- *Corresponding Author:
- M. Dhobi
Department of Pharmacognosy and Phytochemistry, School of Pharmaceutical Sciences, Delhi Pharmaceutical Sciences and Research University, New Delhi-110 017, India
E-mail: mahaveer.pharma@gmail.com
| Date of Received | 23 August 2019 |
| Date of Revised | 29 Decemebr 2019 |
| Date of Accepted | 26 January 2020 |
| Indian J Pharm Sci 2020;82(2):356-361 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The main objective of this investigation was to perform physicochemical and phytochemical standardization and to evaluate the anthelmintic activity of different extracts of the whole plant of Evolvulus alsinoides. Quality control standardization of the whole plant was carried out following the World Health Organization guidelines. Further, 6 different extracts of Evolvulus alsinoides were prepared with n-hexane, petroleum ether, chloroform, acetone, ethanol and water. High performance thin layer chromatography analysis was performed to quantify the content of luteolin in these extracts. In vitro anthelmintic activity was screened on Indian adult earthworm due to its similarities to intestinal roundworms of humans. Doses of 100 mg/ml of each dried extract were mixed with PBS and earthworms were placed in them. Paralysis and/or death of earthworms were taken as a criterion for anthelmintic activity. Most of the physicochemical parameters were found to be within the prescribed limits of World Health Organization guidelines, while, the plant material did not show the presence of any aflatoxins. Ethanol extract of Evolvulus alsinoides showed potent anthelmintic activity and the maximum amount of luteolin was also found to be present in the ethanol extract compared to other extracts. Thus, the potent anthelmintic activity of ethanol extract of Evolvulus alsinoides could possibly be attributed to the presence of luteolin.
Keywords
Anthelmintic, Evolvulus alsinoides, high performance thin layer chromatography, luteolin, standardization
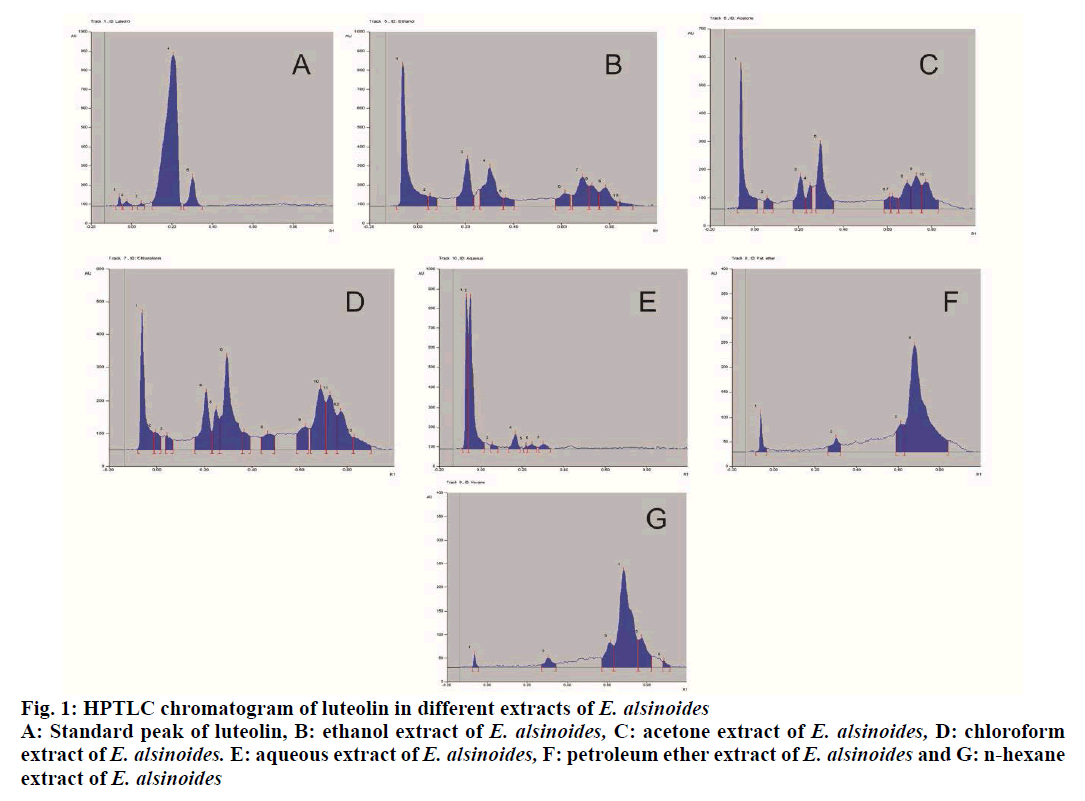
The plant Evolvulus alsinoides L (E. alsinoides), family Convolvulaceae, is a short hairy perennial herb[1]. The branches are 10-30 cm long arising from the top of roots. It is found wild throughout rocky plains of India[2]. The various common names of E. alsinoides in different languages are Dwarf morning glory (English), Shankhavali (Guajarati), Sankhahuli, Shankhapushpi (Hindi), Sankhavela (Marathi) and Visnukranti (Tamil)[3,4]. E. alsinoides is controversial in origin as several other plants including Convolvulus pluricaulis L., Clitoria ternatea L. and Canscora decussata L. are also known as Shankhapushpi in various parts of India[5-7]. The plant E. alsinoides is bitter and is used as a memory enhancing tonic and febrifuge. Leaves of the herb are recommended for asthma and mental disturbances. The herb is used mainly in traditional medicine of East Asia, especially in Ayurveda as a brain tonic and in the treatment of neurodegenerative diseases, amnesia and asthma[8-10]. Worldwide prevalence of helminth infection is around 24 % of the world’s population and is mainly distributed in the tropical and subtropical areas, especially in the regions of sub-Saharan Africa, Americas, China and East Asia. Around 267 million preschool-age children and over 568 million school-age children from the above areas are mainly affected from these infections. Modern medicines used to cure these infections, mainly include antibiotics, which have a long history of many side effects and resistance development[11]. Therefore, people are shifting their focus towards natural remedies mainly herbal therapy. E. alsinoides is reported to be used as anthelmintic agent in traditional systems, however there are no scientific data available on the major active principles responsible for the activity. Hence, an attempt has been made to perform quality control standardization of the plant E. alsinoides and to scientifically justify the traditional use of this plant as an anthelmintic. The dried whole herb of E. alsinoides L. was procured from Tirunelveli District, Tamil Nadu in the month of October 2017. The plant specimen was identified and authenticated at the Division of Plant Exploration and Germplasm Collection, National Herbarium of Cultivated Plants (NHCP), ICAR-National Bureau of Plant Genetic Resources, Pusa Campus, New Delhi-110012. A voucher of the plant specimen (NHCP/NBPGR/2018-03) was deposited in the Museum-Cum-Herbarium of Department of Pharmacognosy, School of Pharmaceutical Sciences, DPSRU, New Delhi under voucher no. 1. Albendazole tablets (Bendex tablets, Cipla) were purchased from a local Pharmacy, Reference standard luteolin (L9283-10 mg) was obtained from the Sigma Aldrich Ltd., Analytical grade solvents were obtained from Central Drug House. Precoated silica gel 60 F254 HPTLC aluminum plates (10×10 cm, 0.2 mm thickness) were obtained from E. Merck Ltd. (Mumbai, India). The whole plant of E. alsinoides was dried, powdered and various physiochemical parameters of the plant powder were evaluated, which included foreign matter, total ash, acid-insoluble ash, water-soluble ash, foaming index, extractive value, loss on drying, swelling index and bitterness value as per the methods described in WHO guidelines[12,13]. The dried plant material was further subjected to determination of heavy metals using atomic adsorption spectroscopy as per the recommended procedure. The plant material was also tested for the presence or absence of aflatoxins B1, G1, B2 and G2. The presence and absence of different pesticides were also determined using gas chromatography coupled with an electron capture detector. The plant material was also tested for presence of any microbial contamination employing total aerobic plate count and tests for the presence/absence of S. aureus, E. coli and Salmonella spp.[12]. These tests were performed at the Standard Analytical Laboratory (ND), Pvt. Ltd., ISO 9001-2008 certified situated at 69, Functional Industrial Estate, Patparganj, Delhi, 110092. About 500 g of the coarsely powdered drug of E. alsinoides was individually extracted with different solvents such as n-hexane, petroleum ether, chloroform, acetone and ethanol through Soxhlet apparatus for 6 h. The aqueous extract was prepared by decoction method. Then, the solvents of each extract were removed and recovered under reduced pressure. The dried extracts were preserved in a vacuum desiccator until use. Preliminary phytochemical screenings of the different extracts from E. alsinoides were performed as per standard procedure[14]. Different extracts of the plant E. alsinoides was standardized with Luteolin (Sigma-Aldrich, St. Louis, MO, USA) using High Performance Thin Layer Chromatography (HPTLC). A stock solution of each extracts (5 mg/ml) and Luteolin (1 mg/ml) were prepared in methanol. The mobile phase for developing the chromatogram consisted of toluene, ethyl acetate and formic acid mixture in the ratio 6: 4: 0.3 (v/v/v). The study was carried out using Camag, HPTLC instrumentation (Camag, Mutten, Switzerland) equipped with Linomat V sample applicator, Camag TLC scanner 3, Camag TLC visualizer and WINCATS 4 software for data interpretation. The Rf values were recorded and the developed plate was screened and photo-documented at 366 nm after derivatization with Anisaldehyde sulphuric acid reagent. The Indian adult earthworms belonging to the species Pheritima posthuma, were purchased from High Tech Natural Products (India) Ltd, Dilshad Garden. They were observed to be having the following characteristics; Length - about 3-5 cm, width - 0.1- 0.2 cm, weight - about 0.8-3.04 g. Investigation of in vitro anthelmintic activity was performed as per the method reported by Rajesh. et al.[15] with minor modifications. Adult Indian earth worm, Pheritima posthuma was selected for the study, due to its anatomical and physiological resemblance with the intestinal round worm parasite of human beings[16,17]. Albendazole was used as reference standard for this study. The test samples were prepared by suspending 2.5 g of each extract in 25 ml of phosphate buffer saline (PBS, pH 7.4) to obtain the solution of 100 mg/ml, while albendazole suspension was prepared at 20 mg/ml (the concentration was decided based on the preliminary trials at 10, 25, 50 and 100 mg/ml). The earth worms were washed with normal saline and divided into 8 groups and each group contained 5 earthworms. Treatment pattern of every group is represented in Table 1. The inhibition of motility (paralysis of the worms) and/or mortality (death of the worms) was used as a criterion for the anthelmintic activity of different extracts of E. alsinoides. Paralysis was said to occur when the worms did not move even in normal saline. Death was concluded when the worms lost their motility followed by fading away of their body colour[17-19]. Quality control standardization of medicinal plants is very essential as it helps in proper identification and authentication of the plant. Due to high therapeutic value and low side effects, there has been a surge in the demand for herbal medicine. Therefore, it is essential to have a proper quality control profile of various medicinal plants used in different traditional system of medicine practiced in India. This helps to minimize adulteration of these medicinal plants, which mainly occurs due to improper knowledge regarding different vernacular names, varied geographical conditions, morphological and microscopic features[13,20]. In the present study, quality control standardization of E. alsinoides was performed following the standard guidelines. It is found to be the other source of Shankhpushpi and is an integral part of Dasapushpam, the ten most sacred flowers of Kerala[9,10]. The plant material showed presence of 0.60 % w/w of foreign matter. Generally, foreign matter determination represents adulteration of any kind of foreign matter in plant material, and this was found to be quite low in the plant material used in this investigation. Loss on drying is an essential tool to determine the moisture content in the plant material. Literature revealed that higher the moisture content in the plant material, higher will be the probability of deterioration, which might lead to variation in the content of active constituents[13]. The moisture content of the plant material used was found to be 8.28 % w/w, which was very low and the plant material has lesser chance of deterioration. Swelling index depicts the presence of gums, mucilage, pectin and hemicelluloses in plant material[13]. The results showed a swelling index of 0.91 ml/g, which concluded that the plant material consisted low quantities of swellable constituents. The bitterness value represents degree of bitterness, which was found to be 96.15 mg/100 g in the plant material used. The foaming index of a plant material is the foaming ability of an aqueous decoction of that plant materials and their extracts to form persistent foam, which confirms the presence of saponins in that plant material[20]. The result showed that the length of the foam of powdered root was found to be less than 1 cm therefore; foaming index was reported to be less than 100. The ash values represent the presence of naturally occurring inorganic salts and those adhering or deliberately added to the crude drug as an adulterant. The plant material showed the following values, total ash- 10.45 % w/w, acid-insoluble ash- 1.5 % w/w and water-soluble ash- 4.0 % w/w. The total ash value is an important quantitative standard, which determines the authenticity and purity of plant material and also helps in detecting the presence or absence of any foreign organic matter such as metallic salts and siliceous contaminants. Acid-insoluble ash is a part of total ash, which indicates the presence of silica, especially in a form of sand and siliceous earth whereas, water-soluble ash is the water soluble portion of the total ash[13]. From these results, it could be concluded that the quantity of water-soluble ash was found to be higher as compared to acid-insoluble ash. The extractive values give an idea about the nature of the chemical constituents present in the plant material and is also useful for the quantitative estimation of specific chemical constituents soluble in a solvent used for extraction. The extractive values in different solvents were found to be, water- 7.54, ethanol- 8.01, acetone- 6.32, chloroform- 6.74, petroleum ether- 5.29 and hexane- 4.73 % w/w, respectively. It was observed that the extractive values in polar solvents were found to be higher as compared to non-polar solvents. Heavy metals analysis showed the presence of As, Cd and Pb (Table 2), which were found to be within the prescribed limits of WHO[12]. Heavy metals get accumulate in medicinal plants either through foliage or through root systems, where the main contributing factor includes agricultural expedients such as cadmium-containing dung, organic mercury fungicides and insecticides containing lead and arsenate along with environmental pollutants, industrial and traffic emissions. Heavy metal analysis also provide valuable information regarding metals that are natural essential components of coenzymes, which play a major role in growth, photosynthesis and respiration[1]. From the aflatoxin analysis, it was found that aflatoxins B1, B2, G1 and G2 were not detected in the plant material. Aflatoxins are considered to be highly dangerous contaminants in any plant material and thus the plant E. alsinoides was found to be free from aflatoxins. The microbial count also revealed the presence of very low number of microbes that were within the prescribed limits of WHO guidelines[12]. Preliminary phytochemical screening of plant material is generally performed to identify the chemical nature of the active constituents present in the plant material[13]. Table 3 represents the major phytoconstituents which were identified in different extracts of E. alsinoides. The observation revealed the presence of mainly alkaloids, steroids, in non polar extracts, while polyphenols (tannins and flavonoids), glycosides were reported in polar as well as non polar extracts. Carbohydrates and amino acids were reported in polar extracts only. Preliminary phytochemical screening of different extracts of E. alsinoides revealed the presence of both polar as well as non polar components which have different pharmacological activities. Antioxidant and antiinflammatory activities have been reported to be exerted by flavonoids, which appear to increase capillary permeability and have been used in the treatment of various cardiovascular diseases[21,22]. Phenols neutralize oxygen-derived free radicals therefore, they are attributed as a strong antioxidants and free radical scavengers along with anticarcinogenic, antibacterial, antiinflammatory activities and cardiovascular activities. Tannins have shown to have strong astringent action and are reported to have antiviral, antioxidant, antibacterial and antiinflammatory activities. From literature, alkaloids have been reported to have numerous therapeutic importance in the treatment of parkinsonism, cancer, malaria, pain, inflammation, hypertension along with number of central nervous system disorders[13,20,21]. Luteolin has been reported to be the major flavonoid present in the E. alsinoides[1]. Further, luteolin has also been reported to exhibit potent anthelmintic activity[22] and therefore, it was selected as a biomarker and was quantified in different extracts of E. alsinoides. The results demonstrated well resolved peaks of luteolin in ethanolic, acetone, chloroform and aqueous extracts, while it was not detected in hexane and petroleum ether extracts (fig. 1). The content of luteolin in descending order was, ethanol extract 2.36, chloroform extract 1.68, acetone extract 1.02 and aqueous extract 0.015 % w/w respectively. The present investigation revealed that the n-hexane, petroleum ether, chloroform, acetone, ethanol and aqueous extracts of the plant E. alsinoides possessed anthelmintic activity in addition to its main pharmacological activity of memory enhancing. Further, the best anthelmintic activity was shown by the ethanol extract at the tested dose by causing paralysis and death of the earthworms. Table 4 shows the anthelmintic activities of E. alsinoides extracts on Indian adult earthworms. All extracts showed anthelmintic activity in the increasing order chloroform extract<aqueous extract<petroleum ether extract<n-hexane extract< acetone extract<ethanol extract. Ethanol extract has shown shortest time of paralysis (P=9.88 min) and death (D=21.54 min) at a dose of 100 mg/ml. It was observed that the ethanol extract of E. alsinoides at the dose of 100 mg/ml was more effective than albendazole at 20 mg/ml. Thus, from these results it could be concluded that the anthelmintic potential of E. alsinoides might be attributed to the presence of luteolin as the activity was observed to be maximum in ethanol extract, in which luteolin was present in higher concentration. Thus, the plant E. alsinoides wassuccessfully standardized and confirmed to possess anthelmintic property. All the 6 extracts of E. alsinoides exhibited anthelmintic activity on earthworms. Maximum activity was shown by ethanol extract. Also, luteolin was present in the maximum amount in ethanol extract, which may the contributing factor for the observed activity. Further isolation studies are required on the ethanol extract of E. alsinoides to confirm the action of luteolin present in the extract.
Figure 1: HPTLC chromatogram of luteolin in different extracts of E. alsinoides A: Standard peak of luteolin, B: ethanol extract of E. alsinoides, C: acetone extract of E. alsinoides, D: chloroform extract of E. alsinoides. E: aqueous extract of E. alsinoides, F: petroleum ether extract of E. alsinoides and G: n-hexane extract of E. alsinoides
| Groups | Treatment |
|---|---|
| I | Petri dish containing earthworms were treated with 1% gum acacia. |
| II | Petri dish containing earthworms were treated with albendazole 20 mg/ml. |
| III | Petri dish containing earthworms were treated with aqueous extract 100 mg/ml. |
| IV | Petri dish containing earthworms were treated with ethanol extract 100 mg/ml. |
| V | Petri dish containing earthworms were treated with acetone extract 100 mg/ml. |
| VI | Petri dish containing earthworms were treated with chloroform extract 100 mg/ml. |
| VII | Petri dish containing earthworms were treated with n-hexane extract 100 mg/ml. |
Table 1: Treatment of Earthworms with Vehicle, Standard and different extracts of E. Alsinoides
| Heavy metals | Content | Limit |
|---|---|---|
| Lead | 1.27 ppm | NMT 10 ppm |
| Arsenic | BLQ (LOQ: 0.5 ppm) | NMT 3.0 ppm |
| Cadmium | BLQ (LOQ: 0.1 ppm) | NMT 0.3 ppm |
Table 2: Heavy Metal Analysis of E. Alsinoides
| Phytoconstituents | Extract | |||||
|---|---|---|---|---|---|---|
| WE | EE | CE | HE | AE | PEE | |
| Alkaloids | - | + | + | - | - | - |
| Steroids | - | - | + | + | - | + |
| Anthraquinone glycoside | - | + | - | - | - | - |
| Cardiac glycoside | - | + | - | - | + | - |
| Cyanogenetic glycoside | - | - | - | - | - | - |
| Saponins | - | - | - | - | - | - |
| Polyphenolic compounds | + | + | + | + | - | - |
| Carbohydrates | + | + | - | - | - | - |
| Proteins | + | + | - | - | - | - |
| Amino acid | + | + | - | - | - | - |
Table 3: Preliminary Phytochemical Screening of E. Alsinoides
| Groups | Treatment with concentrations | Average time taken for paralysis of the worms (min) | Average time taken for death of the worms (min) |
|---|---|---|---|
| I | Vehicle (1% gum acacia) | _ | _ |
| II | Standard albendazole (20 mg/ml) | 12.08±0.46 | 29.58±0.43 |
| III | Aqueous extract (100 mg/ml) | 32.82±0.34*a | 47.84±0.25*a |
| IV | Ethanol extract (100 mg/ml) | 9.88±0.26 | 21.54±0.47 |
| V | Acetone extract (100 mg/ml) | 17.86±0.27 | 28.12±0.24 |
| VI | Chloroform extract (100 mg/ml) | 38.38±0.36*a | 58.84±0.33*a |
| VII | Petroleum ether extract (100 mg/ml) | 30.00±0.27*a | 44.00±0.50*a |
| VIII | n-Hexane extract (100 mg/ml) | 28.86±0.67* | 34.55±0.22* |
Table 4: Anthelmintic effects of different Extract of E. Alsinoides
Acknowledgements
The financial support provided by the Delhi Pharmaceutical Sciences and Research University, New Delhi for the present research work is duly acknowledged. Authors thank Dr. Anjula Pandey, Senior Scientific In charge, Division of Plant Exploration and Germplasm Collection, National Herbarium of Cultivated Plants (NHCP), ICAR-National Bureau of Plant Genetic Resources, Pusa Campus, New Delhi, 110012 for authenticating the plant material.
References
- Gupta AK, Tandon N, Sharma M. Quality Standards of Indian Medicinal Plants. New Delhi: ICMR; 2006.
- Goyal PR, Singh KP. Shankhpuspi (Evolvulus alsinoides Linn.): A Medicinal Herb. Int J Mendel 2005;22:124.
- Singh A. Review of Ethnomedicinal Uses and Pharmacology of Evolvulus alsinoides Linn. Ethnobotanical Leaflets 2008;12:734-40.
- Asolkar LV, Kakkar KK, Chakre OI. Glossary of Indian medicinal plants with Active Principles. New Delhi: Publication and Information Directorate, Council of Scientific and Industrial Research; 1992. p.27.
- Shah V, Bole PV. Botanical Identity of Shankhapushpi. Indian J Pharm 1961;23:223-4.
- Sethiya NK, Nahata SH, Dixit VK. An update on Shankhpushpi, a cognition-boosting Ayurvedic medicine. J Chin Integr Med 2009;7(11):1001-22.
- Vasisht K, Dhobi M, Khullar S, Mandal SK, Karan M. Norneolignans from the roots of Clitoria ternatea L. Tetrahedron Lett 2016;57:1758-62.
- Hussain AZ, Kumaresan S. Antiasthma, phytochemical and isolation of compound from Evolvulus alsinoides. 2014;IN 2014CH04990 A 20141024.
- Mehla J, Pahuja M, Dethe SM, Agarwal A, Gupta YK. Amelioration of intracerebroventricular streptozotocin induced cognitive impairment by Evolvulus alsinoides in rats: In vitro and in vivo evidence. Neurochem Int 2012;61(7):1052-64.
- Abubakar K, Ugwah-Oguejiofor CJ, Usman MN, Abubakar SB, Abdulkadir R. Evaluation of the anticonvulsant effect of the methanolic extract of Evolvulus alsinoides in mice. Scholars Acad J Pharm 2013;2(6):436-441.
- WHO. Soil-transmitted helminth infections. World Health Organization, Geneva, Switzerland 2019.
- Anonymous. Quality Control Methods for Medicinal Plant Materials (An Authorized publication of World Health Organization, Geneva). Delhi: A.I.T.B.S. Publishers & Distributors (Regd); 2002.
- Prasad SK, Kumar R, Patel DK, Sahu AN, Hemalatha S. Physicochemical standardization and evaluation of in-vitro antioxidant activity of Aconitum heterophyllum Wall. Asian Pac J Trop Biomed 2012;2:S526-31.
- Trease GE, Evans WC. Pharmacognosy. 15th ed. London: W.B. Saunders Company Ltd; 2002.
- Rajesh R, Chitra K, Paarakh, PM. In vitro anthelmintic activity of aerial parts of Aerva lanata (Linn) Juss. Int J Pharm Sci Drug Res 2010;2:69-271.
- Rajaqkaruna N, Harris CS, Towers GHN. Antimicrobial activity of plants collected from Serpentine outcrops in Sri Lanka. Pharm Biol 2002;40:235-44.
- Yadav AK, Tangpu V. Anthelmintic activity of ripe fruit extract of Solanum myriacanthum Dunal (Solanaceae) against experimentally induced Hymenolepis diminuta (Cestoda) infections in rats. Parasitol Res 2012;110;1047-53.
- Khan A, Tak H, Nazir R, Lone BA. In vitro and in vivo anthelmintic activities of Iris kashmiriana Linn. J Saudi Soc Agric Sci 2018;17:23540.
- Ayyanar M, Ignacimuthu S. Traditional knowledge of Kanitribals in Kouthalai of Tirunelveli hills, Tamil Nadu, India. J Ethnopharmacol 2005;102:246-55.
- Prasad SK, Sahu AN, Hemalatha S. Cytomorphological and physicochemical evaluations of Cryptocoryne spiralis (Retzius) Wydler. J Herbs Spices Med Plants 2012;18:304-7.
- Abou-Arab AAK, Abou DMA. Heavy metals in Egyptian spices and medicinal plants and the effect of processing on their levels. J Agri Food Chem 2000;48:2300-4.
- Wangchuk P, Pearson MS, Giacomin PR, Becker L, Sotillo J, Pickering D, et al. Compounds derived from the Bhutanese Daisy, Ajania nubigena, demonstrate dual anthelmintic activity against Schistosoma mansoni and Trichuris muris. PLoS Neglected Trop Dis 2016;10:1-18.