beclin1 in gastric cancer tissue was measured by immunohistochemistry. Briefly, the samples were blocked by 3 % hydrogen peroxide, 10 % normal goat serum for 10 min, and were incubated with corresponding antibodies at 4° overnight. After incubation with biotin-conjugated secondary antibody (PV6000, ZSGB-BIO, China), the slides were incubated with streptavidin-biotin horseradish peroxidase, which was then followed by incubating with diaminobenzidine (DAB, ZSGB-BIO, China). Further, counterstaining with hematoxylin was performed and light microscope (CKX41SF, OLYMPUS) was used to view the images of stained samples in a single-blinded manner.
Transmission Electron Microscopy (TEM):
After corresponding treatment, cells were fixed with 2.5 % glutaraldehyde in phosphate buffer (pH 7.0) overnight at 4° followed by washing and post-fixation with 1 % Osmium tetroxide (OsO4) in phosphate buffer (pH 7.0) for (1-2) h. Then, the cells were washed again and dehydrated by a gradient series of ethanol (30 %, 50 %, 70 %, 80 %, 90 % and 95 %) for approximately 15 min at each step. After being transferred to absolute ethanol for 20 min, the specimen was placed in 1:1 mixture of absolute acetone for 1 h at room temperature which was then, it was transferred to 1:3 absolute acetone for 3 h followed by transferring to absolute acetone for >12 h. After being heated at 70° overnight, the specimen was placed into Reichert microtome to be sliced into sections from 70-90 nm and stained with uranyl acetate and alkaline lead citrate for 15 min. Finally, the samples were observed under the TEM of HitachiH-7650.
Cav-1 knockdown:
Small interfering Ribonucleic Acid (siRNA)- mediated Cav-1 knockdown had been performed by the HiPerFect transfection reagent (Qiagen, Germany) before the manufacturer’s instructions were carried out. After 105 cells were seeded into 12 well plates. Transfection were carried out by mixing 100 ng of Cav-1 siRNA (#SI00299635, Qiagen) or 100 ng control siRNA (#1022076, Qiagen) with 100 μl serum free media and 6 μl transfection reagent. After vortexed for 30 s and incubated for 15 min, mixture was directly added into cells. 3 h post-transfection, 800 μl complete media was added to the cells, following treatment with concentrations of quercetin at 80 μM for 72 h at 37°.
RNA extraction and quantitative real-time Polymerase Chain Reaction (PCR):
RNA was extracted from corresponding gastric cancer tissue using Total RNA Isolation reagent (TRIzol) (Life Technologies, Thermo Fisher Scientific, USA). Total RNA was reversely transcribed by complementary Deoxyribonucleic Acid (cDNA) synthesis using PrimeScript RT reagent kit (Takara, Japan) at 37° for 30 min followed by 85° for 5 s. qPCR was performed by 7900 thermal cycler (ABI, Thermo Fisher Scientific, USA) with GoTaq Green Master Mix (Promega, USA) at initial denaturation at 95° for 30 s, 40 cycles of denaturation at 95° for 5 s, annealing for 30 s at 60° and extension for 15 s at 72°. The primers used were s: Cav-1, 5’-GCGACCCTAAACACCTCAAC-3’ (forward) and 5’-ATGCCGTCAAAACTGTGTGTC-3’ (reverse); β-actin, 5’-GTCTGCCTTGGTAGTGGATAATG-3’ (forward) and 5’-TCGAGGACGCCCTATCATGG-3’ (reverse). The cycle threshold values were obtained in each sample. Relative levels of messenger RNA (mRNA) were measured by 2-ΔΔCt method[10]. β-actin was used as an internal gene for normalization.
Wound healing assay:
SGC-7901cells were seeded in six-well Transwell plates at 2×105 cells per well at the bottom to grow into monolayer. Fibroblasts cells were exposed to SGC-7901 cell conditioned media by placing the Fibroblasts Transwell inserts into the wells previously plated with SGC-7901 cells. A linear scratch/wound was created on cell monolayers with a sterile pipette tip. Photomicrographs were taken on live cells at 100X magnification and the distance of cell migration was recorded within appropriate times.
Cell invasion assay:
The invasion ability of gastric cancer cells in vitro was evaluated by Matrigel-coated Transwell (BD Biosciences, USA). 5×104 cells in 500 μl serumfree medium were added to the upper chamber, and medium containing 20 % FBS was added to the lower chamber. The cells were left to invade the Matrigel for a certain period of time, and the non-invading cells on the upper surface of the membrane were removed by wiping. Invading cells were fixed and stained with a three-step stain kit (Richard-Allan Scientific, USA). The population of invaded cells was counted under a microscope in five predetermined fields for each membrane at 400X magnification.
Animals:
Ten male nude mice (6 w old) were obtained from vital river laboratory animal technology Co., Ltd., (Beijing), with production license no. SCXK (Beijing) 2012-0001 and certificate no.11400700185355. All animal experiments in this study were approved by the Jiang Su Center for safety evaluation of drug (Approval No: LL- 20160915-01) in accordance to the ‘Regulations for the administration of affairs concerning experimental animals of Jiangsu Province’. The animals were maintained prior to experiments in an animal room under standard conditions (23°±2° temperature, 60 %±10 % humidity, 12 h:12 h lightdark cycle). All efforts were made to minimize the suffering of animals.
Tumor xenografts:
Mixed gastric cancer cells and fibroblasts were resuspended in 200 μl and injected into the dorsal part of right forelimb of nude mice. Tumors were allowed to grow to 80-120 mm3 in size, Quercetin (50 mg/kg/d) or normal saline was used for treatment. Tumor growth was monitored for 14 d after treatment. The mice were sacrificed and tumors were dissected, and the weight and size of tumors were determined by calipers. Tumor volume was calculated using the formula (X2Y)/2, where X and Y are the short and long tumor dimensions, respectively.
Statistical analysis:
All data and images were obtained from three independent experiments. Data were presented as mean±Standard Deviation (SD). Statistical analysis was performed by Statistical Package for the Social Sciences (SPSS) 13.0 and was conducted by oneway Analysis of Variance (ANOVA) followed by a Dunnett’s or Tukey’s post-hoc test where *p<0.05 and **p<0.01 was considered to be statistically significant difference.
Results and Discussion
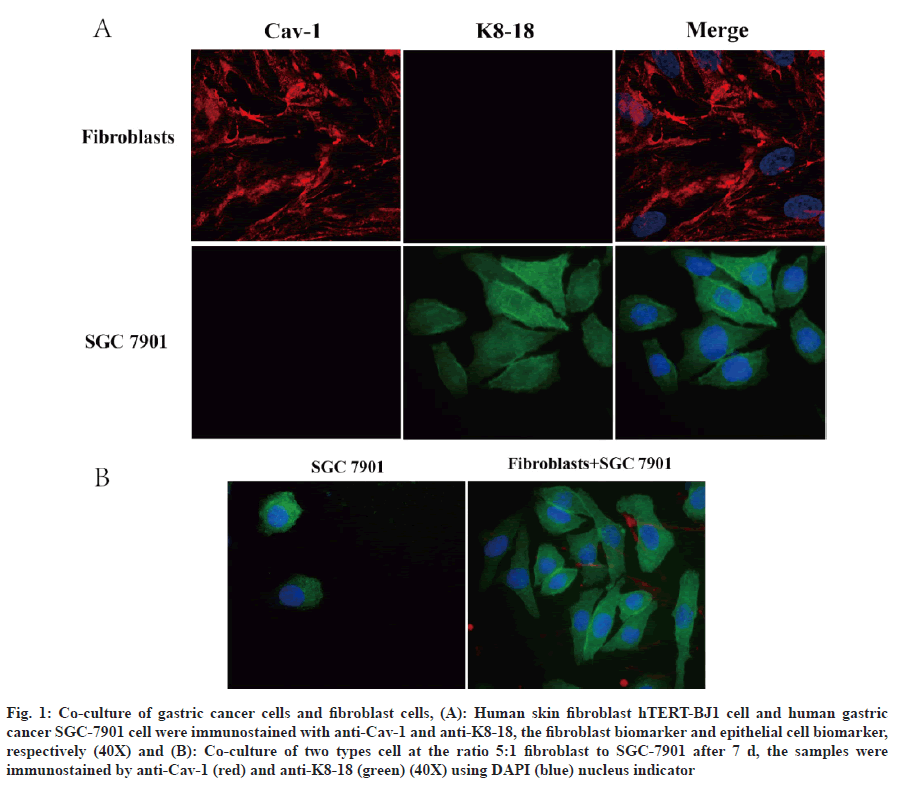
Fibroblasts are the main stromal cells in cancers and are essential for tumorigenesis, tumor development and metastasis which release protective factors for promoting tumor progression[5]. In order to understand the effect of fibroblasts on the growth of gastric cancer, the human gastric cancer cell lines, SGC-7901 and human skin fibroblast cell hTERT-BJ1 were applied to establish a co-culture model for mimicking tumor microenvironment. Firstly, for distinguishing two cell types by immunofluorescence, we used cell type specificity biomarker cytokeratin 8/18 (K8-18) for gastric cancer cell and Cav-1 for fibroblast cells (fig. 1A). It was found that K8-18 clearly labeled the gastric cancer cells[20,21]. Compared to SGC-7901 in monoculture, fibroblast cell:gastric cancer cell at 5:1 ratio, gastric cancer cell retained a high survival rate after 7 d. Further, the co-culture improved the gastric cancer cell morphology as irregular rod-like shape forming net/nest area (fig. 1B).
Fig. 1: Co-culture of gastric cancer cells and fibroblast cells, (A): Human skin fibroblast hTERT-BJ1 cell and human gastric cancer SGC-7901 cell were immunostained with anti-Cav-1 and anti-K8-18, the fibroblast biomarker and epithelial cell biomarker, respectively (40X) and (B): Co-culture of two types cell at the ratio 5:1 fibroblast to SGC-7901 after 7 d, the samples were immunostained by anti-Cav-1 (red) and anti-K8-18 (green) (40X) using DAPI (blue) nucleus indicator
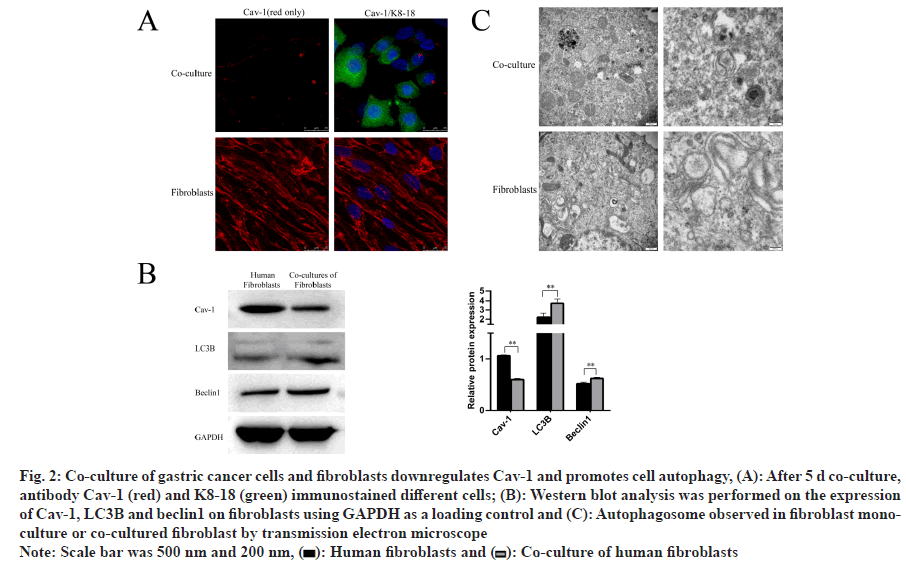
Recent study has reported that down-regulation of Cav-1 in CAFs Cav-1 down regulation might indicate fibroblast to myofibroblast[9]. We found by our team that the expression of Cav-1 decreased by immunofluorescence after 5 d of co-culture compared to fibroblast cell monoculture (fig. 2A). Previous study demonstrated that Cav-1 responded to stress through regulating autophagy and lysosome in breast cancer[11]. Atg8/LC3 is the most widely monitored autophagy-related protein and Beclin1 is an essential partner in the autophagy interactome that signals the inception of autophagy, and autophagy is induced by the release of Beclin1 from B cell lymphoma-2 (Bcl-2). It was also found that Cav-1 decline was accompanied with increased autophagy by Western blotting analysis (fig. 2B). Electron microscopy confirmed this result by showing the autophagosome formation (fig. 2C). This phenomenon has indicated that fibroblast is able to promote gastric cancer growth by downregulating Cav-1 in CAFs, leading to induction of autophagy.
Fig. 2: Co-culture of gastric cancer cells and fibroblasts downregulates Cav-1 and promotes cell autophagy, (A): After 5 d co-culture, antibody Cav-1 (red) and K8-18 (green) immunostained different cells; (B): Western blot analysis was performed on the expression of Cav-1, LC3II and beclin1 on fibroblasts using GAPDH as a loading control and (C): Autophagosome observed in fibroblast monoculture or co-cultured fibroblast by transmission electron microscope
Note: Scale bar was 500 nm and 200 nm, ( ): Human fibroblasts and (
): Human fibroblasts and ( ): Co-culture of human fibroblasts
): Co-culture of human fibroblasts
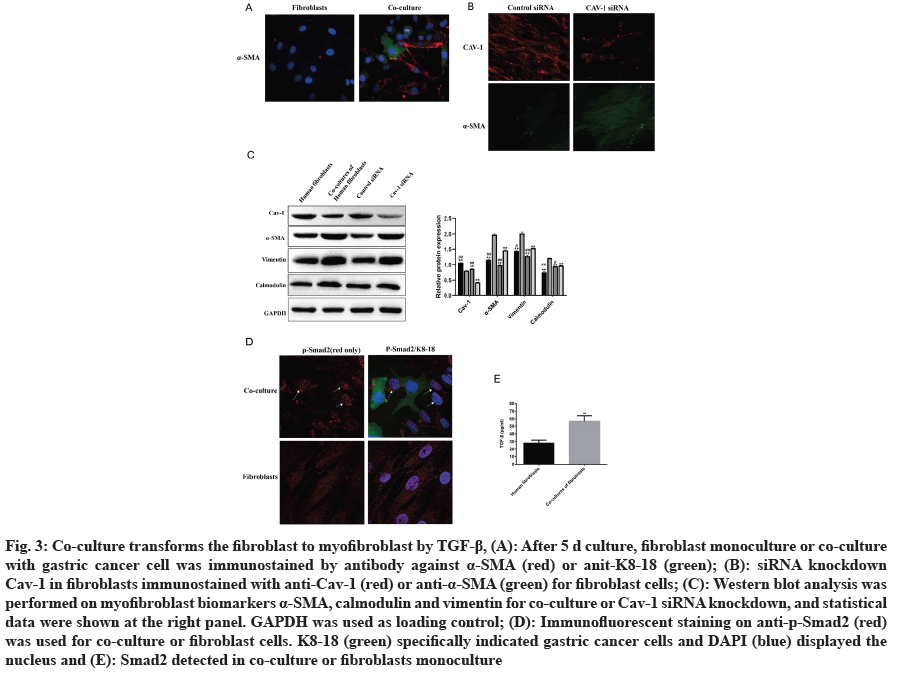
CAFs, including fibroblasts and myofibroblasts, is crucial component within tumor stroma. The expression of α-SMA and vimentin in myofibroblasts increased during tumor progression[7]. The expression of α-SMA expression increased after 5 d in co-culture model compared to fibroblast monoculture (fig. 3A). Knocking down of Cav-1 by siRNA in fibroblast cells also increased the expression of α-SMA (fig. 3B). Analysis indicated that down-regulation of Cav-1 in CAFs promoted fibroblast transformation into myofibroblast (fig. 3C). Several studies have reported that the TGF-β1 is vital for tumor progression by indirectly phosphorylating Smad2 to translocate into nucleus as transcriptional factors[22,23]. p-Smad2 was observed to translocate the nucleus in co-culture model by immunofluorescence (fig. 3D), which was associated with TGF-β1 upregulation (fig. 3E).
Fig. 3: Co-culture transforms the fibroblast to myofibroblast by TGF-β1, (A): After 5 d culture, fibroblast monoculture or co-culture with gastric cancer cell was immunostained by antibody against α-SMA (red) or anit-K8-18 (green); (B): siRNA knockdown Cav-1 in fibroblasts immunostained with anti-Cav-1 (red) or anti-α-SMA (green) for fibroblast cells; (C): Western blot analysis was performed on myofibroblast biomarkers α-SMA, calmodulin and vimentin for co-culture or Cav-1 siRNA knockdown, and statistical data were shown at the right panel. GAPDH was used as loading control; (D): Immunofluorescent staining on anti-p-Smad2 (red) was used for co-culture or fibroblast cells. K8-18 (green) specifically indicated gastric cancer cells and DAPI (blue) displayed the nucleus and (E): Smad2 detected in co-culture or fibroblasts monoculture
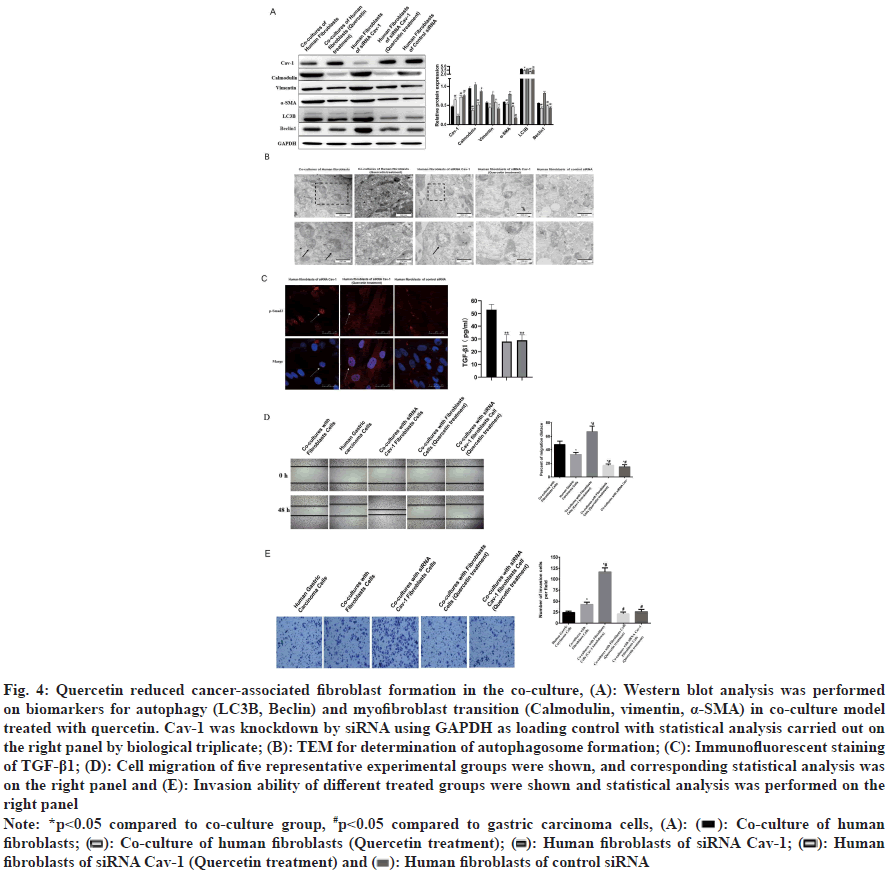
Quercetin, commonly used for treatment of gastric cancer, was a flavonoid with 3, 3’, 4’, 5, 7-pentahydroxyavone structure found in various edible and medicinal plants[20]. Previous study demonstrated that quercetin could protect gastric epithelial cells against oxidative damage by its antioxidant activity as a Reactive Oxygen Species (ROS) scavenger and metal chelator[24], and had pro-apoptotic effect on gastric cancer cell line[19]. Quercetin was used in this study to explore its potential pharmacological mechanism on gastric cancer cells. Western blot analysis showed that quercetin could up regulate Cav-1 protein expression and down regulate myofibroblast transformation and autophagy in the co-culture model (fig. 4A). Electron microscopy confirmed the reduction of autophagosome by quercetin treatment (fig. 4B). Quercetin also reduced TGF-β1 and p-Smad2 translocation into nucleus (fig. 4C). Compared to monoculture of gastric cancer cells, fibroblasts co-culture is capable of increase the migrated and invaded cells and the Cav-1 knockdown could apparently enhance this cell migrated and invaded ability. However, the application of quercetin is able to compromising these results caused by coculture or Cav-1 knockdown (fig. 4D and fig. 4E). Gastric cancer cell migration and invasion were decreased by quercetin treatment (fig. 4D and fig. 4E).
Fig. 4: Quercetin reduced cancer-associated fibroblast formation in the co-culture, (A): Western blot analysis was performed on biomarkers for autophagy (LC3II, Beclin) and myofibroblast transition (Calmodulin, vimentin, α-SMA) in co-culture model treated with quercetin. Cav-1 was knockdown by siRNA using GAPDH as loading control with statistical analysis carried out on the right panel by biological triplicate; (B): TEM for determination of autophagosome formation; (C): Immunofluorescent staining of TGF-β1; (D): Cell migration of five representative experimental groups were shown, and corresponding statistical analysis was on the right panel and (E): Invasion ability of different treated groups were shown and statistical analysis was performed on the right panel
Note: *p<0.05 compared to co-culture group, #p<0.05 compared to gastric carcinoma cells, (A): ( ): Co-culture of human fibroblasts; (
): Co-culture of human fibroblasts; ( ): Co-culture of human fibroblasts (Quercetin treatment); (
): Co-culture of human fibroblasts (Quercetin treatment); ( ): Human fibroblasts of siRNA Cav-1; (
): Human fibroblasts of siRNA Cav-1; ( ): Human fibroblasts of siRNA Cav-1 (Quercetin treatment) and (
): Human fibroblasts of siRNA Cav-1 (Quercetin treatment) and ( ): Human fibroblasts of control siRNA
): Human fibroblasts of control siRNA
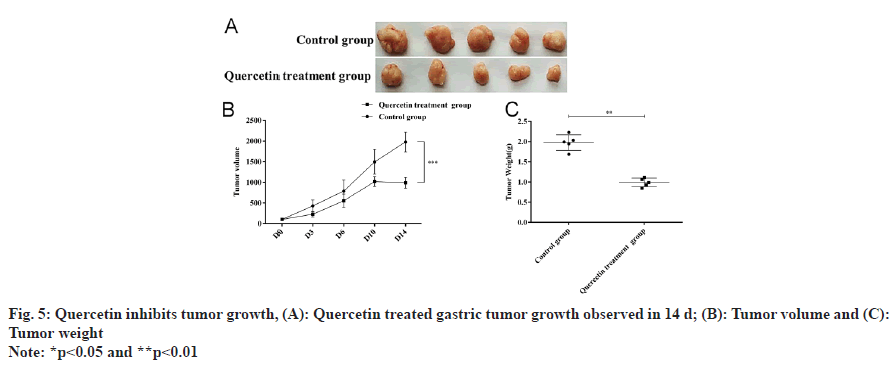
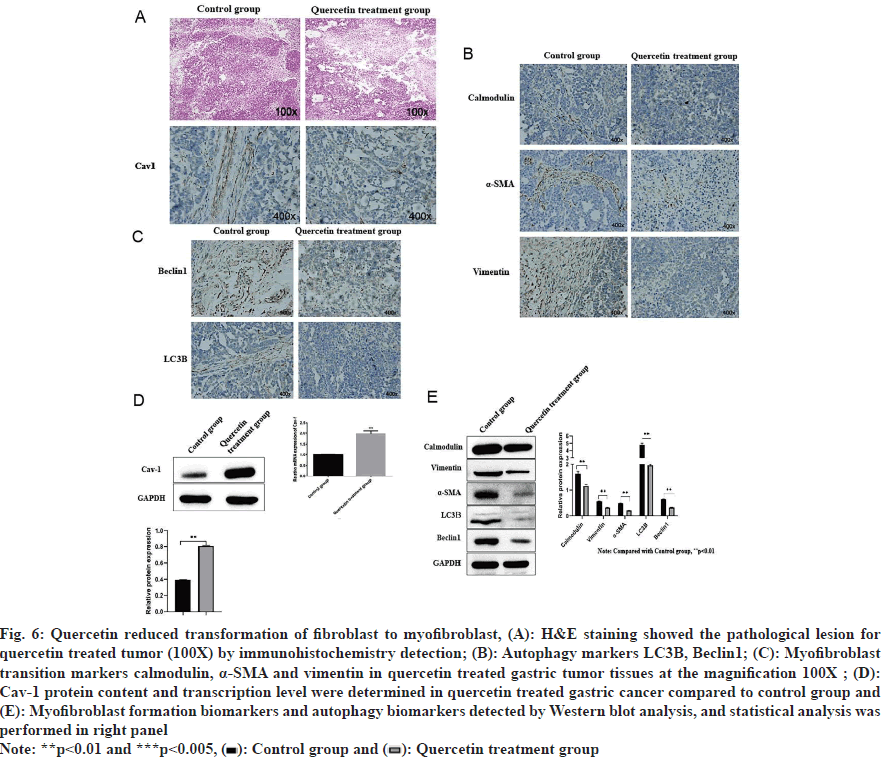
Quercetin treated tumor size reduced after 14 d of treatment (fig. 5A and fig. 5B), and the corresponding tumor weight was also significantly decreased (fig. 5C). Further analysis of tumor tissues by Hematoxylin and Eosin (H&E) staining and immunohistochemical staining showed that histopathology of gastric cancer improved (fig. 6A), and the expression of Cav-1 and calmodulin increased while α-SMA, vimentin, beclin1 and LC3II decreased (fig. 6B and fig. 6C). Corresponding Western blot analysis was consistent with the immunohistochemistry results showing increased Cav-1 expression (fig. 6D) and calmodulin while decreased vimentin, α-SMA, beclin1 and LC3II (fig. 6E) upon quercetin treatment.
Fig. 6: Quercetin reduced transformation of fibroblast to myofibroblast, (A): H&E staining showed the pathological lesion for quercetin treated tumor (100X) by immunohistochemistry detection; (B): Autophagy markers LC3II, Beclin1; (C): Myofibroblast transition markers calmodulin, α-SMA and vimentin in quercetin treated gastric tumor tissues at the magnification 100X ; (D): Cav-1 protein content and transcription level were determined in quercetin treated gastric cancer compared to control group and (E): Myofibroblast formation biomarkers and autophagy biomarkers detected by Western blot analysis, and statistical analysis was performed in right panel
Note: **p<0.01 and ***p<0.005, ( ): Control group and (
): Control group and ( ): Quercetin treatment group
): Quercetin treatment group
Down-regulation of Cav-1 in CAFs in tumor stromal fibroblasts influences the occurrence and progression of tumors. α-SMA, vimentin and calmodulin are the biomarkers of myofibroblast which would be up regulated by TGF-β1 signal pathway. LC3II and Beclin1 are used for detecting autophagy which is associated with downregulation of Cav-1 in CAFs. Fibroblasts in the tumor stroma, known as CAFs, including both fibroblasts and myofibroblasts, is critical for tumor growth because myofibroblasts produce number of important factors that can directly promote growth in the adjacent epithelium. Gastric cancer cells proliferation with fibrosis when the cancer cells invade into the submucosa containing abundant stromal cells. In order to maximally create and imitate the tumor microenvironment in vitro, a co-culture model of human skin immortalized fibroblasts hTERT-BJ1 and gastric cancer cell SGC-7901 was established to evaluate the molecular mechanisms of quercetin for gastric cancer treatment. It was found that this co-culture promoted gastric cancer cell proliferation by downregulating Cav-1 and up regulating myofibroblasts transformation and autophagy. It was also showed that quercetin had the ability to compromise the co-culture and induced those consequences both in vitro and in vivo.
The specific biomarkers for fibroblasts and epithelial cells were used to test the characteristics of each cell line. The research conducted by us revealed that the co-culture facilitated the growth of gastric cancer cells, which is in consistent with previous findings that fibroblasts are capable of promoting MCF breast cancer cell survival[15]. Furthermore, the expression of Cav-1 was reduced when fibroblasts co-cultured with gastric cancer cells. For further exploring the detailed molecular and cellular dynamics in the co-culture, Western blot analysis and electron microscopy were used to detect cell autophagy and related molecules, because relevant report demonstrated that Cav- 1 deficiency could induce autophagy thereby promoting cell survival under starvation[11]. The co-culture decreased expression of Cav-1 and induce autophagy for facilitating cell survival. Lipid raft disruption induced autophagosome formation and lysosome function might be related to the increasing autophagy[11].
Myofibroblasts are spindle-shaped cells and frequently express “mesenchymal” biomarkers, such as α-SMA and vimentin. Besides, myofibroblasts had properties of fibroblasts and smooth muscle cells, and were highly contractile and motile. Especially, fibroblasts in tumor stroma, namely CAFs, includes fibroblasts and myofibroblasts. Importantly, myofibroblasts are particularly associated with scirrhous-type gastric cancer and poor prognosis[25]. We found that co-culture model triggered α-SMA expression which is associated with down-regulation of Cav-1 in CAFs Cav-1 down regulation. The myofibroblasts transformation in this study in consistent with previous study in breast cancer-fibroblast co-culture[15]. Although the comprehensive mechanism of myofibroblasts transformation remains unclear, TGF-β1 and activated phosphorylated Smad2/3 play essential roles in development of the myofibroblastic phenotype from a myriad of precursor cells, including fibroblasts[26]. Immunofluorescence analysis demonstrated that co-culture could stimulate the Smad2 translocation into the nucleus which may serve as transcriptional factors. TGF-β1 content was measured and elevated in co-culture model. Therefore, there is possibility that TGF-β1 activated TGF-β1 receptor to phosphorylate Smad2 thereby translocating to nucleus to induce α-SMA and vimentin expression for myofibroblasts transformation.
Quercetin was used to treat co-culture model and it was found that Cav-1 was up regulated and autophagy biomarkers including calmodulinm, vimentin, α-SMA, LC3B and Beclin1 were downregulated. Furthermore, the cell migration and invasion were also inhibited by quercetin treatment. Quercetin had multiple properties for suppressing gastric cancer, such as antioxidant, anti-inflammatory, anti-allergic or pro-apoptotic effect[18]. Quercetin might up regulate Cav- 1 to compromise myofibroblast oncogenic transformation, autophagy, cell migration and invasion. This finding provided new insights in the functional mechanism of quercetin for the treatment of gastric cancer. In vivo experiment showed that quercetin treatment suppressed tumor growth. The immunohistochemistry and Western blot analysis both indicated that quercetin treated group has a less fibrosis and autophagy which is consistent with in vitro co-culture results.
In conclusion, quercetin up regulates Cav-1 and down regulates fibrosis and autophagy to inhibit cancer-associated fibroblast thereby suppressing gastric cancer growth. This study provides the potential molecular mechanism of using quercetin for cancer treatment.
Acknowledgments:
The research was supported by the National Natural Science Foundation of China (81703943) and Open Project of National Clinical Research Base for Traditional Chinese Medicine(JD2022SZ04).
Author's contributions:
Jing Xing and Yajun Liu performed experiments, collected data and wrote the first draft. Wenqiang Zhu and Lu Zhang performed experiments and collect data. Yi Xu supervised the study. Luzhou Xu and Hong Shen conceived the study and edited the manuscript.
Conflict of interests:
The authors declared no conflict of interests.
References
- Tao L, Yu H, Liang R, Jia R, Wang J, Jiang K, et al. Rev-erbα inhibits proliferation by reducing glycolytic flux and pentose phosphate pathway in human gastric cancer cells. Oncogenesis 2019;8(10):57.
[Crossref] [Google Scholar] [PubMed]
- Zhang W, Ding X, Cheng H, Yin C, Yan J, Mou Z, et al. Dual-targeted gold nanoprism for recognition of early apoptosis, dual-model imaging and precise cancer photothermal therapy. Theranostics 2019;9(19):5610-25.
[Crossref] [Google Scholar] [PubMed]
- Dajas F. Life or death: Neuroprotective and anticancer effects of quercetin. J Ethnopharmacol 2012;143(2):383-96.
[Crossref] [Google Scholar] [PubMed]
- Aizawa T, Karasawa H, Funayama R, Shirota M, Suzuki T, Maeda S, et al. Cancer?associated fibroblasts secrete Wnt2 to promote cancer progression in colorectal cancer. Cancer Med 2019;8(14):6370-82.
[Crossref] [Google Scholar] [PubMed]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 2006;6(5):392-401.
[Crossref] [Google Scholar] [PubMed]
- Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Cunha GR, Hein P, et al. Carcinoma-associated fibroblasts stimulate tumor progression of initiated human epithelium. Breast Cancer Res 2000.
[Crossref] [Google Scholar] [PubMed]
- Brenmoehl J, Miller SN, Hofmann C, Vogl D, Falk W, Scholmerich J, et al. Transforming growth factor-β1 induces intestinal myofibroblast differentiation and modulates their migration. World J Gastroenterol 2009;15(12):1431-42.
[Crossref] [Google Scholar] [PubMed]
- Otsuji E, Kuriu Y, Okamoto K, Ochiai T, Ichikawa D, Hagiwara A, et al. Outcome of surgical treatment for patients with scirrhous carcinoma of the stomach. Am J Surg 2004;188(3):327-32.
[Crossref] [Google Scholar] [PubMed]
- Nassar ZD, Hill MM, Parton RG, Parat MO. Caveola-forming proteins caveolin-1 and PTRF in prostate cancer. Nat Rev Urol 2013;10(9):529-36.
[Crossref] [Google Scholar] [PubMed]
- Sloan EK, Ciocca DR, Pouliot N, Natoli A, Restall C, Henderson MA, et al. Stromal cell expression of caveolin-1 predicts outcome in breast cancer. Am J Pathol 2009;174(6):2035-43.
[Crossref] [Google Scholar] [PubMed]
- Shi Y, Tan SH, Ng S, Zhou J, Yang ND, Koo GB, et al. Critical role of CAV1/caveolin-1 in cell stress responses in human breast cancer cells via modulation of lysosomal function and autophagy. Autophagy 2015;11(5):769-84.
[Crossref] [Google Scholar] [PubMed]
- Yamao T, Yamashita YI, Yamamura K, Nakao Y, Tsukamoto M, Nakagawa S, et al. Cellular senescence, represented by expression of caveolin-1, in cancer-associated fibroblasts promotes tumor invasion in pancreatic cancer. Ann Surg Oncol 2019;26:1552-9. [Crossref]
[Google Scholar] [PubMed]
- Lawrence JC, Saslowsky DE, Edwardson JM, Henderson RM. Real-time analysis of the effects of cholesterol on lipid raft behavior using atomic force microscopy. Biophys J 2003;84(3):1827-32.
[Crossref] [Google Scholar] [PubMed]
- Shi XY, Xiong LX, Xiao L, Meng C, Qi GY, Li WL. Downregulation of caveolin?1 upregulates the expression of growth factors and regulators in co?culture of fibroblasts with cancer cells. Mol Med Rep 2016;13(1):744-52.
[Crossref] [Google Scholar] [PubMed]
- Martinez-Outschoorn UE, Pavlides S, Whitaker-Menezes D, Daumer KM, Milliman JN, Chiavarina B, et al. Tumor cells induce the cancer associated fibroblast phenotype via caveolin-1 degradation: Implications for breast cancer and DCIS therapy with autophagy inhibitors. Cell Cycle 2010;9(12):2423-33.
[Crossref] [Google Scholar] [PubMed]
- Guido C, Whitaker-Menezes D, Capparelli C, Balliet R, Lin Z, Pestell RG, et al. Metabolic reprogramming of cancer-associated fibroblasts by TGF-β1 drives tumor growth: connecting TGF-β1 signaling with “Warburg-like” cancer metabolism and L-lactate production. Cell Cycle 2012;11(16):3019-35.
[Crossref] [Google Scholar] [PubMed]
- Martinez-Outschoorn UE, Whitaker-Menezes D, Lin Z, Flomenberg N, Howell A, Pestell RG, et al. Cytokine production and inflammation drive autophagy in the tumor microenvironment: Role of stromal caveolin-1 as a key regulator. Cell Cycle 2011;10(11):1784-93.
[Crossref] [Google Scholar] [PubMed]
- Haghi A, Azimi H, Rahimi R. A comprehensive review on pharmacotherapeutics of three phytochemicals, curcumin, quercetin, and allicin, in the treatment of gastric cancer. J Gastrointest Cancer 2017;48:314-20.
[Crossref] [Google Scholar] [PubMed]
- Borska S, Chmielewska M, Wysocka T, Drag-Zalesinska M, Zabel M, Dziegiel P. In vitro effect of quercetin on human gastric carcinoma: Targeting cancer cells death and MDR. Food Chem Toxicol 2012;50(9):3375-83.
[Crossref] [Google Scholar] [PubMed]
- Zhang L, Kong Y, Wu D, Zhang H, Wu J, Chen J, et al. Three flavonoids targeting the β?hydroxyacyl?acyl carrier protein dehydratase from Helicobacter pylori: Crystal structure characterization with enzymatic inhibition assay. Protein Sci 2008;17(11):1971-8.
[Crossref] [Google Scholar] [PubMed]
- Karantza V. Keratins in health and cancer: More than mere epithelial cell markers. Oncogene 2011;30(2):127-38.
[Crossref] [Google Scholar] [PubMed]
- Bilici A. Cytokeratin 18 for chemotherapy efficacy in gastric cancer. Transl Gastrointestinal Cancer 2015;4:200-6.
- Guido C, Whitaker-Menezes D, Capparelli C, Balliet R, Lin Z, Pestell RG, et al. Metabolic reprogramming of cancer-associated fibroblasts by TGF-β1 drives tumor growth: Connecting TGF-β1 signaling with “Warburg-like” cancer metabolism and L-lactate production. Cell Cycle 2012;11(16):3019-35.
[Crossref] [Google Scholar] [PubMed]
- Bishayee K, Ghosh S, Mukherjee A, Sadhukhan R, Mondal J, Khuda?Bukhsh AR. Quercetin induces cytochrome?c release and ROS accumulation to promote apoptosis and arrest the cell cycle in G2/M, in cervical carcinoma: Signal cascade and drug?DNA interaction. Cell Prolif 2013;46(2):153-63.
[Crossref] [Google Scholar] [PubMed]
- Kinugasa S, Abe SI, Tachibana M, Hishikawa Y, Yoshimura H, Monden N, et al. Overexpression of transforming growth factor-β1 in scirrhous carcinoma of the stomach correlates with decreased survival. Oncology 1998;55(6):582-7.
[Crossref] [Google Scholar] [PubMed]
- Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res 2010;70(23):9621-30.
[Crossref] [Google Scholar] [PubMed]