- *Corresponding Author:
- K. Murugan
Plant Biochemistry and Molecular Biology Laboratory, Department of Botany, University College, Thiruvananthapuram, Kerala-695 034, India
E-mail: harimurukan@gmail.com
| Date of Submission | 21 December 2016 |
| Date of Revision | 06 April 2017 |
| Date of Acceptance | 13 November 2017 |
| Indian J Pharm Sci 2018;80(1):52-64 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Bridelia retusa is traditionally used as an astringent and for treating rheumatic pains. Present study revealed that 2,4-dichlorophenoxyacetic acid alone or in combination with kinetin-fortified Murashige and Skoog medium showed good response in terms of callus from leaf explants of B. retusa. Growth hormones, pH, light, and carbon source influenced secondary metabolic pathways. The highest callus induction was 98.9 % with 2.5 mg/l N6-benzyladenine+2 mg/l 2,4-dichlorophenoxyacetic acid. The optimal fresh and dry weights of the calli were 1.9±0.04 and 0.45±0.03 g, respectively. The calli incubated in light on Murashige and Skoog medium with 4 % glucose containing 2.5 mg/l benzyladenine and 2 mg/l 2,4-dichlorophenoxyacetic acid at pH 3.5 yielded 2.8 mg/g of anthocyanins. Murashige and Skoog medium with glucose was optimal compared to sucrose for anthocyanin synthesis. Addition of kinetin inhibited anthocyanin accumulation. Pigmented calli transferred to half strength Murashige and Skoog medium with NH+/NO3 (1:4 ratio), 70 g/l sucrose and supplementation with benzyladenine+2,4-dichlorophenoxyacetic acid produced remarkable biomass production with anthocyanin synthesis when compared with the initial culture conditions. Suspension cultures of Murashige and Skoog medium containing 2.5 mg/l 2,4-dichlorophenoxyacetic acid and 2 mg/l benzyladenine at pH 5.0 induced anthocyanin synthesis into the medium with pH 4.4–4.6. HCl-water and HCl-ethanol extractions for 90 min was attempted to obtain the maximum amount of anthocyanin content. Temperature factors and pH against anthocyanin extraction and stability was analyzed. Enhancement in the degradation rate constant with a corresponding decline in the t1/2 values was seen with the increasing temperature at pH 1 and 4. Fractionation of anthocyanin was carried by high performance liquid chromatography coupled mass spectrometry revealed 09 fractions comprising acylated cyanidins and two peonidins. The major compounds were cyanidin 3-p-coumaroyl and feruloyl diglucoside-5-glucosides. Thus, the calli from leaf explants could be a good source for anthocyanin synthesis.
Keywords
Bridelia retusa, callus culture, phytohormones, anthocyanin, cyanidins, peonidins
Anthocyanins are water-soluble secondary metabolites, belonging to flavonoids, polyphenolic molecules containing 15 C chains with an aromatic ring, and one or more sugar groups attached at diverse positions to form hydroxylated basic structure. They occur mainly as glycosides of anthocyanidins. Six anthocyanidins are common in leaves, stems, roots, flowers and fruit such as pelargonidin, cyanidin, peonidin, delphinidin, petunidin and malvidin. Due to dietary antioxidant properties, many works have been attempted to analyse the properties of anthocyanins extracts from many plant species.
In vitro cell cultures are capable of synthesizing and accumulating diverse phytochemicals with medicinal or nutritional value. Alkaloids, saponins, anthraquinones, polyphenols and terpenes have been synthesized via in vitro culture. Among them anthocyanins have significance as natural dye and as antioxidant [1]. Characterization of promoter sequences, regulatory genes and transcription factors involved in anthocyanin synthesis facilitates the in vitro synthesis effectively [2]. Genetic engineering of anthocyanin synthesis emerges as an interesting industrial proposal, since this can eliminate seasonality, geographical and annual variations in plants, and also impact in yields due to pests and pathogens.
Bridelia retusa, commonly known as spinous kino tree; characterized by rigid leathery leaves with straight parallel lateral veins and spines on the bark of young twigs. The species is distributed mostly along hotter parts, throughout India. Medicinally, the plant is pungent, bitter, used for the removal of urinary concretions. Root and bark are ideal astringent and used against rheumatism. Chemical and pharmacological studies of different parts of Bridelia retusa have revealed the presence of sesquiterpenes, triterpenoids, flavonoids, and phenolic compounds [3]. Antinociceptive and antiinflammatory activities of the species due to the presence of gallic acid and ellagic acid as revealed by HPLC analysis from methanolic extract [4]. Banerjee and Bond [5] analysed the phenolic compounds and its antioxidant potential from this species. Islam et al. [6] screened the in vitro antioxidant, brine shrimp lethality bioassay and antimicrobial activities of extracts of B. retusa. Saurabh and Kala [7] attempted three solvent extracts such as chloroform, acetone and ethanol and showed presence of saponins. Steroids were reported in all the four solvent extracts except acetone extract. Flavonoids, phenol, saponin and tannins were present in polar solvents. Most of phytoconstituents were reported in polar solvent ethanol. The terpenoids were present in non-polar as well as polar solvents. This was further supported by Tatiya and Saluja [8]. Tribals used the bark aqueous extract, as curative against many ailments including wound healing. On preliminary analysis it was found rich in anthocyanins when compared to other phytochemicals [9]. In this scenario, the aim of the present study included in vitro callus culture, design of optimal extraction methods from suspension culture, purification and fractionation of the anthocyanins from B. retusa. Furthermore, the thermal stability of extracted anthocyanins in aqueous solutions against different pH was evaluated.
Materials and Methods
In vitro callus culture
B. retusa collected from its natural habitats i.e., deciduous forests of Ponmudi hills was used as source of explants-stem tip (0.5 cm) and leaf (0.5 cm2). Cultures were initiated on Murashige and Skoog (MS) medium [9] with 30 g/l sucrose and fortified with different phytohormones applied singly or in combinations such as naphthaleneacetic acid (NAA), 2,4-dichlorophenoxyacetic acid (2,4-D; 0.5-4 mg/l), N6-benzyladenine (BA; 0.5-3 mg/l). pH of the media was adjusted to 4 prior to adding 8 g/l agar, autoclaved (121°, 104 KPa) for 15 min and dispensed into 8×7 cm flasks (30 ml of culture medium per flask) closed with polypropylene caps. Five flasks containing four explants, each were cultured per treatment and each experiment was repeated twice. Cultures were incubated in a growth chamber at 26±2° under 16 h photoperiod provided by cool-white fluorescent tubes (45 μmol×m-2×s-1). Subcultures to media with the same composition were performed after 30 d of culture. Callus biomass accumulation was estimated after 60 d of culture based on fresh (FW) and dry (DW) weight measurements. Dry mass was obtained after drying at 45° to constant weight. Stock callus cultures were maintained under the same physical conditions described above with subcultures at 20-d intervals.
Cell suspension culture
Cell suspension culture was carried by transferring 3 g of friable calli into 250 ml Erlenmeyer flasks containing 100 ml of fresh half strength MS liquid medium [10] supplemented with different concentrations of 2,4-D (0.5-2.5 mg/l) and BA (3 mg/l) and sucrose (30 g/l), pH 4.8. The suspension cultures were regularly subcultured in the MS liquid medium at 20-60 d intervals agitated on a rotary shaker (110 rpm, 25°) and kept in dark. For evaluation of growth curve, the cells were separated from the stock by filtration under suction. About 1±0.1 g cells were further inoculated into 50 ml of fresh MS liquid medium in a flask. Growth of cell suspension culture, cell viability and anthocyanin content were determined with sets of flasks harvested at regular intervals from day 0 of subculture up to 60 d. Cells were isolated from the medium by filtration using nylon mesh and weighed as fresh weight. Cells viability was determined by incubating 2 ml samples in 0.25 % Evan’s blue stain for 5 min and then at least 500 cells were counted and this was repeated thrice.
Induction of anthocyanin synthesis
About 2 g of cell suspension were subjected to different temperatures (22° or 36°); light intensities (45, 67 and 80 μmol×m-2×s-1); different carbon source: sucrose, glucose, maltose, fructose concentrations (1, 2, 3 and 4 %, respectively); different total nitrogen concentrations (50, 60, 70 and 80 mM) and different ratios of NH4+ to NO3-(1:1; 1:2; 1:3; 1:4). Cell biomass and anthocyanin content were quantified and each experiment was repeated thrice.
Purification of anthocyanin
About 50 g of fresh cells were extracted with 25 ml of two different extraction solvents: 0.01 % (v/v) HCl-acidified water and 0.01 % (v/v) HCl-acidified ethanol. The extraction was done at room temperature with constant shaking at 100 rpm for 60 min. The extract was filtered through Whatman No.1 paper, and the residue was subjected to extraction until it becomes colourless. Filtrates were mixed and used for anthocyanins purification. Suitable extraction solvent was identified according to the highest amount of anthocyanin content obtained. Similarly, the extraction ratios (sample:solvent; 1:10, 1:15 and 1:20) and extraction periods 30 to 120 min were studied [11-14].
After optimal extraction, the sample was filtered through Whatman No.1 paper, and then dried by rotary evaporator at 40° under vacuum conditions. The concentrated sample was loaded onto a C18 open chromatographic column of silica. Elution was performed using three solutions with specific properties geared to optimal anthocyanin purification. The sample was initially eluted with 0.01 % HCl-acidified distilled water to eliminate organic acid and sugar, followed by ethyl acetate to remove phenolic compounds and finally by acidified ethanol (1 % w/v citric acid, pH 2.9). The purified anthocyanin fractions were collected for further analysis.
Temperature stability and pH
pH of the purified anthocyanin solution was adjusted to 1 and 4 by 0.1 M HCl and 7.0 by 0.1 M NaOH at 25°. The absorbance of all samples was measured between 400 to 700 nm. The assay was based on the colour absorbance within 3 min. The 5 ml purified anthocyanin (100 μg/ml) at the stable pH as determined previously was incubated in a water bath at the controlled temperatures of 20, 40, 60 and 80°. Samples were periodically collected during 1-8 h. Each sample was cooled in an ice bath prior to absorbance measurement for evaluating the anthocyanin degradation. Anthocyanin (percent remaining)=A1 or A2 or A3/Aoh×100, where, Aoh=absorbance at time 0 h; A1, A2 or A3=absorbance at time 1 h, 1.5 h or 2 h at different temperatures.
High performance liquid chromatography/mass spectrometry (HPLC/MS)
The purified anthocyanins were mixed with 0.5 % HCl, and then syringe filtered (0.45 μm). The injection volume was 10 μl. The flow rate was 0.8 ml/min and maintained at 35°. The mobile phases comprise of 0.05 % (v/v) trifluoroacetic acid (TFA, solvent A) in distilled water and 100 % acetonitrile (solvent B). The gradient elution program was performed as follows: solvent A at 95-80 % from 0 to 20 min, at 80-60 % from 20 to 50 min. The chromatogram was then compared with the standard chromatogram using cyanidin-3- glucoside.
Statistical analysis
All experiments were carried out in three replications and presented as mean±standard deviation (SD). The data were statistically analysed by one-way analysis of variance (ANOVA). The level of statistical significance was determined at p<0.05.
Results and Discussion
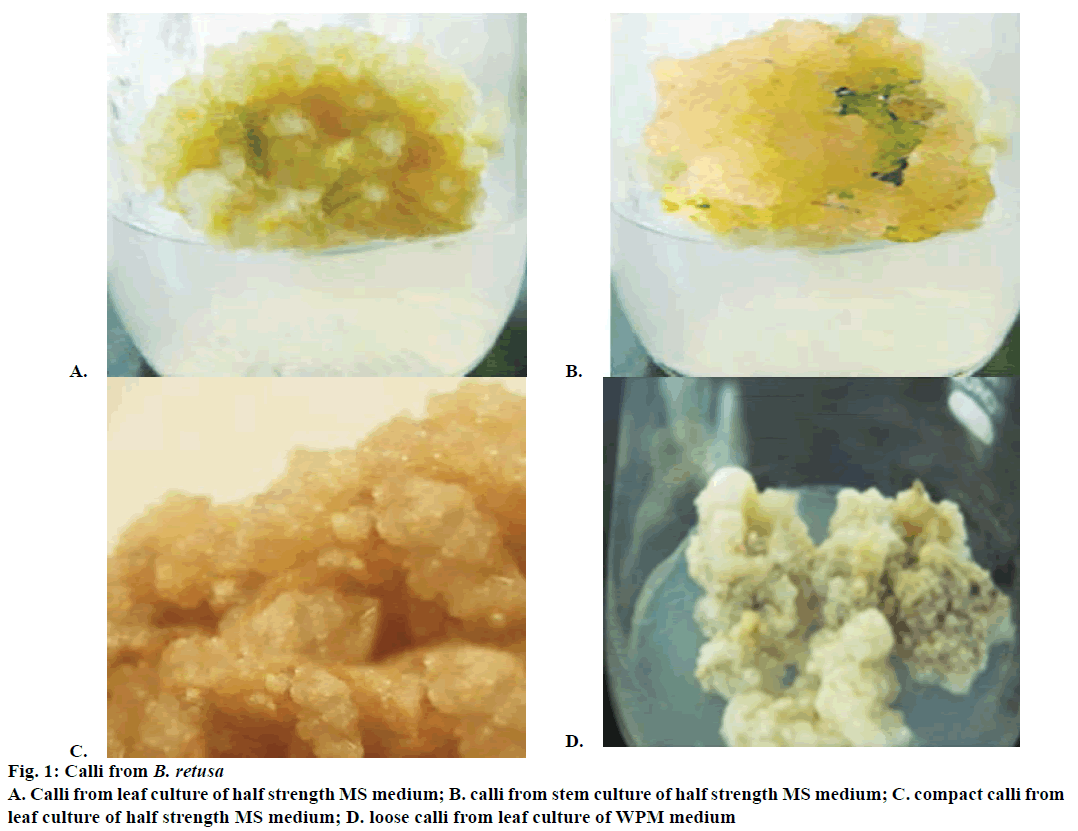
Remarkable callus induction was noticed in B. retusa after 6th w of inoculation. Generally, the calli formed were creamy. The percent of callus induction ranged from 17.8 to 98.9. The highest callus induction i.e., 98.9 % was found with the leaf explants cultured on MS medium supplemented with 2 mg/l 2,4-D and 2.5 mg/l BA (Table 1). The calli yielded from such treatment were found to be completely friable. NAA also induced calli optimally in combinations with BA. However, the calli yielded from such treatment were found to be loose (Figure 1A). 2,4-D and BA recorded the optimal callus fresh and dry weights of 1.7±0.02 and 0.65± 0.03 g, respectively with leaf explant. Meanwhile, in the case of stem the respective values were 1.2±0.04 and 0.59±0.02. After 4th w of sub-culturing, the calli were proliferated and enlarged profusely (Figure 1B). Most of the calli formed during this phase were found to be compact (Figure 1C). Leaves cultured on Woody plant medium (WPM) yielded insignificant brownish calli compared to those cultured on MS medium (Figure 1D).
| Callus induction | Leaf | Stem | ||||||
|---|---|---|---|---|---|---|---|---|
| NAA (mg/l-1) |
BA (mg/l-1) |
2,4-D | Leaf (%) | Stem (%) | Freshweight (g) |
Dryweight (g) |
Freshweight (g) |
Dryweight (g) |
| 0.5 | 0.2 | 17.8±0.8 | 12±0.88 | 1.3 ±0.05 | 0.66±0.01 | 1.08 ±0.25 | 0.56±0.3 | |
| 0.5 | 1 | 27±0.3 | 20.5±0.32 | 1.1±0.04 | 0.49±0.06 | 1.03±0.01 | 0.5±0.08 | |
| 0.5 | 5 | 31±0.05 | 22±0.16 | 0.93±0.02 | 0.32±0.2 | 0.99±0.03 | 0.39±0.09 | |
| 1 | 0.2 | 24.7±0.2 | 20±0.28 | 0.78±0.09 | 0.31±0.05 | 0.61±0.1 | 0.29±0.06 | |
| 1 | 1 | 45.4±0.13 | 35±0.33 | 0.79±0.05 | 0.28±0.06 | 0.59±0.12 | 0.25±0.04 | |
| 1 | 5 | 50±0.9 | 40±0.27 | 0.78±0.32 | 0.26±0.1 | 0.58±0.13 | 0.24±0.01 | |
| 2 | 0.2 | 24±0.4 | 19±0.54 | 0.74±0.16 | 0.21±0.2 | 0.54±0.06 | 0.24±0.001 | |
| 2 | 1 | 39±0.5 | 25.6±0.16 | 0.63±0.09 | 0.13±0.08 | 0.43±0.09 | 0.12±0.05 | |
| 2 | 5 | 42±0.25 | 30±0.63 | 0.58±0.03 | 0.11±0.07 | 0.38±0.03 | 0.10±0.007 | |
| 0.5 | 0.25 | 21±0.44 | 15.7±0.05 | 0.97±0.04 | 0.43±0.03 | 0.67±0.04 | 0.4±0.008 | |
| 1 | 0.5 | 50.7±0.26 | 41±0.22 | 1.08±0.01 | 0.5±0.07 | 0.9±0.08 | 0.45±0.05 | |
| 1.5 | 1 | 71.4±0.77 | 65±0.17 | 1.2±0.08 | 0.56±0.01 | 1±0.02 | 0.46±0.002 | |
| 2 | 1.5 | 88±0.83 | 72±0.94 | 1.3±0.03 | 0.59±0.02 | 1.02±0.07 | 0.52±0.005 | |
| 2.5 | 2 | 98.9±0.17 | 92±0.27 | 1.7±0.02 | 0.65±0.03 | 1.2±0.04 | 0.59±0.02 | |
| 3 | 2.5 | 98±0.29 | 90±0.19 | 1.55±0.01 | 0.61±0.09 | 1.1±0.05 | 0.57±0.009 | |
Table 1: Effects of NAA, BA and 2,4-D on callus induction (%) from leaf and stem explants and on fresh and dry weight of calli of B. retusa in the ms medium
Anthocyanin formation was initiated in the leaf calli from 25 to 30 d in the MS medium with 2,4-D. Higher concentrations of 2,4-D (>2.5 mg/l) reduced anthocyanin synthesis. MS medium with BA induced optimal callus formation with poor anthocyanin content. Similarly, NAA and BA treatments also showed poor anthocyanin synthesis. 2,4-D or NAA in combination with BA showed optimal anthocyanin synthesis when compared to medium fortified with 2,4-D or NAA only. Medium containing 2 mg/l 2,4-D and 2.5 mg/l BA developed 1.7±0.02 g callus from leaf explant (60 d) with the highest amount of anthocyanin (3.4 mg/g; Table 1). Meanwhile, stem calli yielded less amount of anthocyanin content i.e., 2.1±0.09. Replacement of BA with kin resulted poor amount of anthocyanin in the calli. BA and NAA combination was inferior to 2,4-D and BA in anthocyanin synthesis (Table 1). Meanwhile, other combinations of NAA and BA or NAA and 2,4-D did not yield remarkable anthocyanin level, but showed ideal callus growth (Table 1). Sub-culturing of calli in different media with respective hormones showed the anthocyanin level more or less in a similar manner.
Effect of pH, light and carbon source, negative correlation was noticed between pH and anthocyanin content i.e., higher the pH lower the anthocyanin content (Table 2). Liquid MS medium containing 2,4-D and BA with pH 4.2 showed the highest anthocyanin formation followed by pH 4.4 (Table 2). At higher pH, i.e. 5.0, anthocyanin formation was marginal.
| Temperature (°) |
Light intensity (µmol×m-2×s-1) |
Anthocyanin content (mg/g, FW) |
Biomass (g) | pH | Anthocyanin level (mg/g) |
|---|---|---|---|---|---|
| 22 | 80 | 6±0.08 | 0.67±0.02 | 3.4 | 2.8±0.01 |
| 67 | 4±0.12 | 0.55±0.13 | |||
| 45 | 3±0.36 | 0.53±0.04 | 3.6 | 3.4±0.08 | |
| 24 | 80 | 7±0.07 | 4.9±0.21 | ||
| 67 | 6.2±0.55 | 2.98±0.08 | 3.8 | 4.8±0.05 | |
| 45 | 3±0.29 | 1.73±0.05 | |||
| 26 | 80 | 6.8±0.16 | 8.3±0.03 | ||
| 67 | 4±0.37 | 2±0.05 | 4 | 5.1±0.03 | |
| 45 | 3±0.65 | 1.77±0.07 | |||
| 28 | 80 | 7.3±0.28 | 14.8±0.34 | ||
| 67 | 4.1±0.48 | 3±0.045 | 4.2 | 7.9±0.09 | |
| 45 | 2.8±0.63 | 2.3±0.08 | |||
| 30 | 80 | 8.2±0.78 | 22.7±0.093 | 4.4 | 6.6±0.04 |
| 67 | 4±0.37 | 5.3±0.07 | |||
| 45 | 3±0.21 | 3±0.056 | 4.6 | 4±0.06 | |
| 32 | 80 | 6.7±0.29 | 9±0.04 | ||
| 67 | 4±0.03 | 6±0.03 | |||
| 45 | 3±0.17 | 2.8±0.01 | 4.8 | 3.4±0.05 | |
| 34 | 80 | 5.2±0.21 | 4±0.01 | ||
| 67 | 3.7±0.34 | 2.5±0.08 | |||
| 45 | 2.4±0.11 | 1±0.01 | 5 | 2.5±0.02 | |
| 36 | 80 | 3.5±0.23 | 4±0.03 | ||
| 67 | 2±0.07 | 2.3±0.26 | 5.2 | 1.7±0.41 | |
| 45 | 1.4±0.028 | 1.5±0.37 |
Table 2: Anthocyanin content in callus cultures of B. retusa against different temperature and light intensity and pH
Similarly, cells were analysed for anthocyanin level against different regimes of light irradiation and temperature. Initially anthocyanin content increased gradually with temperature and reached the peak at 30°. Temperature above 30° showed a negative impact on pigment accumulation irrespective of the light irradiation. Meanwhile, increasing on light irradiation had a positive impact on anthocyanin synthesis (Table 2). Cells at 30° and 80 μmol×m-2×s-1 achieved the highest anthocyanin content (8.2±0.78 mg/g, FW) and biomass accumulation (22.7±0.093) i.e. room temperature connected with high light irradiation is the best physical parameter to anthocyanin synthesis. Liquid MS medium with BA and 2,4-D at pH 4.2 were further analysed for different carbohydrate sources and concentrations required for anthocyanin synthesis (Table 3). Low carbon source, the suspension culture showed poor rate of proliferation and turned colourless. Out of the various carbon sources tested, MS medium having 4 % glucose produced the maximum anthocyanin (8.4 mg/g) and was followed by 3 % sucrose (Table 3). Subsequent sub-culturing displayed a stable yield of anthocyanin. The anthocyanin formation was accelerated significantly with glucose and sucrose. Meanwhile, fructose and maltose was insignificant towards anthocyanins induction.
| Concentrations (%) | ||||||
|---|---|---|---|---|---|---|
| Carbon source | 1 | 2 | 3 | 4 | 5 | |
| Glucose | 4.9 ±0.85 | 6.2±0.43 | 7.4±0.72 | 8.4±1.1 | 8.5±0.93 | |
| Fructose | 2.4±0.04 | 3.6±0.05 | 4.1±0.92 | 4.6±0.03 | 4.5±0.08 | |
| Maltose | 2.8±0.02 | 3.3±0.03 | 4.6±0.77 | 5±0.32 | 5.3±0.33 | |
| Sucrose | 3.4±0.2 | 4.92±0.27 | 5.8±0.25 | 6.5±0.26 | 6.9±0.21 | |
| Nitrogen concentrations (mM) | 50 | 60 | 70 | 80 | ||
| Anthocyanin (mg/g) | 6.8±1.4 | 8.5±1.4 | 6±0.15 | 6±0.98 | ||
| NH4:NO3 ratio | 1:1 | 1:2 | 1:3 | 1:4 | ||
| Anthocyanin (mg/g) | 3.7±0.39 | 5.6±0.65 | 7.9±0.25 | 8.7±0.19 | ||
Table 3: Effect of carbohydrate sources, nitrogen dose and NH4:NO3 ratio on anthocyanin content of B. retusa
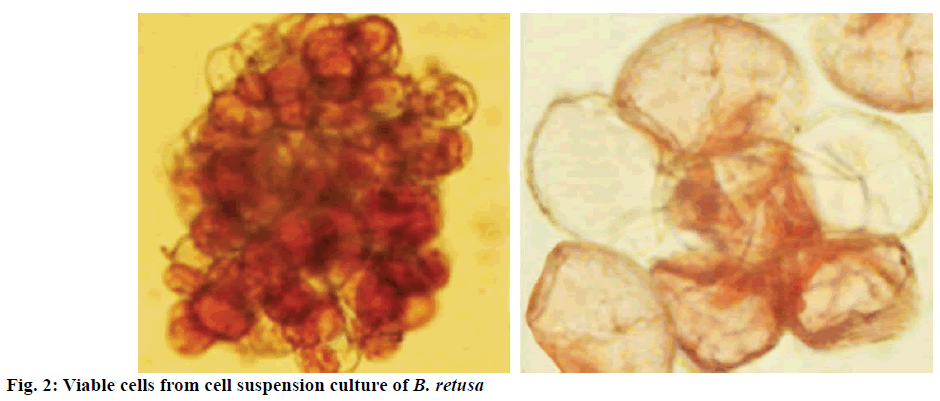
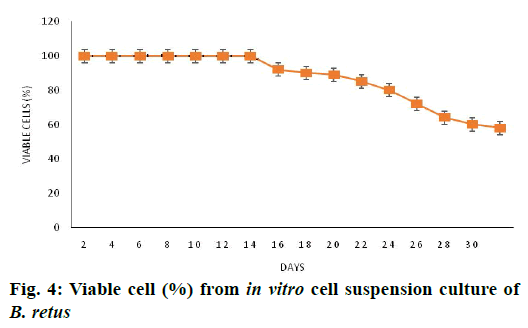
In the present study, friable and fresh calli clumps grown on 2.5 mg/l BA and 2.0 mg/l 2,4-D were used for initiating cell suspension culture. Cell growth was measured in terms of fresh mass of cells in the liquid MS medium supplemented with 2.5 mg/l BA and 2.0 mg/l 2,4-D once in 2 d (Figure 2). The growth of suspension cultures indicated that the growth rate of cells was slow initially (3 d-lag phase). However, a marked increase was seen from 6th d onwards in terms of mass (exponential phase). Maximum fresh weight was reached on 20th d and was about 20-fold higher than the initial mass. Subsequently, the rate of growth was stable (stationary phase). Later, a gradual reduction in cell density was noticed. Based on the results, subculturing to new fresh media was carried between 15 and 20 d of incubation i.e. the end of exponential growth phase. This may be due to the exhaustion of nutrients in the medium or accumulation of toxic metabolites. The cell viability was 80 % on 22 d of culture (Figure 2) and then marginally declined.
Phytohormones are compounds used in in vitro cultures as signalling molecules for initiating plant growth and development. Auxin, cytokinin, 2,4-D are proven phytohormones in inducing callus and cell proliferation in suspension cultures in many medicinal plant species. The present results in B. retusa revealed that 2,4-D in the culture media was critical to induce callus and cell suspension formation. The effectiveness of 2,4-D in callus or cell growth induction is through stimulating cell division and parallelly suppressing organogenesis. Further, the MS medium fortified with 2,4-D alone displayed significant delay in the initiation of calli or cells. Therefore, BA is also added to induce additional physiological impact. The optimum callus and cell suspension induction was noticed in MS medium fortified with 2 mg/l 2,4-D and 2.5 mg/l BA.
Variation was also noticed with the media in callus induction in B. retusa i.e. calli isolated from WPM culture media were poor with loose calli than MS media. Maximum anthocyanins content was noticed with HCl-acidified ethanol extraction (0.01 %) i.e. 8.6± 1.3 mg/g at 90 min in the sample-solvent ratio of 1:20. The amount in terms of yield of recovery decreases at the subsequent extractions when compared to first extraction. About 4.63±0.09 mg/g anthocyanins were obtained with the ratio 1:100 for 90 min. Increase in time of extraction is directly related with anthocyanins content with all the ratios (1:20 to 1:100). Usually, by enhancing extraction time from 90 to 120 min with the optimum ratio of solvent to sample (1:20-1:100) increase the yield of anthocyanins. However, in the present study the extraction time using acidified ethanol as solvent, the recovery of yield was high from first to third extraction (Table 4). Generally, anthocyanins were extracted with various solvents like water, ethanol, methanol or acetone and marginal amount of acid to obtain the flavylium cationic form which is relatively stable in acid medium (Table 4). Preferable solvents are water and ethanol because of less toxic, especially from food and consumable samples, rather than using methanol or acetone.
| Anthocyanin (mg/g) extraction by HCl-acidified ethanol | |||||
| Ratio | Time (min) | 1st extraction | 2nd extraction | 3rd extraction | Total amount |
| 30 | 2±0.04 | 1±0.07 | 0.89±0.001 | 3.89±0.5 | |
| 1:20 | 60 | 2.8±0.66 | 1.4±0.03 | 0.81±0.01 | 5.01±0.22 |
| 90 | 5.2±0.88 | 2.1±0.13 | 1.3±0.06 | 8.6±1.3 | |
| 1:40 | 30 | 1.82±0.22 | 0.89±0.03 | 0.75±0.02 | 3.46±0.07 |
| 60 | 2.2±0.05 | 0.9±0.01 | 0.79±0.01 | 3.89±0.042 | |
| 90 | 3±0.62 | 1.4±0.09 | 0.71±0.03 | 5.11±0.62 | |
| 1:60 | 30 | 1.73±0.35 | 0.76±0.009 | 0.67±0.03 | 3.16±0.08 |
| 60 | 2.3±0.07 | 0.87±0.004 | 0.68±0.005 | 3.85±0.15 | |
| 90 | 2.78±0.05 | 1.27±0.07 | 0.85±0.01 | 4.9±0.68 | |
| 1:100 | 30 | 1.61±0.08 | 0.65±0.003 | 0.60±0.02 | 2.86±0.08 |
| 60 | 2.1±0.03 | 0.80±0.04 | 0.65±0.002 | 3.55±0.06 | |
| 90 | 2.65±0.04 | 1.18±0.02 | 0.8±0.08 | 4.63±0.09 | |
| Anthocyanin (mg/g) extraction by HCl-acidified water | |||||
| Ratio | Time (min) | 1st extraction | 2nd extraction | 3rd extraction | Total amount |
| 30 | 1.7±0.02 | 0.72±0.05 | 0.59±0.031 | 3.01±0.03 | |
| 1:20 | 60 | 1.7±0.007 | 0.8±0.04 | 0.61±0.003 | 3.11±0.09 |
| 90 | 3.3±0.15 | 1.56±0.003 | 1±0.06 | 5.86±0.63 | |
| 1:40 | 30 | 1±0.09 | 0.66±0.002 | 0.54±0.007 | 2.2±0.005 |
| 60 | 1.5±0.01 | 0.77±0.04 | 0.65±0.07 | 2.92±0.022 | |
| 90 | 4.01±0.064 | 1.1±0.09 | 0.61±0.008 | 5.31±0.62 | |
| 1:60 | 30 | 1.53±0.05 | 0.65±0.023 | 0.61±0.009 | 2.79±0.001 |
| 60 | 1.88±0.002 | 0.75±0.023 | 0.6±0.012 | 3.23±0.02 | |
| 90 | 2.55±0.026 | 1.15±0.033 | 0.6±0.005 | 4.33±0.056 | |
| 1:100 | 30 | 1.54±0.016 | 0.61±0.045 | 0.560±0.047 | 2.71±0.014 |
| 60 | 1.77±0.016 | 0.67±0.028 | 0.56±0.019 | 3±0.018 | |
| 90 | 2.41±0.012 | 1±0.032 | 0.53±0.029 | 3.94±0.016 | |
Table 4: Extraction of Anthocyanin by 0.01% HCL-Acidified Ethanol and Water (Thrice) at Different Ratios and Extraction Times
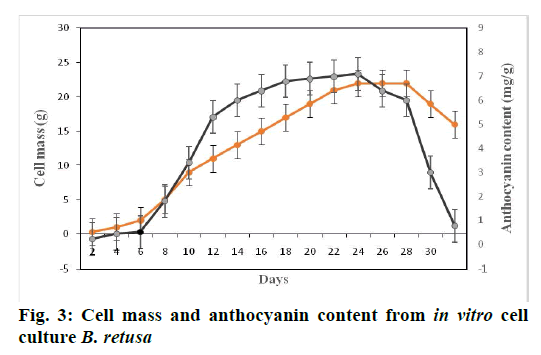
Anthocyanin extracted by using acidified water and acidified ethanol using 1:20 and 1:100 ratio for 90 min was filtered through Whatman No.1 paper and then evaporated under vacuum. Crude concentrated anthocyanin was loaded to C18 column of silica. Elution was performed using three solutions such as 0.01 % HCl acidified distilled water to eliminate organic acid and sugar compounds, followed by ethyl acetate to exclude phenol compounds and finally by acidified ethanol. The purified anthocyanin fractions were collected for subsequent analysis. To evaluate, the ideal period of cell suspension culture for anthocyanin, cells were collected regularly at 2 d intervals throughout the experimental periods. Quantity of anthocyanin increased proportionally with days and reached maximum level at 22nd d (7.1±0.04 mg/g, FW), after which the levels declined. Subsequently, the cells became brownish when retained on the same medium without sub-culturing, probably due to degradation of anthocyanin (Figures 3 and 4). About 60 mM nitrogen concentration yielded an optimal anthocyanin synthesis when compared with other concentrations (Table 3), without altering the biomass remarkably. Similarly, 1:4 ratio of NH4+:NO3" enhanced the anthocyanin content when compared to MS medium ratio 1:2 or others (Table 4).
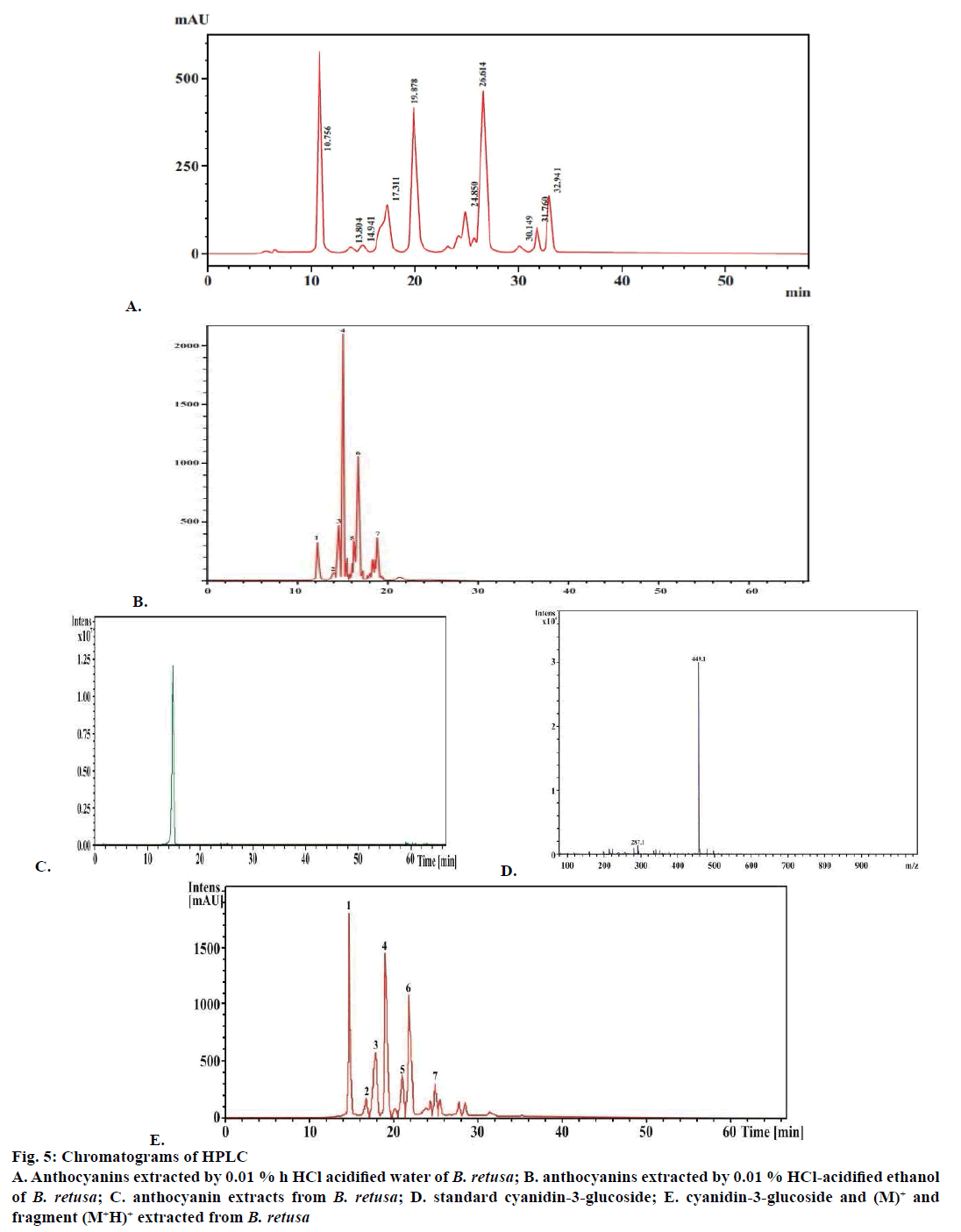
The determination cyanidin-3-glucoside content of purified anthocyanin was analysed by HPLC at 520 nm at the retention time 10.8 min was the peak of cyanidin- 3-glucoside as extracted by acidified water (1:20) and acidified ethanol (1:100; Figure 5A, B, C and D) by comparing with the standard with the retention time of 10.562 min (Figure 5C and D). The amount of cyanidin- 3-glucoside by acidified water (1:20) and acidified ethanol (1:20) were 288 and 260 mg/g, respectively.
Figure 5: Chromatograms of HPLC
A. Anthocyanins extracted by 0.01 % h HCl acidified water of B. retusa; B. anthocyanins extracted by 0.01 % HCl-acidified ethanol of B. retusa; C. anthocyanin extracts from B. retusa; D. standard cyanidin-3-glucoside; E. cyanidin-3-glucoside and (M)+ and fragment (M+H)+ extracted from B. retusa
Identification of anthocyanins from reverse phase HPLC/MS analysis were used to identify the anthocyanins at 520 nm indicated the cyanidin- 3-glucosides eluted at peak 1 as compared to the standard. This was confirmed by retention time, spectroscopic characteristic, and fragmentation pattern between sample extract and the standard solution (Figure 5E). Peak 1 was cyanidin-3-glucoside with molecular ion (M+H)+ at m/z 449 and a fragment ion (M+H-162) at m/z 287 (Figure 5E). The molecular ion (M+) and fragment (M+H)+ from HPLC/MS analysis indicated 7 anthocyanin fractious. Non-acylated forms such as cyanidin-3-glucoside (449 and 287), pelargonidin-3-glucoside (433 and 271), peonidin-3-glucoside (463 and 301) and malonyl derivatives or ethylmalonyl derivatives (acylated forms) includes cyanidin-3-(6-malonylglucoside) (353 and 287), pelargonidin-3-(6-malonylglucoside) (519 and 271), peonidin-3-(6-malonylglucoside) (549 and 301) and cyanidin-3-(6-ethylmalonylglucoside) (563 and 287), respectively.
The stability of anthocyanin is evaluated against pH in relation to colour, spectral features and absorbance of light (Table 5). Significant light absorbance was noticed with pH 1 at 520 nm and subsequently, it was decreased with increasing pH. Intense red colour was changed in to orange at higher pH 4. Similarly, at pH 7 and 9, the maximum absorbance at 570-630 nm and colour changed from light purple to blue.
| pH | Temperature (c) | k (h-1) | T1/2 (h) |
|---|---|---|---|
| 1 | 20 | 0.21 (0.95) | 240 |
| 40 | 0.23 (0.98) | 216.3 | |
| 60 | 0.67 (0.97) | 73.8 | |
| 80 | 4.52 (0.97) | 11.6 | |
| 4 | 20 | 0.86 (0.86) | 59.2 |
| 40 | 0.96 (0.96) | 53 | |
| 60 | 1.51 (0.91) | 30.5 | |
| 80 | 4.65 (0.97) | 10 |
Table 5: Kinetic Parameters of Disintegration of Anthocyanins at Various Temperatures
Thermal labile property coupled with zero-order reaction kinetics from 20° to 80° in aqueous solutions at pH 1 and pH 4 was also evaluated. Enhancement in the degradation rate constant (k) at increasing temperature coupled with an analogous decline in the t1/2 values was seen at pH 1 and 4 (Table 5). Thus, it is possible to interpret that pH had a pertaining role on the thermal stability of anthocyanins of B. retusa. At pH 1, anthocyanin degradation was almost insignificant (100 %) with the temperature range from 20 to 40° but was changed at 80° (66 %) within 8 h. Meanwhile, the anthocyanin degradation was remarkable at pH 4 than at pH 1 with the temperature range from 20 to 60° within a period of 8 h. When the temperature was raised to 80°, the rate of degradation (60 %) was not much different at pH 1 compared to pH 4.
Callus and cell suspension culture coupled with anthocyanin synthesis was established in B. retusa. Hormonal combinations vary among the plant species for establishing in vitro cultures. For example, the induction of somatic embryogenesis in red pigmented callus of Euphorbia pulcherrima was achieved on MS supplemented with NAA (2.69 μM) and 6-(γ,γ- dimethylallylamino)purine (2iP). Reduced level of NAA (0.54 μM) in the same medium caused maturation of somatic embryoids [15]. Liu et al. [16] reported 5 to 120 mg/l of thidiazuron increased the regeneration frequency and improved the quality of the regenerated buds within 35 d culture on MS medium. Significant in vitro culture of Parkia biglobosa was in the medium supplemented with 1 mg/l BA and 0.2 mg/l NAA as the optimum growth regulator combination for callus induction [17]. Catapan et al. [18] reported the best basal media for callus proliferation was 0.62 μM BA supplemented in MS1/2 medium. The explants-derived callus cultures of MS medium fortified with 21.48 μM α-NAA was superior for callus growth. Medium with 2.22 μM BA and 2.26 μM 2,4-D at pH 3.5 yielded the highest amount of anthocyanins. Suspension cultures of MS medium containing 2.26 μM 2,4-D and 2.22 μM BA at pH 5.0 initiated anthocyanins. Eugenio et al. [19] cultured diverse ornamental cacti using different media and hormonal combinations. Soliman et al. [20] noticed the best medium for callus induction in fig species was MS supplemented with 2 mg/l 2,4-D and 0.2 mg/l kin. Similarly, the callus failed to induce organogenesis on media containing a combination of BA and kinetin. Remarkable shoot and root formation percent was obtained with 2 mg/l thidiazuron and 4 mg/l 6-(γ,γ-dimethylallylamino)purine (2iP) and 1 mg/l indole-3-butyric acid, respectively.
Cell suspension culture and the purification of anthocyanin in B. retusa was at par with purified anthocyanins from red cabbage, purple-fleshed potato, fruit residues of Vaccinium uliginosum and mulberry anthocyanins [11-14,21,22].
Temperature above 30° showed a negative impact on pigment accumulation irrespective of the light irradiation in B. retusa. This may be due to the hydrolysis of glycosidic bonds of glucosidases, resulting in pigment degradation and formation of other degraded products [23]. Similarly, Narayan et al. [24] studied temperature induction versus anthocyanin synthesis in carrot and revealed that it was speciesdependent i.e. cultures of Daucus carota revealed the peak anthocyanin content at 30° when compared to lower and higher temperatures.
Cells at 30° and 80 μmol×m-2×s-1 achieved the highest anthocyanin content (8.2±0.78 mg/g, FW) and biomass accumulation (22.7±0.093) i.e. room temperatures coupled with high light irradiation are the best physical parameters to anthocyanin synthesis [25]. Nakamura et al. [26] reported a reverse trend i.e. darkness promote anthocyanin accumulation in strawberry. Generally, light activate the key enzymes involved in anthocyanin biosynthesis, like phenylalanine ammonia-lyase and chalcone synthase, whose expression is increased in response to high irradiation. For example, Zhang et al. [27] noticed such an activation of enzymes in grapes with light irradiance correlated with anthocyanin content.
Addition of sugars in the culture medium plays multiple roles like source of energy, structural components and also metabolic signals regulating the gene expression [28]. Vitrac et al. [29] and Narayan and Venkataraman [30] reported that sucrose signal transduction leading to anthocyanin synthesis via the phosphorylation of hexoses by hexokinase in grapes and carrot.
The present findings revealed that the supplementation of BA at an optimum concentration with 2,4-D is required to produce calli or cell culture with viable cell morphology. Meanwhile, Saikia et al. [31] reported that the hormonal combination of 2,4-D and kinetin was found to be effective in producing optimum callus induction in Aquilaria malaccensis i.e. 70-73 %. However, Rashid et al. [32] noticed that the addition of kinetin affected the callus formation negatively in Triticum aestivum. In the present study, MS medium fortified with BA together with 2,4-D (2 mg/l) induced callus formation significantly and the values are statistically significant at 5 % level. Narayan et al. [24] studied the effect of phytohormones in anthocyanin synthesis i.e. 2,4-D has been shown to inhibit the production of anthocyanins in carrot. Contradictorily, in the present study 2,4-D induced biomass and anthocyanin content along with the combination BA.
Effective plant regeneration with compact callus was reported in Cinnamomum tamala with WPM medium by Sharma and Nautiyal [33]. However, Aviles et al. [34] noticed friable calli in Juglans regia cultured on MS medium with poor browning.
In contrast with the present study in B. retusa, Behbahani et al. [35] showed significant callus induction from leaf explants with 2 mg/l 2,4-D in WPM medium, in Barringtonia racmosa than in MS medium. Therefore, it has been justified that nutritive media influence the morphogenic responses among the species being cultured and the explants employed. Similarly, the duration related with induction response of callus formation also showed variation. Further studies were carried to analyse the impact of medium components towards callus morphogenic responses such as macro and micronutrients available in different basal culture media MS medium differ from WPM medium in terms of potassium nitrate and also other nutrients such as ammonium nitrate and calcium chloride. Similarly, WPM medium contain calcium nitrate, potassium sulphate and manganese sulphate [36]. Therefore, these nutrients regulate the in vitro culture significantly among the species.
Zhou et al. [37] noticed similar observation in Prunus incisa due to nutrient depletion or oxidative stress by toxic by-products in the culture medium or phytochemicals synthesized by stressed cells. Narayan and Venkataraman [30] also noticed alleviation of the anthocyanin content in carrot by NH4+ to NO3- ratio. Generally, callus cultures comprises a mixture of subpopulations of cells differing in their morphoforms, gene expression, morphogenetic caliber, and thus, in the ability to produce anthocyanin [38]. Cisse et al. [39] extracted anthocyanins of H. sabdariffa by water at the ratio of 1:25 (sample: solvent), with a yield of 88 %. Todaro et al. [40] also confirmed the extraction optimization in eggplant using different solvent, acid concentration, temperature, time of extraction and solvent-to-solid ratios as independent variables for anthocyanin recovery. de-Teresa S et al. [41] identified 9 anthocyanins from purple corn such as cyanidin- 3-glucoside, pelargonidin-3-glucoside, peonidin-3- glucoside, and their respective malonyl derivatives. The other three were produced during the industrial extraction process identified as the corresponding ethylmalonyl derivatives. Yang and Zhai [42] found that cyanidin-3-glucoside, pelargonidin-3-glucoside, and peonidin-3-glucoside were the major components in Chinese purple corn seed extracts with other minor anthocyanin fractions. Meanwhile, six kinds of anthocyanin were extracted and were identified from Chinese purple corn cob such as cyanidin-3-glucoside, pelargonidin-3-glucoside and peonidin-3-glucoside, and their respective malonated counterparts using HPLCMS analysis. Moreno et al. [43] identified anthocyanins from Mexican purple corn kernels as cyanidin-3- glucoside, pelargonidin-3-glucoside, peonidin-3- glucoside, cyanidin-3-(6′-malonylglucoside) and cyanidin- 3-(3′,6′- dimalonylglucoside).
In an aqueous solution at pH 1-3 the flavylium cation is red colored, however, at pH 5 the resultant carbinol pseudo base is colourless and at pH 7-8 the blue purple quinoidal base is formed [44]. Jie et al. [45] found that thermal stability of purple-fleshed sweet potato anthocyanins in aqueous solution against diverse pH values followed a first-order kinetics model, which may due to structural variation and types of anthocyanin fractions. Present results were in comparable with that of Kadsura cocinea anthocyanin i.e., gradual decrease from 97.9 to 88 % from 20° to 80° within 3 h [46]. The stability of anthocyanin from B. retusa at various pH and temperature suggests that it is optimal to use it in acidic foods at low temperature to retain its stability of colour in the products. Based on the results, an ideal medium formulation was designed for anthocyanin synthesis was established by combining MS1/2 with 1:4 ratio of NH4+ to NO3", 4 % glucose and supplementation with 2 mg/l 2,4-D and 2 mg/l BA at 30° and 80 μmol×m-2×s-1 light irradiance. The culture conditions established in the present work induced the formation of friable fastgrowing calli from leaf explants of B. retusa.
An efficient callus and cell suspension induction protocol via leaf was established from B. retusa, a medicinal plant. The effects of plant growth regulators and culture media on the induction and formation of calli had been elucidated from this study. Production of calli, anthocyanin extraction from cell suspension culture and various parameters for enhancing anthocyanin synthesis were standardized. Subsequently, anthocyanin was purified, characterized and fractionated by HPLC-MS. Future studies are planned to enhance anthocyanin content by elicitor treatment and evaluation of its biological properties. Therefore, the success obtained with callus and suspension cultures of B. retusa, where anthocyanin production was associated to high growth rates on the same medium, makes this protocol a suitable and reliable system for in vitro anthocyanin production without affecting in vivo plants.
Acknowledgements
The authors acknowledge the Kerala State Council for Science, Technology and Environment (KSCSTE), Govt. of Kerala for providing funding in connection with the major project.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Gomez-Zeledon J, Jimenez VM. Producciónin vitrode antocianinas–revisión. ActaBiolColomb 2011;16:3-20.
- Streisfeld MA, Rausher MD. Altered trans-regulatory control of gene expression in multiple anthocyanin genes contributes to adaptive flower color evolution in Mimulusaurantiacus. MolBiolEvol 2009;26:433-44.
- Rupali B, Dhawale N, Pawar N. Phytochemical screening and antimicrobial activity of medicinal plant BrideliaretusaLin. (leaves extract). Int J Adv Res 2015;3(1):385-87.
- Kumar T, Jain V. Antinociceptive and anti-inflammatory activities of Brideliaretusamethanolic fruit extract in experimental animals. Sci World J 2014;2:1-12.
- Banerjee SK, Bond CG. Total phenolic content and antioxidant activity of extracts of BrideliaRetusaSpreng bark: impact of dielectric constant and geographical location. J Med Plants Res 2011;5(5):817-22.
- Islam T, Hasan MR, Roy A, Md. Shafiqul Islam, Md. AfazUddin, Md. Ariful Islam, et al. Screening of in vitroantioxidant, brine shrimp lethality bioassay and antimicrobial activities of extracts of Brideliaretusa(L) Spreng. fruit. Int J Pharm 2015;5(4):1058-67.
- Saurabh BK, Kala KS. Pharmacognostic and preliminary phytochemical investigation on bark of BrideliaretusaSpreng. Int J Pharm Clin Res 2009;1(1):35-39.
- Tatiya AU, Saluja AK.Evaluation of phytochemical standards and in vitroantioxidant activity of tannins rich fraction of Stem Bark of Brideliaretusa(L). Int J PharmTech Res 2010;2:649-55.
- Dhal NK, Panda SS, Muduli SD. Traditional uses of medicinal plants by native people in Nawarangpur district, Odisha, India. Asian J Plant Sci Res 2015;5(2):27-33.
- MurashigeT, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 1962;15:473-97.
- Jampani C,RaghavaraoKSMS.Extraction and purification of anthocyanins from red cabbage. Food Bioprod Process 2012;90(4):615-23.
- Heinonen J, Farahmandazad H, Vuorinen A, Kallio H, Yang B, Sainio T. Extraction and purification of anthocyanins from purple-fleshed potato. Food Bioprod Process 2016;99:136-46.
- Hua Z, Yuesheng D, Ge X, Menglu L, Liya D, LiJia A, et al. Extraction and purification of anthocyanins from the fruit residues of VacciniumuliginosumLinn. J Chromat Separation Techniq 2013;4(2):1-5.
- Liu X, Xiao G, Chen W, Xu Y, Wu J. Quantification and purification of mulberry anthocyanins with macroporous resins. J Biomed Biotechnol 2004;1(5):326-31.
- Jasrai YT, Thaker KN, D'SouzaMC. In vitro propagation of Euphorbia pulcherrimaWilld.through somatic embryogenesis. Plant Tissue Cult 2003;13:31-6.
- Liu Y, Tong X, Hui W, Liu T, Chen X, Li J, et al. Efficient culture protocol for plant regeneration from petiole explants of physiologically mature trees of JatrophacurcasL. BiotechnolBiotechnol Equip 2016;29:479-88.
- Ntui VO, Uyoh EA, Urua IS, Ogbu U, Okpako EC. Regeneration of ParkiabiglobosaBenth.: An important tree species of Africa. J MicrobiolBiotechnol Res 2012;2:169-77.
- Catapan E, Otuki MF, Viana AM. In vitroculture of Phyllanthusstipulatus(Euphorbiaceae). Rev Bras Bot 2001;24:25-34.
- Eugenio P, María del S, Rafael R, Neftalí O. Tissue culture of ornamental cacti.SciAgric 2015;72:540-61.
- Soliman HI, Gabr M, Abdallah N. Efficient transformation and regeneration of fig (FicuscaricaL.) via somatic embryogenesis. GM Crops 2010;1:40-51.
- Qui JA, Castro-Concha LA, Garcıa-Sosa K, Pena-Rodrıuez LM, Miranda-Ham ML. Differential effects of phytotoxic metabolites from Alternariatageticaon, Tageteserectacell cultures. J Gen Plant Pathol 2009;75:331-9.
- Mathur S, Shekhawat GS. Establishment and characterization of Stevia rebaudiana(Bertoni) cell suspension culture: an in vitroapproach for production of stevioside. ActaPhysiol Plant 2013;35:931-9.
- Schiozer AL, Barata LES. Stability of natural pigments and dyes. Rev Fitos 2007;3:6-23.
- Narayan MS, Thimmaraju R, Bhagyalakshmi B. Interplay of growth regulators during solid-state and liquid-state batch cultivation of anthocyanin producing cell line of Daucuscarota. Process Biochem2005;40:351-8.
- Chalker-Scott L.Environmental significance of anthocyanins in plant stress response, PhotochemPhotobiol 1999;70:1-9.
- Nakamura, Takeuchi Y, Miyanaga K, Seki MS, Furusaki. High anthocyanin accumulation in the dark by strawberry (Fragariaananassa) callus. BiotechnolLett 1999;21:695-9.
- Zhang W, Curtin C, Kikuchi M, Franco C. Integration of jasmonic acid and light irradiation for enhancement of anthocyanin biosynthesis in Vitisviniferasuspension cultures. Plant Sci 2002;162:459-68.
- Jang JC, Leon P, Sheen J. Hexokinase as a sugar sensor in higher plants. Plant Cell 1997;9:5-19.
- Vitrac X, Larronde F, Krisa S, Decendit A, Deffieux G, Merillon JM. Sugar sensing and Ca2þ-calmodulin requirement in Vitisviniferacells producing anthocyanins. Phytochemistry 2000;53:659-5.
- Narayan MS, Venkataraman LV. Effect of sugar and nitrogen on the production of anthocyanin in cultured carrot (Daucuscarota) cells. J Food Sci 2002;67:84-6.
- Saikia M, Shrivastava K, Singh SS. Effect of culture media and growth hormones on callus induction in AquilariamalaccensisLam., a medicinally and commercially important tree species of North East India. Asian J BiolSci2013;6:96-105.
- Rashid U, Ali S, Ali GM, Ayub N, Masood MS. Establishment of an efficient callus induction and plant regeneration system in Pakistani wheat (Triticumaestivum)cultivars. ElectronJ Biotechnol2009;12:4-5.
- Sharma G, Nautiyal AR. Influence of explants type and plant growth regulators on in vitro multiple shoots regeneration of a laurel from Himalaya. Nat Sci2009;7:1-7.
- Aviles F, Rios D, Gonzalez R, Sanchez-Olate M. Effect of culture medium in callogenesis from adult walnut leaves (JuglansregiaL.). Chil J Agr Res 2009;69:460-7.
- Behbahani M, Shanehsazzadeh M, Hessami MJ. Optimization of callus and cell suspension cultures of Barringtoniaracmosa(Lecythidaceae family) for lycopene production. SciAgric2011;68:69-76.
- Saad AIM, Elshahed AM. Plant tissue culture media. In: Leva A, Rinaldi LMR, editors. Recent advances in plant in vitro culture.Rijeka, Croatia: InTechOpen; 2012.
- Zhou S, Sauve RJ, Howard EF. Identification of a cell wall peroxidase in red calli of PrunusincisaThunb. Plant Cell Rep2002;21:380-4.
- Ceoldo S, Levi M, Marcono AM, Baldan G, Giarola M, Guzzo F. Image analysis and in vivoimaging as tools for investigation of productivity dynamics in anthocyanin-producing cell cultures of Daucuscarota. New Phytol 2005;166:339-52.
- Cisse M, Bohuon P, Sambe F, Kan C, Sakho M, Dornier M. Aqueous extraction of anthocyanins from Hibiscus sabdariffa: Experimental kinetics and modeling. J Food Eng2012;109:16-21.
- Todaro A, Cimino F, Rapisarda P, Catalano AE, Barbagallo RN, Spagna G. Recovery of anthocyanins from eggplant peel. Food Chem2009;114:434-9.
- de-Teresa S, Santos-Buelga C, Rivas-Gonzalo JC. LC/MS analysis of anthocyanins from purple corn cob. J Sci Food Agric 2002;82:1003-6.
- Yang Z, Zhai W. Identification and antioxidant activity of anthocyanins extracted from the seed and cob of purple corn (Zea mays L.). Innov Food SciEmergTechnol2010;11:169-76.
- Moreno YS, Sanchez GS, Hernandez DR, Lobato NR. Characterization of anthocyanin extracts from maize kernels. J ChromatogrSci 2005;43:483-7.
- Vayupharp B, LaksanalamaiV. Antioxidant properties and color stability of anthocyanin purified extracts from Thai waxy purple corn cob. J Food Nutri Res 2015;3:629-36.
- Jie L, Xio-ding L, Yun Z, Zheng-dong Z, Zhi-ya Q, Meng L, et al. Identification and thermal stability of purple-fleshed sweet potato anthocyanins in aqueous solutions with various pH values and fruit juices. Food Chem2013;136:1429-34.
- Sun J, Yao J, Huang S, Long X, Wang J, Garcia-García E. Antioxidant activity of polyphenol and anthocyanin extracts from fruits of Kadsuracoccinea(Lem.). Food Chem2009;117:276-81.