- *Corresponding Author:
- Yachana Mishra
School of Bioengineering and Biosciences, Lovely Professional University, Phagwara, Punjab 144411, India
E-mail: yachanamishra@gmail.com
| Date of Received | 22 December 2023 |
| Date of Revision | 26 June 2024 |
| Date of Acceptance | 22 October 2024 |
| Indian J Pharm Sci 2024;86(5):1564-1574 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Cancer is among the main reasons of mortality worldwide. Early diagnosis offers the best chance of recovery for cancer patients. Scientists are still unable to successfully treat cancer spreading to various vital organs, despite the considerable strides in understanding the complex mechanisms leading to cancer formation and metastasis in recent years. The processes of tumor invasion, carcinogenesis and metastasis are still unclear because of the complexity of cancer cells and the microenvironment of tumors. Therefore, to understand the intricate molecular information underpinning the biological behaviours of tumors, it is imperative to develop a novel technology for cancer diagnosis and real-time observation right away. Quantum dots are luminescent semiconductor nanocrystals with a nanometer size range. Due to their unusual optical properties, including excellent brightness, simultaneous detection of many signals, long-term stability and adjustable emission spectra, they appeal as potential diagnostic and therapeutic systems in cancer. Due to their high quantum yields, low toxicity, biocompatibility and flexibility for various surface modifications, quantum dots are currently promising for bioanalytical investigations. The capacity of quantum dots for multiplexed sensing with various emission wavelengths to simultaneously detect a variety of biomarkers of illness is intriguing.
Keywords
Quantum dots, cancer, nanocrystals, therapeutic system, drug delivery, diagnosis
Cancer is one of the leading causes of death worldwide[1]. According to estimates, 9.6 million fatalities in 2018 (or 1 in every 6 deaths) were attributed to cancer. World Health Organization (WHO) stated that around 70 % of cancer-related fatalities occurred in Low- to Middle-Income Nations (LMINs). Researchers have predicted that by 2030, there will be 16-18 million more instances of cancer annually, 60 % of which will occur in emerging nations. According to the WHO, only 12 nations are anticipated to achieve a reduction of one-third in early cancer death by 2030[2]. More money must be spent on treating non-communicable illnesses like cancer if sustainable development goals are to be met[3].
The increasing prevalence of cancer puts enormous physical, emotional and financial strains on people, families and ultimately, the world's health systems. Many cancer patients globally cannot obtain a timely diagnosis and treatment because health agencies in LMINs are the least prepared to handle this load. Only one out of every five LMINs possesses the data required informing the cancer treatment and mitigation strategy, although the global cost of treatment for cancer in 2010 was predicted to be 1.16 trillion USD. According to reports, between 30 % and 50 % of all cancer cases are preventable and may be treated with a long-term, cost-effective plan. The prognosis-quality therapy and survivorship care are increasing the survival rates of cancer patients in many different nations[4]. Due to the intricacy of cancer cells and the Tumor Microenvironment (TME), the processes of carcinogenesis, cancer invasion and metastasis are yet unknown. Therefore, it is essential to create a unique method for cancer diagnosis and real-time monitoring immediately to comprehend the complex molecular data underlying the biological behaviors of tumors[5-7].
Engineered fluorescent Quantum Dots (QDs) show distinct optical as well as chemical characteristics and have demonstrated considerable promise as prospective platforms for biological applications[8]. The clinical utility of QD-based nanotechnology in cancer diagnosis and detection including the principal difficulties in adapting QD-based detection techniques for clinical applications and encouraging future approaches are discussed in this review.
Worldwide Cancer Statistics
Because of the aging and expanding global population, as well as the rising prevalence of cancer-causing activities, the global cancer burden is still rising[9,10]. As per the World Cancer Report 2014, the global cancer burden increased to approximately 4 million new cases per year, with a projected increase to 22 million cases annually during the following 20 y. Cancer deaths are expected to increase from 8.2 million to 13 million annually throughout this time frame. Despite recent advancements in early diagnosis and surgery-centered multidisciplinary treatments, the clinical outcome is still far from satisfactory. This is largely because of the complex cancer development process, which is a multifactor and multistep continuum, not just a disease of imbalance with a variety of molecular dysfunction and cell signalling disturbance, but a disease of imbalance with a variety of molecular dysfunction and cancer-favoring TME[11].
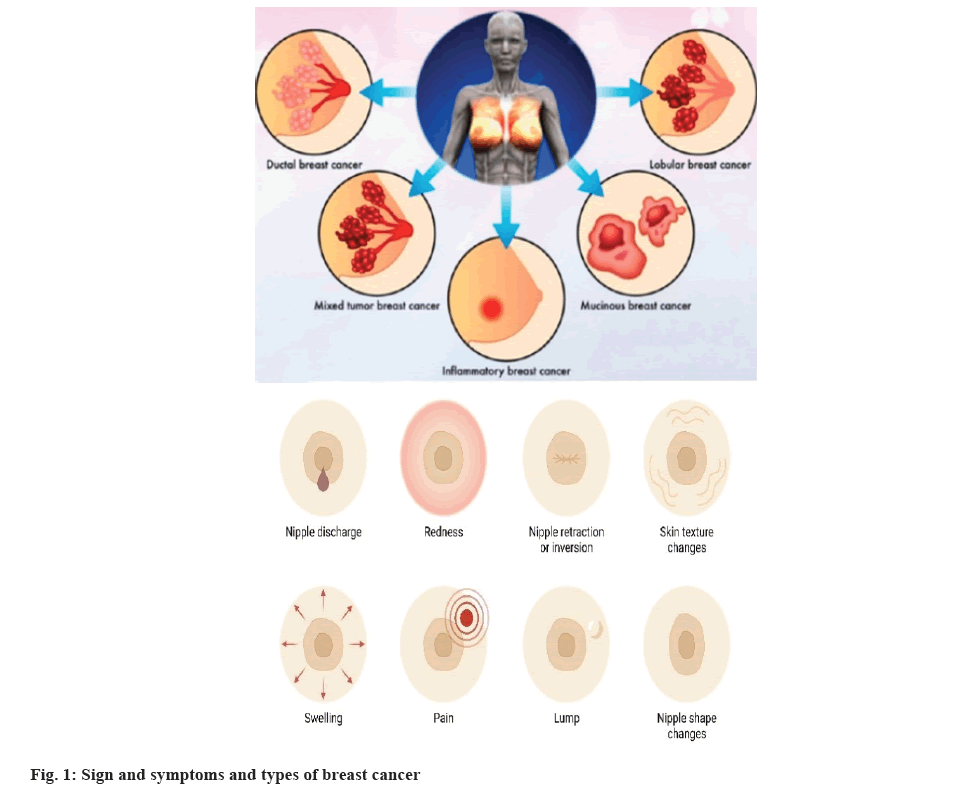
Breast Cancer (BC) is affecting about 2.1 million females annually. In 2018, 627 000 women died from BC, making up about 15 % of all women's cancer-related fatalities[12]. Typically, BC is classified according to how easy it can expand. Ductal carcinoma in situ begins in a milk duct but does not spread to the other breast tissue. Invasive or infiltrating types of BC i.e., invasive lobular carcinoma and invasive ductal carcinoma might spread to the breast tissue around them. The invasive ductal carcinoma constitutes up to 70 %-80 % of all BCs[13]. Inflammatory BC and triple-negative BC are also types of invasive BC. Inflammatory BC makes up 1 %-5 % of all BCs and is a rare kind of invasive BC. Fig. 1 shows various types as well as signs and symptoms of BC in females[14].
BC is becoming more serious cause of death in women. In this regard, scientists are working at the cellular level using cutting-edge nanomaterials to create early diagnostic and therapy techniques for BC[15-18]. However, the requirement for its execution in clinical practice is impeccable[14].
QDs: Emerging Theranostic Agents
Because of their potential for application in the diagnosis and treatment of cancer, QDs are gaining attention[19]. Fluorescence probes made of semiconductor QDs have proven to be useful in the detection and treatment of cancer. They were a unique fluorophore that, due to their size, exceptional stability, lack of photobleaching and water solubility, may replace conventional organic dyes[20]. Successful advancements in the nanotechnology fields[21] and cancer nanomedicine have been developed as a result of formulation development, molecular biology and bioimaging[22,23].
QDs, luminous nanocrystals are utilized for both imaging and delivery of various bioactive in a controlled pattern. These fluorescent entities with integrated carrier and imaging functionalities enable the administration of diagnostic and therapeutic agents. QDs are a very hopeful technique for personalized medicine because of their high sensitivity, unique optical features and flexible surface chemistry[24]. Given the stubborn nature of cancer, researchers' main objective and greatest difficulty are to combine detection and therapy (theranostics) utilizing Graphene QDs (GQDs). These have gained notoriety quickly in the fields of materials science and biomedicine[14]. It is mentioned that different surface alterations on QDs have a direct impact on their attributes, such as their toxicity and optical capabilities. These materials are employed in clinically targeted molecular treatment and imaging due to the positive outcomes[25].
Fabrication techniques of QDs:
The QDs can be prepared by bottom-up and top-down methods. The use of cheap, non-toxic raw materials, simple post-processing steps, straightforward operations, rapid reactions and renewable resources are only a few advantages of the environmentally friendly synthesis of QDs. Prospective uses for these nanomaterials in biomedical and clinical sciences include bioimaging, diagnostics, bioanalytical testing and biosensors[26].
All of the top-down and bottom-up approaches proposed for the synthesis of QDs may be divided into three categories i.e., physical, chemical and biological methods[27-29]. Depending on the production process, the size of QDs can range from a few nm to a few μm and via careful growth processes, the particle size distribution can be regulated within 2 %[30]. The methods used to create QDs vary depending on whether they are cadmium-based or cadmium-free and they range from conventional methods to cutting-edge methods.
Features of QDs:
Nanoscale semiconductor crystals known as QDs are intriguing materials in a variety of scientific fields, including biology[31,32]. QDs were initially identified by a Russian scientist named Alexei Ekimov in the 1980s. These substances are made up of elements from groups II-VI or group III-V of the periodic table having physical dimensions less than the exciton's Bohr radius[33,34]. Their utility is getting better more than 20 y after they were first introduced.
QDs are exceptional prospects for in vitro and in vivo imaging because of their irreplaceable optical features. The inorganic core of the QDs is responsible for the optical and semiconductor capabilities. Before ligands are applied to the active core surface of a QD, it is frequently passivated by another inorganic shell. This enhances the optical characteristics of QDs because the shell's material has a wider band gap than the core, preventing electrons and holes from entering the shell[35]. Due to the confinement of electron-hole pairs (excitons) inside the nanocrystal grain boundaries, QDs exhibit distinctive visual features. QDs are ideal for a wide range of biomedical applications due to their unique photo-physical characteristics, which include broad absorption spectra and size-tunable narrow emission spectra, size- and composition-tunable light emission, high ?uorescence quantum yields, enormous absorption extinction coefficients, photo-chemical robustness, barrier properties to the photo bleach effect, large stokes shift and decreased fluorescence intermittency[36]. A few nanometers in size, QDs are luminous semiconductors nanocrystals[37,38]. It's critical to do more research on several significant issues to better understand the risks associated with the development and use of QDs in cancer treatment[39]. Due to their low toxicity, biocompatibility, high quantum yields and versatility for various surface modifications, silicon QDs, non-blinking QDs and QDs with decreased size and regulated valence are currently particularly tempting for use in bioanalytical applications. The potential for multiplexed sensing employing QDs with different emission wavelengths to simultaneously detect a variety of biomarkers of illness is intriguing[40]. QDs have gotten a lot of interest since Ekimov and Efros classified them as a class of nanomaterials in the early 1980s. Although the earliest research focused on CdSe-based nanocrystals, the field has subsequently expanded to encompass a variety of classes of nanoparticles with different chemical compositions for their core, shell and passivation[41].
QDs as Cancer Diagnostic Agents
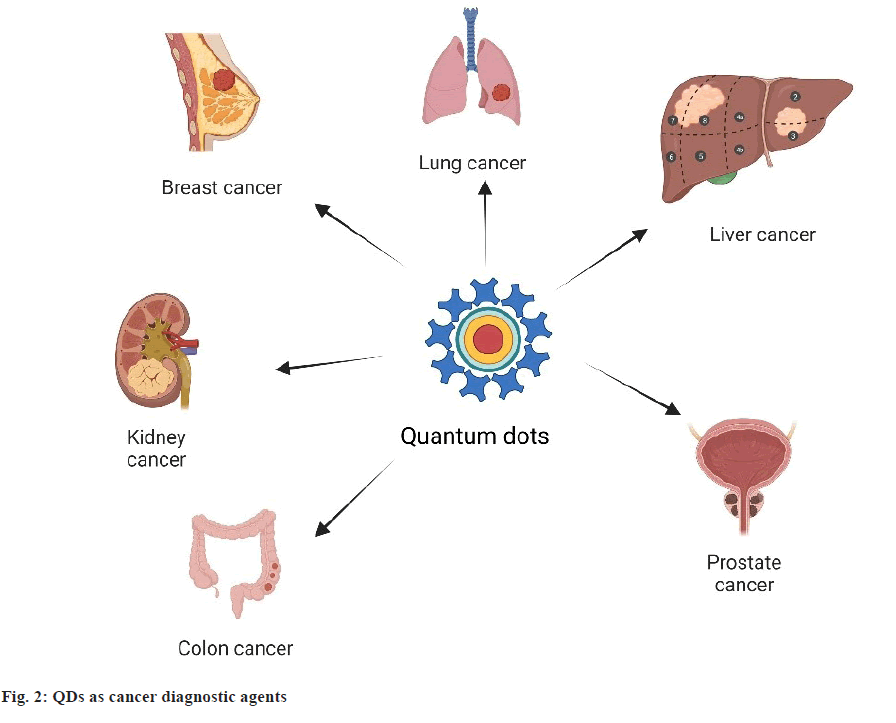
Due to the rising rates of cancer, cardiovascular disease, neurodegenerative disorders, autoimmune diseases and numerous infections worldwide, it is essential to develop strategies that can quickly and accurately detect the ultralow concentrations of relevant biomarkers, pathogens, toxins and pharmaceuticals in biological matrices. Many research efforts are now required to construct biosensors for their early identification and treatment using nanomaterials like QDs (fig. 2). These nanomaterials successfully enhance the repeatability, selection and sensitivities of the sensing performance[42].
BC:
The preferred diagnostic techniques for BC continue to be pathological laboratory tests like blood tests and biopsies, as well as biomedical diagnostic tests like computed tomography scans, positron emission tomography scans, ultrasound imaging, Magnetic Resonance Imaging (MRI) and mammography. Gene expression profiling is frequently employed as a diagnostic tool for the index of BC cell lines in place of immunohistochemical techniques[43,44].
In BC, QDs-based imaging investigations were carried out, including tissue imaging for assessing prognostic biomarkers and researching relationships between biomarkers, in vivo imaging for showing BC xenograft tumor, recognizing BC metastases and mapping the axillary lymphatic system[15,6]. Future applications of QDs-based imaging on clinical BC will mostly be focused on tissue investigation, particularly in BC molecular pathology and will include a variety of biomarkers[45].
The limitations of the many genomic expression approaches, including OncotypeDx, MammaPrint and BC Index (BCI), often include the requirement that BC has not yet spread to the lymph nodes for them to be effective. The inherited mutation of the BC gene is the genetic cause of BC (BRCA1 or BRCA2). About 25 %-30 % of invasive BC patients have elevated levels of HER2, a 185 kDa protein also known as c-erbB2 or HER2/neu, which is strongly associated with more aggressive tumor biological characteristics[46]. The first to create QDs-based strategies for HER2 targeting in mice mammary tumor sections and human BC cells (SK-BR-3). Additionally, they created a technique to concurrently detect HER2 and human anti-nuclear antigens in a single BC cell using several QD colors. The usefulness of HER2 detection using QDs for BC has been supported by a couple of researches. HER2 over-expressing BC alive mice model was tracked using a single particle QD coupled with an anti-HER2 antibody, which effectively detected five delivery processes as follows. HER2 binding to the cell membrane, traveling from the cell membrane to the perinuclear region, occurring during extravasation, first inside the circulation within a blood vessel and in the perinuclear region[47].
Twenty-five distinct subtypes, including HHR, LHR-LTH2, LHR-HTH2, NHR-LTH2 and NHR-HTH2 have been identified using information from QDs-based quantitative spectral analysis of HER2, ER and PR. HHR stands for high hormone receptors expression, LHR for low hormone receptors expression, NHR for negative hormone receptors expression, LTH2 for low HER2 total load and HTH2 for high HER2 total load[48,49].
Thomas Ashworth, an Austrian scientist, first identified Circulating Tumor Cells (CTCs) in patient cadavers in 1869[50]. Since they are released from primary tumors into circulation to relocate to distant organs, CTCs play a crucial role in metastases and their number is highly correlated with clinical prognosis[51]. CTCs have not entered common clinical practice despite being discovered more than a century ago, mostly due to a lack of technology to isolate these incredibly uncommon cells. QDs have been created as a brand-new class of fluorescent probes with several distinctive features for therapeutic use. Numerous initiatives have been taken to increase target sensitivity and specificity[52-55]. It has been suggested that particular cancer cells can be captured and separated using built-in bifunctional nanospheres and further trifunctional nanospheres using both QDs and magnetic nanoparticles. Then, wheat germ agglutinin changed the trifunctional nanospheres to readily collect prostate cancer cells without harm. Later, these monoclonal antibody-based probes were used to target a variety of cancer cells, including leukemia cells and prostate cancer cells. For these two types of cancer cells, the capture efficiencies were 96 % and 97 %, respectively, after 25 min. And using this approach, it was possible to quickly identify a small percentage of cancer cells (approximately 0.01 %) intermingled with a vast population of normal cells in culture[56].
Fluorescent labeling of aptamers is a useful technique for cell imaging and aptamer tracking. The aptamer SL2-B, which is targeted towards the Heparin-Binding Domain (HBD) of the VEGF165 protein, was coupled to QDs to produce the QD-SL2-B aptamer conjugate. The photobleaching impact of the QDs and the QD-aptamer combination was evaluated before incubating the cells[57].
QDs are intravenously injected into orthotopic breast and pancreatic tumors in mice using the tumor-penetrating iRGD peptide. The extravascular cancer cells and fibroblast still hold intact QDs after quenching the excess QDs, producing a tumor-specific signal[58].
Prostate cancer:
Sensitive and multicolor imaging of cancer cells under in vivo settings using QDs probes coated with Prostate-Specific Membrane Antigen (PSMA), a significant marker of Prostate Cancer (PC), has been described[59]. Additionally, the same group investigated E-cadherin, high-molecular-weight cytokeratin, p63 and -methyl acyl CoA racemase in situ detection to perform QD-based high-throughput digital mapping of molecular, cellular and glandular changes on surgical PC specimens[60] which, particularly at complicated and dubious illness loci, is not accessible by H&E and IHC techniques. Shi et al., demonstrated the higher quality of multiplexed QDs for the detecting of PSMA in grown PC cells[61].
Ovarian cancer:
The epithelial antigen CA125, a helpful tumor marker for ovarian cancer, may also be found using QDs in various specimen types, including fixed cells, tissue slices and xenograft pieces. The signals from the QDs probes have been more distinct and brighter than those from the traditional organic dye. The simultaneous detection of BRAF and BRCA Deoxyribonucleic Acid (DNA) using a "nano-on-micro" technology-based multiplexed DNA sensor was also reported by Ai et al. This method has significant promise for the early identification of diseases such as BC, ovarian cancer and papillary thyroid carcinoma[62-64].
Pancreatic cancer:
Bombesin (BN) receptors in the healthy pancreas, which are absent in pancreatic cancer, have been designed as a target by Montet et al., instead of targeting upregulated or overexpressed molecules in cancer cells[65]. The BN-Cy5.5 nanoparticle offers a potential method for imaging pancreatic cancer by reducing the T2 signal of a healthy pancreas and improving the capacity to see the tumor in a model of pancreatic cancer using MRI. Multifunctional QDs have become efficient materials for simultaneous cancer diagnostics, targeting and therapy. The main barrier to clinical translation for QDs is still toxicology[37].
Liver cancer:
To target liver tumors, Yu et al., created customized QDs-Anti-AFP probes. As these probes gathered inside the tumor, they fluoresced, allowing for active tumor-targeted and spectroscopic hepatoma imaging[66]. Additionally, the QDs-based pictures could demonstrate lung metastasis, recurrence, and subcutaneous tumor progression.
Lung cancer:
A method for quantifying protein expression, including EGFR, pan-cytokeratin and E-cadherin, of lung cancer tissues from xenograft models, was developed and validated by Ghazani et al. This method provided an automated mathematical tool to remove auto-fluorescence, normalize tumor protein normalized to cellular content, and produce a thorough profile of tumor-derived antigen on a tissue microarray[67]. When compared to conventional IHC, QDs-IHC had a greater sensitivity for detecting caveolin-1 and PCNA in lung cancer[37,68-70].
Other solid tumors:
Recent developments in the conjugation of QDs and the extracellular domain of EGFR proteins[71] allow for the rapid identification of tumor type, grade and chemoresistance by biological markers characteristics and enable nanoparticle-based, mechanistic studies of the role of activated EGFR in the growth and invasiveness of brain tumors as extracellular domain of EGFR proteins could induce receptor activation to enable the precise detection of intracellular[72]. Multicolor QDs can also be utilized to diagnose Hodgkin's lymphoma[73]. Four protein indicators (CD15, CD30, CD45 and CD Pax5) that were imaged multiplexed enabled the quick identification and discrimination of the uncommon Hodgkin's and Reed-Sternberg cells from encroaching immune cells[38,74,75].
QDs in Treatment of Cancers
With QDs-based probes, in vitro and in vivo molecular imaging has seen significant developments. However, the intriguing physical and chemical properties of well-known QDs may also be related to their potentially harmful effects on living cells and tissues. Further research on some crucial issues is necessary to appropriately quantify the risks associated with the manufacturing and use of QDs in treating cancer[76]. The literature has examined the use of QDs for anticancer therapy using drug administration, gene delivery, and the recently established Photodynamic Therapy (PDT)[10,24,77,78].
In combination with fluorescence imaging, modified QDs may effectively infiltrate cells and be employed as therapeutic agents to treat various cancers[79]. In the pharmaceutical business, QDs can be employed as tools for cell labeling, immunolabeling, medicine delivery and diagnostics[80]. Applications for silicon QD-based in vitro and in vivo imaging and drug delivery techniques are also discussed[81].
A QD aptamer-Doxorubicin (Dox) conjugate has been described by Bagalkot et al., as a targeted cancer imaging, treatment and sensing system. This straightforward multipurpose nanoparticle technology can both give Dox to the desired PC cells and detect Dox delivery by turning on the fluorescence of QD[82].
Moving nano-diagnostic technologies from the lab to society scales will require further work. Due to their smaller size (10-150 nm), GQDs exhibit selective extravasation capacity from the circulation into tumor tissue in the event of passive targeting of tumors. They may be able to positively accumulate in tumor tissue due to Nanoparticles' (NPs') increased permeability and retention effective response[83]. Many researchers are working to make further progress in the areas of reducing the potential toxicity, improving biocompatibility and improving the capacity of GQDs to load drugs. Recent studies have shown the effectiveness of combining GQDs with rare-earth up-converting NPs for synergistic PDT/PTT and bioimaging applications in cancer therapy to solve the aforementioned issues[84]. It is reasonable to assume that GQDs-based nanoplatforms can enhance theranostic techniques in practical and biocompatible ways given the wealth of research reports on GQDs. However, there is always a "silver line" that must be crossed before an innovation is accepted and that is the point at which the newly developed materials become well-known or are used in common biomedical applications.
Challenges in QDs-based Detection Techniques
Nanotechnology holds enormous promise for both fundamental and therapeutic cancer research. Particularly with conjugated QDs in targeting metastasis and in quantitative detection of molecular targets, unique surface and size features of QDs offer significant potential in enhancing the clinical application and excite enthusiasm for breaking past present technical limits. Due to the lack of clinical experience with their use, innovative QDs-based technologies have prompted concerns about biosafety, repeatability and dependability when compared to current conventional technologies. In the case of QDs, several surface modifications are said to affect properties including toxicity and optical properties directly. Due to the promising outcomes, these materials are employed in clinics for specialized molecular therapy and imaging. Several issues must be resolved before the widespread use of QDs in a therapeutic setting. Proposals are suggested and investigated with respect to the existing obstacle facing QDs to propose an appropriate approach for the therapeutic application of these materials[25].
Biosafety issues of QDs
Before incorporating QDs into clinical use, we must first overcome the obstacle of reducing the QDs' cytotoxicity. QDs include potentially harmful metal atoms and because of colloidal effects and photon-induced free radical production, they may unexpectedly cause cytotoxicity[85]. There aren't many papers or sources of information on the cytotoxicity or biosafety of QDs, which is partially related to the novelty of nanotechnology[86]. These issues might not have a significant impact on the advancement of in vitro applications, but they pose significant obstacles to in vivo cancer imaging in humans. Although more attention has been paid to how QDs affect human health and the environment[87].
Cytotoxicity:
Numerous QDs have some level of cytotoxicity. The size, capping material, dosage, surface chemistry, coating bioactivity and QD exposure pathway are the key determinants of QD cytotoxicity. Target cells and tissues may experience a harmful effect from the remaining organic compounds[88]. As an illustration, Wistar rats were exposed to 0.52 mg cd/m3 for 5 d (6 h/d) by nasal delivery. After 3 d of exposure, Cd-based QDs were discovered upon a histological investigation of the clinical variables in blood, Bronchoalveolar Lavage (BAL) fluid and lung tissue. Without harming the central nervous system, the Cd-based QDs were able to trigger local neutrophil inflammation in the lungs[89]. Due to the protective effect of the ZnS shell preventing the escape of Cd ions from the inner side, a significant buildup of QDs was seen in the spleen. In Drosophila melanogaster, the long-term toxicity of CdSe-ZnS QDs with surface coating and the genotoxic effects of QDs in vivo were investigated. Additionally, the toxicity of QDs is examined, along with modifications for toxicity reduction. Real publications and patents are used to analyze how QDs are used in biomedicine[90].
The cytotoxicity of QDs has been attributed to several different causes. These mechanisms, which have been previously described, can be summed up as free Cd desorption (degradation of the core of QDs); production of free radicals and interaction of QDs with intracellular components. Since the inner metal core of some types of QDs is made of hazardous metal (such as Cd, As, Se, Te, and Pb), the stability of the outer surface layers of QDs directly affects their biocompatibility[91].
In vivo toxicity:
The toxicity caused by the leaking of heavy metal ions like cadmium, lead or arsenic from contained QDs (II-IV, IV-VI, or III-V groups) into biological systems is one of the key obstacles to the widespread use of QDs for in vivo investigations. If the QD surfaces are not adequately shielded by ligands or covered by shells, the issue becomes more challenging. Until recently, several in vitro and in vivo research have been conducted to examine the cell- and tissue-toxicity of QDs[92,93]. As was already established, research has revealed that QDs are not necessarily safe[94-98] and when utilized as imaging and therapeutic agents, they have various impacts on biological systems at the cellular, subcellular, and molecular levels[99]. QDs may negatively impact cell viability, growth and proliferation in addition to causing various DNA damages[100].
Surface modifications of QDs
The use of QDs for biological applications has certain significant limitations, as has already been addressed in the literature. One of their drawbacks is typically associated with the release of cadmium, lead, or arsenic from their structural components into the biological environment. Their poor solubility is a further constraint. Today, surface alterations have been suggested to solve the QD problems outlined above. Several strategies have been put forth in this area, including surface ligand exchange and covering QDs with biocompatible compounds (e.g., polymer layer)[101-103].
Conclusion
To improve the properties of QDs for particular applications, several synthesis methods and tactics have been developed during the past 10 y. Recently, this family of materials has demonstrated considerable promise in various biological applications, including tissue engineering, regenerative medicine, and cancer treatment. Additionally, QDs have lately been recognized as helpful tools in several scientific fields, including real-time dual-targeted medication administration and molecular imaging of adipose regions in obese patients. QDs may successfully interact with many biomolecules because of their relatively high surface area and distinctive molecular structure. Both developed and stem cells' ultimate fates in the human body may be significantly influenced by these interactions. Due to a notable improvement in the creation of chemotherapeutic and protein-based medications, applications of QDs in cancer medicine have drawn a lot of interest among other developments.
Nanotechnology-based on QDs is a huge field. Despite the difficulties in clinical translation, QDs represent scientific advances that might transform cancer diagnostics. Although it is clear that QDs-based in vivo molecular imaging has benefits, their biosafety still has to be thoroughly investigated and assessed. QDs are currently used often in vitro for the detection of cancer-associated proteins, the capture of CTCs, and molecular pathology. These applications highlight the dynamic process of cancer formation and enable the creation of a new platform for a better understanding of tumor heterogeneity.
Despite this, the biggest obstacle for researchers will be the in vivo or clinical usage of QDs. Even though cell-based investigations of QDs offer some important advantages over conventional diagnostic approaches, there are still major obstacles to overcome before these approaches can be effectively applied in clinical settings. The majority of QD research relies on in vitro testing. To realize their potential and determine their safety and effectiveness profile, these investigations should be continued in animal models and clinical trials. To speed up the application of QDs in cancer detection and diagnosis in clinically relevant contexts, interdisciplinary partnerships combining oncologists, bioinformatics scientists, chemists, physicists, biomedical engineers, biologists, and pathologists are urgently needed.
Conflict of interests:
The authors declared no conflict of interests.
References
- Islami F, Miller KD, Siegel RL, Zheng Z, Zhao J, Han X, et al. National and state estimates of lost earnings from cancer deaths in the United States. JAMA Oncol 2019;5(9):e191460.
[Crossref] [Google Scholar] [PubMed]
- Hulvat MC. Cancer incidence and trends. Surg Clin North Am 2020;100(3):469-81.
[Crossref] [Google Scholar] [PubMed]
- World Health Organization. WHO report on cancer: Setting priorities, investing wisely and providing care for all. World Health Organization; 2020.
- World Health Organization. Don't let tobacco take your breath away: Choose health, not tobacco: 31 May. World tobacco day; 2019.
- El-Tanani M, Nsairat H, Mishra V, Mishra Y, Aljabali AA, Serrano-Aroca Á, et al. Ran GTPase and its importance in cellular signaling and malignant phenotype. Int J Mol Sci 2023;24(4):3065.
[Crossref] [Google Scholar] [PubMed]
- El-Tanani M, Al Khatib AO, Al-Najjar BO, Shakya AK, El-Tanani Y, Lee YF, et al. Cellular and molecular basis of therapeutic approaches to breast cancer. Cell Signal 2023;101:110492.
[Crossref] [Google Scholar] [PubMed]
- El-Tanani M, Platt-Higgins A, Lee YF, Al Khatib AO, Haggag Y, Sutherland M, et al. Matrix metalloproteinase 2 is a target of the RAN-GTP pathway and mediates migration, invasion and metastasis in human breast cancer. Life Sci 2022;310:121046.
[Crossref] [Google Scholar] [PubMed]
- Fang M, Chen M, Liu L, Li Y. Applications of quantum dots in cancer detection and diagnosis: A review. J Biomed Nanotechnol 2017;13(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet?Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65(2):87-108.
[Crossref] [Google Scholar] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011;144(5):646-74.
[Crossref] [Google Scholar] [PubMed]
- Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global cancer observatory. Cancer Today 2018;23(7):323-6.
- Wenzel C, Bartsch R, Hussian D, Pluschnig U, Altorjai G, Zielinski CC, et al. Invasive ductal carcinoma and invasive lobular carcinoma of breast differ in response following neoadjuvant therapy with epidoxorubicin and docetaxel+ G-CSF. Breast Cancer Res Treat 2007;104:109-14.
[Crossref] [Google Scholar] [PubMed]
- Tade RS, Patil PO. Theranostic prospects of graphene quantum dots in breast cancer. ACS Biomater Sci Eng 2020;6(11):5987-6008.
- Mishra Y, Amin HI, Mishra V, Vyas M, Prabhakar PK, Gupta M, et al. Application of nanotechnology to herbal antioxidants as improved phytomedicine: An expanding horizon. Biomed Pharmacother 2022;153:113413.
[Crossref] [Google Scholar] [PubMed]
- Mishra Y, Mishra V, Tambuwala MM. Tumor adhesion molecule targeting for breast cancer nanomedicine. In: Targeted nanomedicine for breast cancer therapy 2022;257-80.
- Mishra V, Kesharwani P, Jain NK. Biomedical applications and toxicological aspects of functionalized carbon nanotubes. Crit Rev Ther Drug Carrier Syst 2018;35(4):293-330.
[Crossref] [Google Scholar] [PubMed]
- Kesharwani P, Mishra V, Jain NK. Validating the anticancer potential of carbon nanotube-based therapeutics through cell line testing. Drug Discov Today 2015;20(9):1049-60.
[Crossref] [Google Scholar] [PubMed]
- Madani SY, Shabani F, Dwek MV, Seifalian AM. Conjugation of quantum dots on carbon nanotubes for medical diagnosis and treatment. Int J Nanomed 2013;8:941-50.
[Crossref] [Google Scholar] [PubMed]
- Babu LT, Paira P. Current application of quantum dots (QD) in cancer therapy: A review. Mini Rev Med Chem 2017;17(14):1406-15.
[Crossref] [Google Scholar] [PubMed]
- Rompicherla NC, Joshi P, Shetty A, Sudhakar K, Amin HI, Mishra Y, et al. Design, formulation, and evaluation of aloe vera gel-based capsaicin transemulgel for osteoarthritis. Pharmaceutics 2022;14(9):1812.
[Crossref] [Google Scholar] [PubMed]
- Mishra V, Kesharwani P, Amin MC, Iyer A, editors. Nanotechnology-based approaches for targeting and delivery of drugs and genes. Academic Press; 2017.
- Patil A, Mishra V, Thakur S, Riyaz B, Kaur A, Khursheed R, et al. Nanotechnology derived nanotools in biomedical perspectives: An update. Curr Nanosci 2019;15(2):137-46.
- Tripathi SK, Kaur G, Khurana RK, Kapoor S, Singh B. Quantum dots and their potential role in cancer theranostics. Criti Rev Ther Drug Carrier Syst 2015;32(6):461-502.
[Crossref] [Google Scholar] [PubMed]
- Kargozar S, Hoseini SJ, Milan PB, Hooshmand S, Kim HW, Mozafari M. Quantum dots: A review from concept to clinic. Biotechnol J 2020;15(12):2000117.
[Crossref] [Google Scholar] [PubMed]
- Iravani S, Varma RS. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots: A review. Environ Chem Lett 2020;18:703-27.
[Crossref] [Google Scholar] [PubMed]
- de Souza JA, Hunt B, Asirwa FC, Adebamowo C, Lopes G. Global health equity: cancer care outcome disparities in high-, middle-, and low-income countries. J Clin Oncol 2016;34(1):6-13.
[Crossref] [Google Scholar] [PubMed]
- Faramarzi MA, Sadighi A. Insights into biogenic and chemical production of inorganic nanomaterials and nanostructures. Adv Colloid Interface Sci 2013;189:1-20.
[Crossref] [Google Scholar] [PubMed]
- Kargozar S, Ramakrishna S, Mozafari M. Chemistry of biomaterials: Future prospects. Curr Opin Biomed Eng 2019;10:181-90.
- Franke D, Harris DK, Xie L, Jensen KF, Bawendi MG. The unexpected influence of precursor conversion rate in the synthesis of III–V quantum dots. Angew Chem Int Ed Engl 2015;127(48):14507-11.
[Crossref] [Google Scholar] [PubMed]
- Zheng XT, Ananthanarayanan A, Luo KQ, Chen P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015;11(14):1620-36.
[Crossref] [Google Scholar] [PubMed]
- Kargozar S, Mozafari M. Nanotechnology and nanomedicine: Start small, think big. Mater Today Proc 2018;5(7):15492-500.
- Zhang Y, Liu Y, Li C, Chen X, Wang Q. Controlled synthesis of Ag2S quantum dots and experimental determination of the exciton Bohr radius. J Phys Chem C 2014;118(9):4918-23.
- Mishra V, Gurnany E, Mansoori MH. Quantum dots in targeted delivery of bioactives and imaging. In: Nanotechnology-based approaches for targeting and delivery of drugs and genes. 2017;427-50.
- Reshma VG, Mohanan PV. Quantum dots: Applications and safety consequences. J Lumin 2019;205:287-98.
- Shukla S. Recent developments in biomedical applications of quantum dots. Adv Mater Rev 2014;1(1):2-12.
- Luo G, Long J, Zhang B, Liu C, Ji S, Xu J, et al. Quantum dots in cancer therapy. Expert Opin Drug Deliv 2012;9(1):47-58.
[Crossref] [Google Scholar] [PubMed]
- Riyaz B, Sudhakar K, Mishra V. Quantum dot-based drug delivery for lung cancer. In: Nanotechnology-based targeted drug delivery systems for lung cancer. 2019;311-326.
- Wagner MK, Li F, Li J, Li XF, Le XC. Use of quantum dots in the development of assays for cancer biomarkers. Anal Bioanal Chem 2010;397:3213-24.
[Crossref] [Google Scholar] [PubMed]
- Kamila S, McEwan C, Costley D, Atchison J, Sheng Y, Hamilton GR, et al. Diagnostic and therapeutic applications of quantum dots in nanomedicine. Light-responsive nanostructured systems for applications in nanomedicine. 2016:203-24.
[Crossref] [Google Scholar] [PubMed]
- Mansuriya BD, Altintas Z. Applications of graphene quantum dots in biomedical sensors. Sensors 2020;20(4):1072.
[Crossref] [Google Scholar] [PubMed]
- Chavez KJ, Garimella SV, Lipkowitz S. Triple negative breast cancer cell lines: One tool in the search for better treatment of triple negative breast cancer. Breast Dis 2010;32(1-2):35-48.
[Crossref] [Google Scholar] [PubMed]
- Reis-Filho JS, Pusztai L. Gene expression profiling in breast cancer: Classification, prognostication, and prediction. Lancet 2011;378(9805):1812-23.
[Crossref] [Google Scholar] [PubMed]
- Wang LW, Peng CW, Chen C, Li Y. Quantum dots-based tissue and in vivo imaging in breast cancer researches: Current status and future perspectives. Breast Cancer Res Treat 2015;151:7-17.
[Crossref] [Google Scholar] [PubMed]
- Chen C, Xia HS, Gong YP, Peng J, Peng CW, Hu MB, et al. The quantitative detection of total HER2 load by quantum dots and the identification of a new subtype of breast cancer with different 5-year prognosis. Biomaterials 2010;31:8818-25.
[Crossref] [Google Scholar] [PubMed]
- Tada H, Higuchi H, Wanatabe TM, Ohuchi N. In vivo real-time tracking of single quantum dots conjugated with monoclonal anti-HER2 antibody in tumors of mice. Cancer Res 2007;67(3):1138-44.
[Crossref] [Google Scholar] [PubMed]
- Chen C, Peng J, Xia HS, Yang GF, Wu QS, Chen LD, et al. Quantum dots-based immunofluorescence technology for the quantitative determination of HER2 expression in breast cancer. Biomaterials 2009;30(15):2912-8.
[Crossref] [Google Scholar] [PubMed]
- Chen C, Sun SR, Gong YP, Qi CB, Peng CW, Yang XQ, et al. Quantum dots-based molecular classification of breast cancer by quantitative spectroanalysis of hormone receptors and HER2. Biomaterials 2011;32(30):7592-9.
[Crossref] [Google Scholar] [PubMed]
- Arya SK, Lim B, Rahman AR. Enrichment, detection and clinical significance of circulating tumor cells. Lab Chip 2013;13(11):1995-2027.
[Crossref] [Google Scholar] [PubMed]
- Wen CY, Wu LL, Zhang ZL, Liu YL, Wei SZ, Hu J, et al. Quick-response magnetic nanospheres for rapid, efficient capture and sensitive detection of circulating tumor cells. ACS Nano 2014;8(1):941-9.
[Crossref] [Google Scholar] [PubMed]
- Di Corato R, Bigall NC, Ragusa A, Dorfs D, Genovese A, Marotta R, et al. Multifunctional nanobeads based on quantum dots and magnetic nanoparticles: Synthesis and cancer cell targeting and sorting. ACS Nano 2011;5(2):1109-21.
[Crossref] [Google Scholar] [PubMed]
- Hsieh YH, Lai LJ, Liu SJ, Liang KS. Rapid and sensitive detection of cancer cells by coupling with quantum dots and immunomagnetic separation at low concentrations. Biosens Bioelectron 2011;26(10):4249-52.
[Crossref] [Google Scholar] [PubMed]
- Gazouli M, Lyberopoulou A, Pericleous P, Rizos S, Aravantinos G, Nikiteas N, et al. Development of a quantum-dot-labelled magnetic immunoassay method for circulating colorectal cancer cell detection. World J Gastroenterol 2012;18(32):4419-26.
[Crossref] [Google Scholar] [PubMed]
- Lee HJ, Cho HY, Oh JH, Namkoong K, Lee JG, Park JM, et al. Simultaneous capture and in situ analysis of circulating tumor cells using multiple hybrid nanoparticles. Biosensor Bioelectron 2013;47:508-14.
- Song EQ, Hu J, Wen CY, Tian ZQ, Yu X, Zhang ZL, et al. Fluorescent-magnetic-biotargeting multifunctional nanobioprobes for detecting and isolating multiple types of tumor cells. ACS Nano 2011;5(2):761-70.
[Crossref] [Google Scholar] [PubMed]
- Kaur H. Aptamer conjugated quantum dots for imaging cellular uptake in cancer cells. J Nanosci Nanotechnol 2019;19(7):3798-803.
[Crossref] [Google Scholar] [PubMed]
- Liu X, Braun GB, Qin M, Ruoslahti E, Sugahara KN. In vivo cation exchange in quantum dots for tumor-specific imaging. Nat Commun 2017;8(1):343.
- Khan R, Arshad F, Hassan IU, Naikoo GA, Pedram MZ, Zedegan MS, et al. Advances in nanomaterial-based immunosensors for prostate cancer screening. Biomed Pharmacother 2022;155:113649.
[Crossref] [Google Scholar] [PubMed]
- Liu J, Lau SK, Varma VA, Kairdolf BA, Nie S. Multiplexed detection and characterization of rare tumor cells in Hodgkin’s lymphoma with multicolor quantum dots. Anal Chem 2010;82(14):6237-43.
[Crossref] [Google Scholar] [PubMed]
- Shi C, Zhou G, Zhu Y, Su Y, Cheng T, Zhau HE, et al. Quantum dots-based multiplexed immunohistochemistry of protein expression in human prostate cancer cells. Eur J Histochem 2008;52(2):127-34.
[Crossref] [Google Scholar] [PubMed]
- Ai X, Ma Q, Su X. Multiplex DNA sensor for BRAF and BRCA detection. Anal Biochem 2013;438(1):22-8.
[Crossref] [Google Scholar] [PubMed]
- Chaurasiya S, Mishra V. Biodegradable nanoparticles as theranostics of ovarian cancer: An overview. J Pharm Pharmacol 2018;70(4):435-49.
[Crossref] [Google Scholar] [PubMed]
- Jain NK, Tare MS, Mishra V, Tripathi PK. The development, characterization and in vivo anti-ovarian cancer activity of poly (propylene imine)(PPI)-antibody conjugates containing encapsulated paclitaxel. Nanomedicine 2015;11(1):207-18.
[Crossref] [Google Scholar] [PubMed]
- Montet X, Weissleder R, Josephson L. Imaging pancreatic cancer with a peptide−nanoparticle conjugate targeted to normal pancreas. Bioconjug Chem 2006;17(4):905-11.
[Crossref] [Google Scholar] [PubMed]
- Yu X, Chen L, Deng Y, Li K, Wang Q, Li Y, et al. Fluorescence analysis with quantum dot probes for hepatoma under one-and two-photon excitation. J Fluoresc 2007;17:243-7.
[Crossref] [Google Scholar] [PubMed]
- Ghazani AA, Lee JA, Klostranec J, Xiang Q, Dacosta RS, Wilson BC, et al. High throughput quantification of protein expression of cancer antigens in tissue microarray using quantum dot nanocrystals. Nano Lett 2006;6(12):2881-6.
[Crossref] [Google Scholar] [PubMed]
- Chen H, Xue J, Zhang Y, Zhu X, Gao J, Yu B. Comparison of quantum dots immunofluorescence histochemistry and conventional immunohistochemistry for the detection of caveolin-1 and PCNA in the lung cancer tissue microarray. J Mol Histol 2009;40:261-8.
[Crossref] [Google Scholar] [PubMed]
- Kaur P, Mishra V, Shunmugaperumal T, Goyal AK, Ghosh G, Rath G. Inhalable spray dried lipidnanoparticles for the co-delivery of paclitaxel and doxorubicin in lung cancer. J Drug Deliv Sci Technol 2020;56:101502.
- Sudhakar K, Mishra V, Riyaz B, Jain A, Charyulu RN, Jain S. Hydrogel-based drug delivery for lung cancer. In: Nanotechnology-Based Targeted Drug Delivery Systems for Lung Cancer. 2019;293-310.
- Dudu V, Rotari V, Vazquez M. Targeted extracellular nanoparticles enable intracellular detection of activated epidermal growth factor receptor in living brain cancer cells. Nanomedicine 2011;7(6):896-903.
[Crossref] [Google Scholar] [PubMed]
- Meco D, Servidei T, Riccardi A, Ferlini C, Cusano G, Zannoni GF, et al. Antitumor effect in medulloblastoma cells by gefitinib: Ectopic HER2 overexpression enhances gefitinib effects in vivo. Neuro Oncol 2009;11(3):250-9.
[Crossref] [Google Scholar] [PubMed]
- Liu J, Lau SK, Varma VA, Moffitt RA, Caldwell M, Liu T, et al. Molecular mapping of tumor heterogeneity on clinical tissue specimens with multiplexed quantum dots. ACS Nano 2010;4(5):2755-65.
[Crossref] [Google Scholar] [PubMed]
- Bakshi HA, Quinn GA, Nasef MM, Mishra V, Aljabali AA, El-Tanani M, et al. Crocin inhibits angiogenesis and metastasis in colon cancer via TNF-α/NF-kB/VEGF pathways. Cells 2022;11(9):1502.
[Crossref] [Google Scholar] [PubMed]
- Mishra V, Kesharwani P. Dendrimer technologies for brain tumor. Drug Discov Today 2016;21(5):766-78.
[Crossref] [Google Scholar] [PubMed]
- Dwivedi N, Shah J, Mishra V, Mohd Amin MC, Iyer AK, Tekade RK, et al. Dendrimer-mediated approaches for the treatment of brain tumor. J Biomater Sci Polym Ed 2016;27(7):557-80.
[Crossref] [Google Scholar] [PubMed]
- Gupta M, Mishra Y, Mishra V, Tambuwala MM. Current update on anticancer effects of icariin: A journey of the last ten years. EXCLI J 2022;21:680-6.
[Crossref] [Google Scholar] [PubMed]
- Jain A, Jain A, Parajuli P, Mishra V, Ghoshal G, Singh B, et al. Recent advances in galactose-engineered nanocarriers for the site-specific delivery of siRNA and anticancer drugs. Drug Discov Today 2018;23(5):960-73.
[Crossref] [Google Scholar] [PubMed]
- Qiao LL, Yao WJ, Zhang ZQ, Yang X, Zhao MX. The biological activity research of the nano-drugs based on 5-fluorouracil-modified quantum dots. Int J Nanomed 2020;15:2765-76.
[Crossref] [Google Scholar] [PubMed]
- Gour A, Ramteke S, Jain NK. Pharmaceutical applications of quantum dots. AAPS Pharm Sci Tech 2021;22(7):233.
[Crossref] [Google Scholar] [PubMed]
- Chinnathambi S, Chen S, Ganesan S, Hanagata N. Silicon quantum dots for biological applications. Adv Healthc Mater 2014;3(1):10-29.
[Crossref] [Google Scholar] [PubMed]
- Bagalkot V, Zhang L, Levy-Nissenbaum E, Jon S, Kantoff PW, Langer R, et al. Quantum dot− aptamer conjugates for synchronous cancer imaging, therapy, and sensing of drug delivery based on bi-fluorescence resonance energy transfer. Nano Lett 2007;7(10):3065-70.
[Crossref] [Google Scholar] [PubMed]
- Bazak R, Houri M, El Achy S, Hussein W, Refaat T. Passive targeting of nanoparticles to cancer: A comprehensive review of the literature. Mol Clin Oncol 2014;2(6):904-8.
[Crossref] [Google Scholar] [PubMed]
- Laurenti M, Paez-Perez M, Algarra M, Alonso-Cristobal P, Lopez-Cabarcos E, Mendez-Gonzalez D, et al. Enhancement of the upconversion emission by visible-to-near-infrared fluorescent graphene quantum dots for miRNA detection. ACS Appl Mater Interfaces 2016;8(20):12644-51.
[Crossref] [Google Scholar] [PubMed]
- Smith AM, Nie S. Next-generation quantum dots. Nat Biotechnol 2009;27(8):732-3.
[Crossref] [Google Scholar] [PubMed]
- Zrazhevskiy P, Gao X. Multifunctional quantum dots for personalized medicine. Nano Today 2009;4(5):414-28.
[Crossref] [Google Scholar] [PubMed]
- Mahendra S, Zhu H, Colvin VL, Alvarez PJ. Quantum dot weathering results in microbial toxicity. Environ Sci Technol 2008;42(24):9424-30.
[Crossref] [Google Scholar] [PubMed]
- Derfus AM, Chan WC, Bhatia SN. Intracellular delivery of quantum dots for live cell labeling and organelle tracking. Adv Mater 2004;16(12):961-6.
- Rzigalinski BA, Strobl JS. Cadmium-containing nanoparticles: Perspectives on pharmacology and toxicology of quantum dots. Toxicol Appl Pharmacol 2009;238(3):280-8.
[Crossref] [Google Scholar] [PubMed]
- Pohanka M. Quantum dots in the therapy: Current trends and perspectives. Mini Rev Med Chem 2017;17(8):650-6.
[Crossref] [Google Scholar] [PubMed]
- Zhou J, Liu Y, Tang J, Tang W. Surface ligands engineering of semiconductor quantum dots for chemosensory and biological applications. Mater Today 2017;20:360-76.
- Kominkova M, Milosavljevic V, Vitek P, Polanska H, Cihalova K, Dostalova S, et al. Comparative study on toxicity of extracellularly biosynthesized and laboratory synthesized CdTe quantum dots. J Biotechnol 2017;241:193-200.
[Crossref] [Google Scholar] [PubMed]
- Zhang W, Yang L, Kuang H, Yang P, Aguilar ZP, Wang A, et al. Acute toxicity of quantum dots on late pregnancy mice: Effects of nanoscale size and surface coating. J Hazard Mater 2016;318:61-9.
[Crossref] [Google Scholar] [PubMed]
- Paesano L, Perotti A, Buschini A, Carubbi C, Marmiroli M, Maestri E, et al. Data on HepG2 cells changes following exposure to cadmium sulphide quantum dots (CdS QDs). Data Brief 2017;11:72-97.
[Crossref] [Google Scholar] [PubMed]
- Fan JW, Vankayala R, Chang CL, Chang CH, Chiang CS, Hwang KC. Preparation, cytotoxicity and in vivo bioimaging of highly luminescent water-soluble silicon quantum dots. Nanotechnology 2015;26(21):215703.
[Crossref] [Google Scholar] [PubMed]
- Liu J, Hu R, Liu J, Zhang B, Wang Y, Liu X, et al. Cytotoxicity assessment of functionalized CdSe, CdTe and InP quantum dots in two human cancer cell models. Mater Sci Eng C Mater Biol Appl 2015;57:222-31.
[Crossref] [Google Scholar] [PubMed]
- Chen CW, Wu DY, Chan YC, Lin CC, Chung PH, Hsiao M, et al. Evaluations of the chemical stability and cytotoxicity of CuInS2 and CuInS2/ZnS core/shell quantum dots. J Phys Chem C 2015;119(5):2852-60.
- Yuan X, Liu Z, Guo Z, Ji Y, Jin M, Wang X. Cellular distribution and cytotoxicity of graphene quantum dots with different functional groups. Nanoscale Res Lett 2014;9:1-9.
[Crossref] [Google Scholar] [PubMed]
- Sun H, Zhang F, Wei H, Yang B. The effects of composition and surface chemistry on the toxicity of quantum dots. J Mater Chem B 2013;1(47):6485-94.
[Crossref] [Google Scholar] [PubMed]
- Yan M, Zhang Y, Xu K, Fu T, Qin H, Zheng X. An in vitro study of vascular endothelial toxicity of CdTe quantum dots. Toxicology 2011;282(3):94-103.
[Crossref] [Google Scholar] [PubMed]
- Yao J, Yang M, Duan Y. Chemistry, biology, and medicine of fluorescent nanomaterials and related systems: New insights into biosensing, bioimaging, genomics, diagnostics, and therapy. Chem Rev 2014;114(12):6130-78.
[Crossref] [Google Scholar] [PubMed]
- Chen C, Peng J, Xia H, Wu Q, Zeng L, Xu H, et al. Quantum-dot-based immunofluorescent imaging of HER2 and ER provides new insights into breast cancer heterogeneity. Nanotechnology 2010;21(9):095101.
[Crossref] [Google Scholar] [PubMed]
- Derfus AM, Chan WC, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett 2004;4(1):11-8.
[Crossref] [Google Scholar] [PubMed]
- Yu X, Chen L, Li K, Li Y, Xiao S, Luo X, et al. Immunofluorescence detection with quantum dot bioconjugates for hepatoma in vivo. J Biomed Opt 2007;12(1):014008.
[Crossref] [Google Scholar] [PubMed]