- *Corresponding Author:
- Z. Fan
Department of Pharmacy, Graduate School, Hebei North University, Qiaodong, Zhangjiakou 075000, China
E-mail: kzzaiwenfan@163.com
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “1-15” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Protocadherin beta 2 belongs to the procalcitonin family and is implicated in the progression of various tumors. It is linked to unfavorable prognosis in patients suffering from different types of cancers, whereas limited investigation was conducted on protocadherin beta 2 in gastric cancer. This study identified the key genes involved in tumor microenvironment of gastric cancer, ultimately pinpointing protocadherin beta 2 as the main focus of investigation. Analysis of survival data in the Cancer Genome Atlas and Gene Expression Omnibus databases indicated that patients with gastric cancer who exhibited elevated levels of protocadherin beta 2 had higher likelihood of experiencing unfavorable survival outcomes. Furthermore, the expression of protocadherin beta 2 demonstrated a strong association with tumor staging and grading. Gene set enrichment analysis indicated that protocadherin beta 2 was primarily linked to cell adhesion molecules, transforming growth factor-beta signaling pathway, phosphoinositide-3-kinase-protein kinase B signaling pathway and antigen-antibody presentation. Analysis of immune infiltration revealed a robust association between the expression of protocadherin beta 2 and infiltration of immune cells into tumors, which was found to be significant in relation to immune checkpoint inhibitors, leukocyte antigens; negatively correlated with microsatellite instability index, tumor mutation burden index and tumor immune dysfunction and exclusion scores. The analysis of drug prediction indicated that increased expression of protocadherin beta 2 exhibited greater responsiveness to anticancer medications like gemcitabine, 5-fluorouracil and adriamycin. Examining the prognostic and immunotherapeutic significance of protocadherin beta 2 in gastric cancer, this investigation reveals that protocadherin beta 2 influences the tumor microenvironment and prognosis of gastric cancer.
Keywords
Protocadherin beta 2, gastric cancer, prognosis, stomach adenocarcinoma, tumor microenvironment
Stomach Adenocarcinoma (STAD) ranks the 5th most prevalent form of cancer globally and is considered to be a significant disease burden with high fatality rate[1]. Surgery, radiotherapy and chemotherapy can improve the life quality partially, whereas gastric cancer continues to exhibit the traits of low rates of early detection and poor rates of survival. Hence, investigating the pathogenesis and exploring novel tumor indicators holds immense importance in the treatment of gastric cancer.
Tumor Microenvironment (TME) refers to the environment surrounding the tumor cells, comprising primarily of tumor cells, fibroblasts associated with tumor, a variety of cytokines and chemokines, along with immune and inflammatory cells in close proximity which is a sophisticated and interconnected system. Studies have indicated that changes in TME can impact the diversity of STAD, consequently influencing the advancement of STAD. For instance, the administration of Programmed cell Death protein-1/Programmed Death Ligand-1 (PD-1/PD-L1) axis inhibitor therapy greatly enhances the condition in individuals with gastric cancer. Additionally, infiltration of macrophages which is associated with gastric cancer into tissues results in substantial alterations in the distribution of tumor blood vessels, the extent of tumor infiltration and the status of lymph nodes. Hence, identifying the crucial genes of TME and disrupting the essential signaling interactions in TME could potentially serve as a better effective treatment approach for individuals with STAD[2-4].
Protocadherin Beta 2 (PCDHB2) gene belongs to the procalcitonin family and is mainly responsible for producing proteins that facilitate cell adhesion which are dependent on calcium. Several evidences indicate a strong correlation between the development of most tumors and PCDHB2. PCDHB2 has the ability to serve as a modification site for methylation in colon cancer, thereby impacting the biological functions of colon cancer cells and regulating the proliferation, migration, differentiation and homeostasis of tumor cells. This gene is notably overexpressed in the Hepatocellular Carcinoma (HCC) subtype HCCW2, leading to the activation of the Wingless-related integration site (Wnt)-hippo signaling pathway, subsequently resulting in a decreased survival rate for HCC patients[5]. Additionally, PCDHB2 can function as a molecular marker for radiation therapy in non-small cell lung cancer, providing improved guidance for treatment[6]. To sum up, prior research has shown that PCDHB2 plays a vital role in proliferation, infiltration, spread and immune response of cancer cells. Nevertheless, there is a dearth of research concerning PCDHB2 in relation to gastric cancer.
This study examined PCDHB2 gene that is closely associated with the TME of gastric cancer. We analyzed and compared the importance of the variations in expression, prognostic value and clinical staging of PCDHB2 in gastric cancer. Additionally, we discussed about the correlation between PCDHB2 and infiltration of immune cells. Furthermore, cellular experiments confirmed the association between PCDHB2 and migratory and invasive capabilities of gastric cancer. We constructed a model which showcased the role of PCDHB2 in gastric cancer, indicating that PCDHB2 could serve as a novel biomarker for the treatment of gastric cancer.
Materials and Methods
Data extraction:
Transcript levels (log2 counts+1 values) and clinical data of 32 normal tissues and 375 tumor tissues of gastric cancer were downloaded from The Cancer Genome Atlas (TCGA) database based on the University of California Santa Cruz (UCSC) Xena (http://xena.ucsc.edu/) and the Gene Expression Omnibus (GEO) (https://www. ncbi.nlm.nih.gov/geo/) database to download two independent datasets for external validation, including the GSE29998 and GSE84437 datasets. The GSE29998 dataset contains 49 normal and 50 tumor tissues, while GSE84437 dataset contains 433 gastric cancer samples with survival information. The data on somatic mutations were obtained from cBio Cancer Genomics Portal (https://www.cbioportal.org/) database.
Enrichment analysis on the co-expression network of PCDHB2:
Using the R package corrplot (p<0.05), we screened the top 400 genes that showed the strongest correlation with PCDHB2. We performed pathway analyses, using the R package clusterProfiler. Using Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) as references, these genes were further analyzed. Finally, visualization was performed using ChiPlot (http//www.chiplot.online) software.
Analyzing gene sets for enrichment using Gene Set Enrichment Analysis (GSEA):
In order to gain a deeper understanding of the potential mechanism of PCDHB2, we categorized the samples in the TCGA-STAD dataset into two groups based on the median value of PCDHB2. Then we utilized the GSEA package in R programming language for predicting the signaling pathways that could potentially involve PCDHB2.
Evaluating and calculating immunizations and substrates:
TME of gastric cancer was evaluated in this research using the Estimation of STromal and Immune cells in MAlignant Tumor tissues using Expression data (ESTIMATE) algorithm (bioinformatics.mdanderson.org/estimate/). Weighted Gene Co-Expression Network Analysis (WGCNA) has been extensively employed in different areas of oncology to identify the essential genes in tumors. For this research, we utilized WGCNA package in R programming language to combine the top 5000 gene expressions from the TCGA-STAD dataset with the stromal and immune scores of each sample in the TME of gastric cancer. Generally, we created a co-expression network and identified clusters of gastric cancer genes that showed strong correlation with the stromal and immune scores. Furthermore, we employed different algorithms to screen the genes within the core module, aiming to identify central genes that have the potential to be therapeutic targets[7-10]. Cell-type Identification by Estimating Relative Subsets of Ribonucleic Acid (RNA) Transcripts (CIBERSORT) algorithm (https//cibersort.stanford.edu/) computes the degree of infiltration of 22 immune cells in gastric cancer. This calculation is employed to analyze the correlation between target genes and immune cells. Tumor Immune Dysfunction and Exclusion (TIDE) database (http://tide.dfci.harvard.edu/login/) provides forecast related to the effectiveness of immunotherapy for individuals with gastric cancer. Additionally TCGAbiolinks package, which is based on R programming language, retrieves the mutation data for gastric cancer and computes the mutation burden associated with this disease. In the end, we obtained the gastric cancer mutation data using the TCGAbiolinks package in the R programming language. Then we computed the mutation load and assessed the microsatellite instability of gastric cancer.
Sources of data for drug projections:
A database of >200 anticancer drugs Genomics of Drug Sensitivity in Cancer (GDSC) (https://www. cancerrxgene.org/) was downloaded for this study. Using the oncoPredict package in R programming language, we predicted drug susceptibility through the half-maximal Inhibitory Concentration (IC50) (μM) values. Based on the median level of PCDHB2 expression, the samples were divided into two groups and visualized using the Wilcoxon t-test.
Literature for cell source and cell culture:
The process of growing cells in a controlled environment and introducing genetic material into them is termed as cell culture. The epithelial cell lines, Gastric Adenocarcinoma (AGS) and MKN were acquired from White Novelty. AGS cells were grown in Ham'S F-12 medium with the addition of 10 % Fetal Bovine Serum (FBS), while MKN cells were grown in Roswell Park Memorial Institute (RPMI) 1640 medium with the addition of 10 % FBS. The cultures were incubated at 37° temperature with 95 % air using a thermostat and 5 % Carbon dioxide (CO2). Following 70 % cellular adhesion, AGS and MKN cell cultures were subjected to transfection with small interfering (si) PCDHB2 (Beijing Avantage Biotech Co., Ltd.,) following the manufacturer's instructions. The transfection efficiency of PCDHB2 was confirmed through Western Blot (WB) and Real-Time quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) analyses after 72 h.
RNA extraction and RT-PCR analysis:
qRT-PCR was employed to detect gene expression. Total RNA Isolation Reagent (TRIzol) reagent (Ambion, Inc.) was used to isolate total RNA from tumor cells and post-transfected tumor cells, which was then converted to complementary (c) DNA using a reverse transcription kit (R2020S, US Everbright, Inc.). The amplification was performed using the Bio-Rad CFX96 touch instrument for qRT-PCR. The primer sequences for qRT-PCR were as follows, Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH), Forward (F) 5'-GGTTGTCTCCTGCGACTTCA-3'; Reverse (R): 5'-TGGTCCAGGGTTTCTTACTCC-3'; PCDHB2: Forward (F): 5 ' -CGGAGACAACGGAAGGATGGTG-3' and Reverse (R): 5'-TCGGTGACGGTGATGGTGATGGTGATG-3' which was calculated by the 2-ΔΔCt method.
WB assay:
After collecting the treated cells, protein quantification was performed using Bicinchoninic Acid (BCA) assay (B6167, US Everbright, Inc.). Subsequently, protein samples were transferred to Polyvinylidene Difluoride (PVDF) membranes using Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) (20230417, Solarbio Life Science). Following the application of 5 % skimmed milk to seal the membrane, the membrane was left to incubate overnight at 4° with primary antibodies targeting anti-PCDHB2 (OACD06318, Aviva Systems Biology) and anti-Beta (β)-actin (BM0627, Boster Bio). Subsequently, the secondary antibody (bs-40296G-HRP, Bioss) was incubated at 37° for 2 h. Protein levels were visualized using Electrochemiluminescence (ECL) solution (abs920, Absin) and were assessed using Bio-Rad image laboratory software.
Proliferation assay:
The growth potential of gastric cancer cells was evaluated using the Cell Counting Kit-8 (CCK-8) (RX30025, Beijing Rongxing Biotechnology Co., Ltd.) test. The cells were placed in 96-well plates and treated with 10 μl of CCK-8 solution at various time intervals of 4, 24, 48, and 72 h and they were incubated for 2 h at 37°. Afterwards, the measurement of absorbance was conducted at a wavelength of 450 nm. Calculation was done to determine the average Optical Density (OD) of the four wells.
Transwell assay:
The gel matrix (abs9491, Absin) was thinned to a concentration of 200 μg/ml using medium without serum. Subsequently, 100 μl of the thinned gel matrix was introduced into every Transwell chamber for the invasion test. The upper chamber received 200 μl of cell suspension with a concentration of 2×105 cells/ml, while the lower chamber was supplemented with 500 μl of medium containing 20 % FBS. After incubating at 37° for 24 h, the upper layer of the membrane was eliminated using a cotton swab. The chambers were then fixed in paraformaldehyde (WJ1002, Beijing Biotech Co., Ltd.) approximately for 30 min. Subsequently, the chambers were stained with crystalline violet stain (G1062, Solarbio Life Science) and microscopic images were captured after 15 min.
Clone formation experiment:
Cells in the logarithmic growth stage were harvested and the concentration of cells was modified to 5×105 cells/ml for cultivation in 6-well plates with 5×105 cells per well. Cells were permitted to grow until they formed a monolayer, and then the 6-well plates were vertically scratched using a 200 μl pipette tip. Following the Phosphate-Buffered Saline (PBS) wash and removal of the suspended cells, the plates were placed in a 37°, 5 % CO2 environment and incubated with Serum-Free Medium (SFM). Microscopic images were captured at 0 h and 24 h from the initiation of the experiment. The pertinent familial movement was computed using the below mentioned equation.
Cell migration speed=initial scratch width-scratch width after 24 h/initial scratch width×100 %
Statistical analysis:
The differential expression of cancerous and normal tissues of STAD in TCGA database and GEO database was analyzed using the Wilcoxon t-test. Additionally, the differences between PCDHB2, different clinical grades and classifications were compared using Analysis of Variance (ANOVA) or Kruskal-Wallis test. PCDHB2-associated genes were selected; their expression and immunodetection points, immune infiltration coefficients, etc., were calculated using Pearson correlation analysis. To evaluate the impact of PCDHB2 and certain clinical factors on prognostic factors, both univariate and multivariate Cox regression analyses were conducted. Scatter plots, histograms, heat maps, etc. GraphPad Prism 8.4.3 and the ggplot2 package for the R language (version 4.2.2) were utilized to plot the data.
Results and Discussion
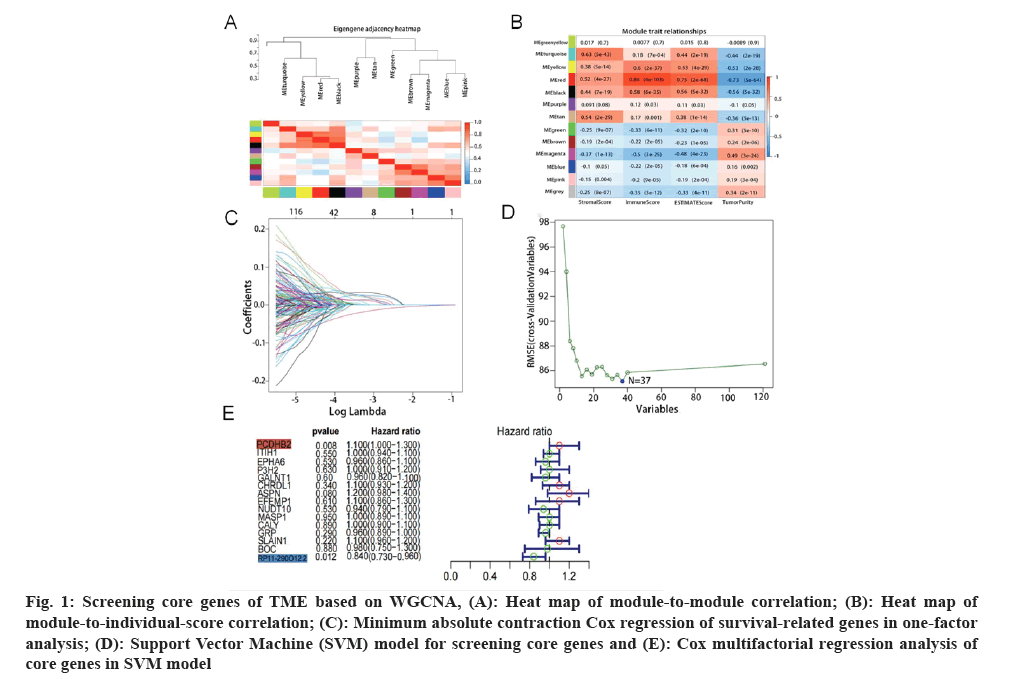
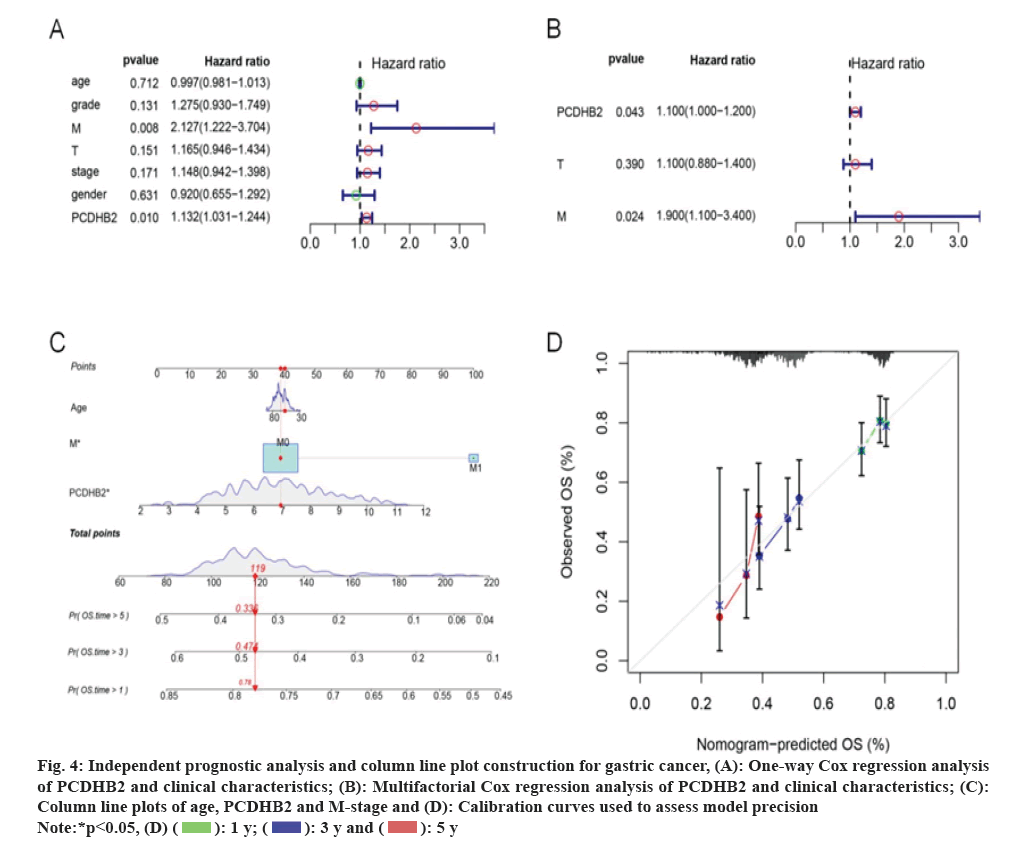
Acquisition of target genes was carried out. Analysis of the immune microenvironment of gastric cancer was used by the WGCNA method. We found that the red module and the turquoise module had a strong correlation with the immune scores and stroma scores (fig. 1A). Firstly, we combined gene expression data with the two modules mentioned above, and then we integrated this data with overall survival data. Using one-way risk regression analysis, we identified genes that affect the survival of gastric cancer. Additionally, we conducted further screening of genes using Least Absolute Shrinkage Selection Operator (LASSO) regression analysis (fig. 1B) and Support Vector Machine (SVM) machine learning method (fig. 1C). After conducting a thorough analysis, we successfully identified 16 genes associated with the prognosis of gastric cancer, namely PCDHB2, Inter-Alpha (α)-Trypsin Inhibitor Heavy chain H1 (ITIH1), Ephrin type receptor A6 (EPHA6), Prolyl 3-Hydroxylase 2 (P3H2), Chordin Like 1 (CHRDL1), and others. Subsequently, we proceeded to perform multifactorial Cox regression analysis on these genes (fig. 1D and fig. 1E). Interestingly, we discovered that PCDHB2 gene solely stood out as the statistically significant risk factor among these 16 genes with Hazard Ratio (HR)>1 and p<0.05. Hence, we concentrated on examining the association between PCDHB2 and the prognosis of gastric cancer in this study.
Fig. 1: Screening core genes of TME based on WGCNA, (A): Heat map of module-to-module correlation; (B): Heat map of module-to-individual-score correlation; (C): Minimum absolute contraction Cox regression of survival-related genes in one-factor analysis; (D): Support Vector Machine (SVM) model for screening core genes and (E): Cox multifactorial regression analysis of core genes in SVM model.
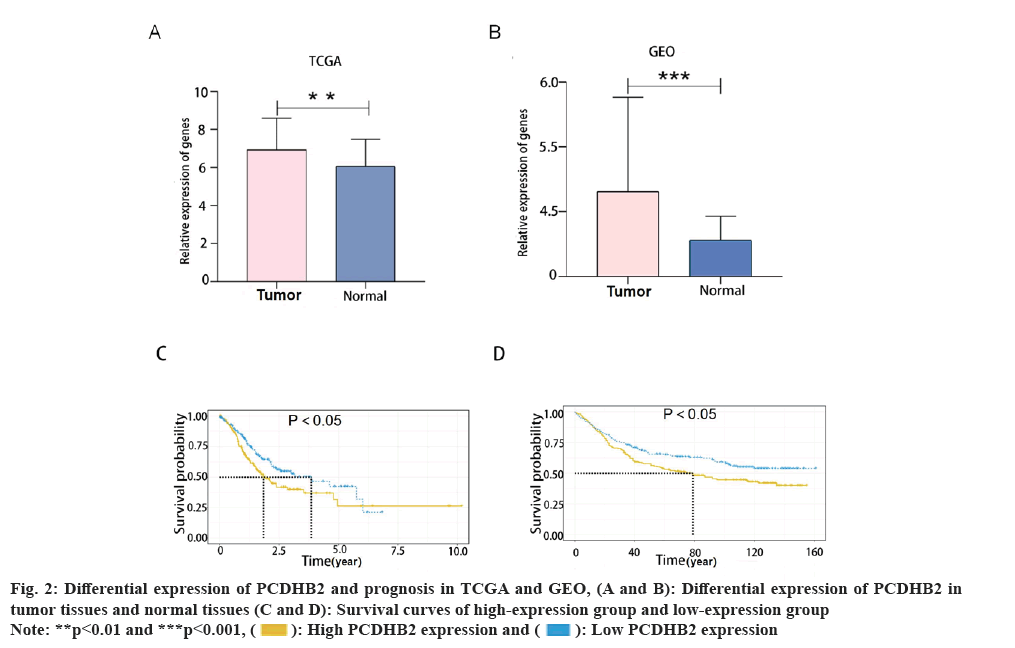
We found that prognosis was adversely affected by the high expression of PCDHB2. This study involved a comparison of the variations in PCDHB2 expression between normal tissues and tumor tissues from the TCGA database and the GEO database (GSE29998) (fig. 2A and fig. 2B). Findings from both the databases indicated a significant upregulation of PCDHB2 in tumor tissues. In order to further examine the predictive significance of PCDHB2 in cancer patients, survival curves for PCDHB2 were generated using the TCGA database and GEO (GSE84437) database. These curves were plotted using the survival package in the R programming language (fig. 2C and fig. 2D). According to the findings from the TCGA database, the high-expression group (PCDHB2) had a considerably lower survival rate compared to the low expression group (PCDHB2). The expression level of the high-expression group was notably lower than the low-expression group (p<0.05). This finding was confirmed using the GSE84437 dataset and the same methodology, and the conclusion remained consistent. The aforementioned research indicated that gastric cancer exhibits a substantial upregulation of PCDHB2, which is associated with a decreased likelihood of survival among patients with gastric cancer.
The expression of PCDHB2 in gastric cancer shows a negative correlation with both extent of Tumor stage (T) and histological grading. According to the aforementioned research, the clinical stage and grading of a tumor are crucial factors that impact the prognosis and survival of patients. The study revealed that a high expression of PCDHB2 is associated with poorer survival rates. Consequently, we utilized R language's pheatmap package to create a heat map illustrating the expression of PCDHB2 and the histological grading of gastric cancer (fig. 3A). In the study, it was discovered that the group with high expression tended to have a lower T stage and grading in comparison to the group with low expression. The heat map revealed that the high-expression group exhibited greater advancement in T staging and grading compared to the low-expression group. In order to further examine the correlation between PCDHB2 and phenotypes like clinical stage and grading, we compared the variations in PCDHB2 expression across gender, age, presence of Metastasis (M) stage, T stage and tumor grading (fig. 3B-3G). The findings indicated a significant statistical association between PCDHB2 expression in T stage and tumor grading. Moreover, the current results demonstrated that elevated PCDHB2 expression primarily correlated with the T stage and tumor grading of the tumor.
PCDHB2 plays a crucial role in forecasting the survival outcome of individuals with gastric cancer. In order to further examine the connection between PCDHB2 and survival, we conducted univariate and multivariate Cox regression analyses to assess the correlation between the clinical characteristics of PCDHB2, T stage, lymph Nodes (N) stage, M stage, age, and overall survival. The findings demonstrated a significant correlation between the survival of gastric cancer and the expression of PCDHB2, as well as the T and N stage, as revealed by the univariate Cox regression analysis (p<0.05, fig. 4A). Furthermore, the independent prognostic indicators for gastric cancer patients, PCDHB2 and M stages were further supported by multivariate regression analysis (p<0.05, fig. 4B). Both univariate and multivariate analyses revealed that the expression of PCDHB2 and M stage were identified as factors that pose a risk to the survival of individuals with gastric cancer. We utilized age, M stage and PCDHB2 expression level to construct nomograms in order to enhance understanding of the correlation and forecast the 1 y, 3 y and 5 y survival rates of individuals with gastric cancer (fig. 4C). According to the study, when the M stage was more progressed and there was an increased expression of PCDHB2, the chances of survival for gastric cancer patients decreased. To evaluate the model, a calibration curve was created, and the findings indicated that the predicted 1 y, 3 y and 5 y survival rates from the column line plot closely matched the actual survival rates (fig. 4D). This outcome provided evidence of the model's reliability. To summarize, the aforementioned data indicate that PCDHB2 serves as a significant indicator for the prognosis of individuals with gastric cancer, enabling the prediction of their overall survival.
Fig. 4: Independent prognostic analysis and column line plot construction for gastric cancer, (A): One-way Cox regression analysis
of PCDHB2 and clinical characteristics; (B): Multifactorial Cox regression analysis of PCDHB2 and clinical characteristics; (C):
Column line plots of age, PCDHB2 and M-stage and (D): Calibration curves used to assess model precision.
 .
.
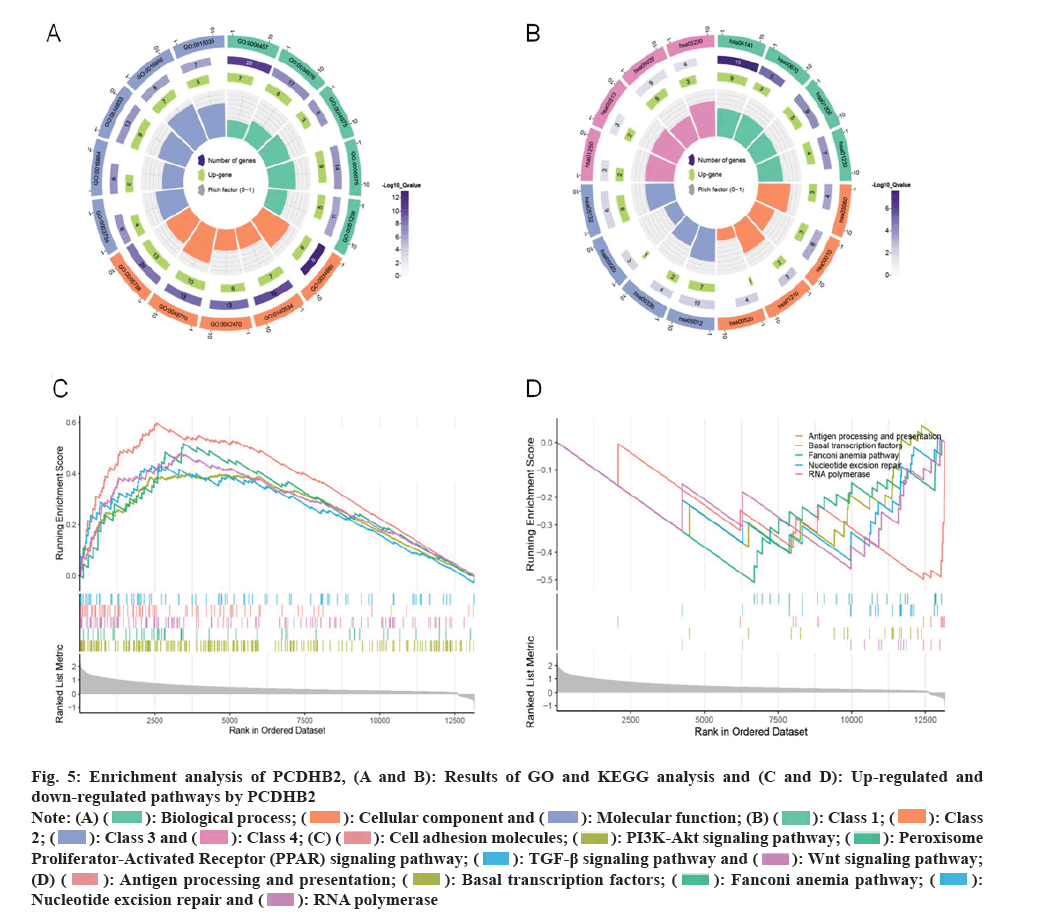
PCDHB2 gene is closely associated with gastric cancer proliferation, growth, and immune infiltration. To evaluate the biological role of the PCDHB2 gene in STAD, we examined the top 400 genes that had the strongest correlation with PCDHB2 in the gastric cancer expression matrix using the Pearson algorithm for GO and KEGG analyses. The GO analysis indicated that PCDHB2 was primarily linked to the molecular functions of cell adhesion, maintenance of hematopoietic stem cells, and the Wnt signaling pathway (fig. 5A). On the other hand, the KEGG analysis revealed that PCDHB2 was mainly enriched in the Ras-Associated Protein-1 (RAP1) signaling pathway, Hedgehog signaling pathway, Mitogen-Activated Protein Kinase (MAPK) signaling pathway, and others (fig. 5B). Furthermore, the GSEA enrichment analysis revealed that PCDHB2 primarily enhanced the expression of cell adhesion molecules, the Transforming Growth Factor (TGF)-β signaling pathway, the Phosphoinositide-3-Kinase-protein Kinase B (PI3K-Akt) signaling pathway, and other related pathways. In contrast, PCDHB2 primarily suppressed activities like nucleotide excision repair, antigen processing, and presentation (fig. 5C and fig. 5D). The findings suggested that the PCDHB2 gene had a strong correlation with the proliferation, growth, and immune infiltration of gastric cancer.
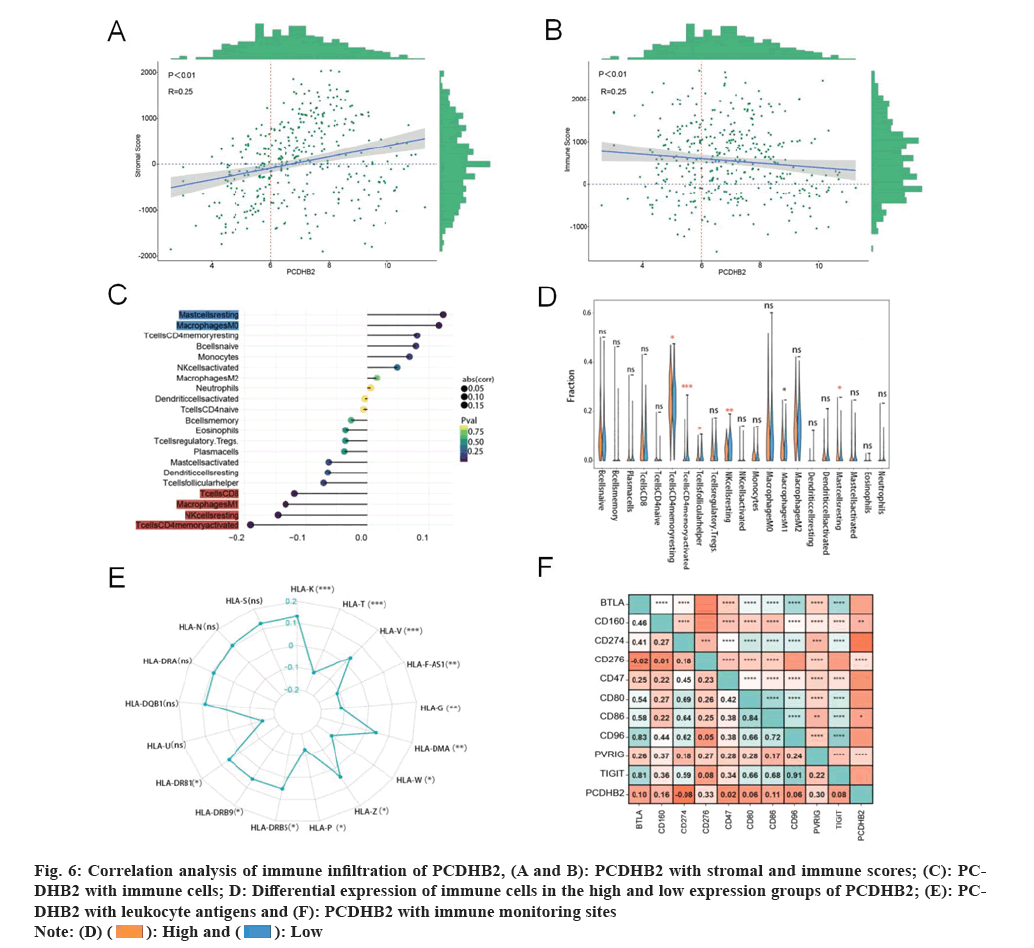
The presence of PCDHB2 is linked to the infiltration of immune cells in tissues affected by gastric cancer. Analysis of enrichment revealed a strong association between PCDHB2 and immunity against gastric cancer. Then, we evaluated the significance of PCDHB2 in the TME of gastric cancer by examining the association between PCDHB2 expression level and gastric cancer immunity score, stroma score, expression level of certain immune detection markers, leukocyte antigen expression level, and variation in immune cell infiltration between high and low PCDHB2 expression groups. Significant differences were observed in the immune cell resting memory Cluster of Differentiation 4 (CD4) T cells, activation, T cells follicular helper cells, Natural Killer (NK) cells, M1 macrophages, and resting mast cells, as indicated by the results (fig. 6A-6F). Analysis of immune cell correlation revealed a significant negative correlation between the expression of PCDHB2 and CD8 T cells, M1, resting NK cells and CD4 T cells memory activation. Additionally, PCDHB2 expression exhibited a significant positive correlation with resting mast cells and macrophage M0 expression. The correlation between expression and resting mast cells, macrophage M0 expression was significantly positive. On the other hand, PCDHB2 expression showed a significant negative correlation with the immune infiltration score, whereas a significant positive correlation with the stromal score. Furthermore, the correlation analysis of immune monitoring points revealed a significant positive correlation between PCDHB2 and the immune detection points of CD276, Poliovirus Receptor-related Immunoglobulin domain-containing (PVRIG), CD160, and CD86. The correlation analysis of leukocyte antigens revealed a significant positive correlation between PCDHB2 and Human Leukocyte Antigen (HLA)-K, HLA-T, HLA-V, HLA-F-Antisense (AS) RNA 1, HLA-G, HLA-W, HLA-Z, HLA-P and HLA-major histocompatibility complex, Class II (DRB9), while a significant negative correlation was observed with HLA-DMα and HLA-DRβ5 (fig. 6E). The available data indicates that PCDHB2 may cause disruption in the TME of gastric cancer and the individuals with elevated PCDHB2 expression in gastric cancer are more susceptible to immune evasion.
Fig. 6: Correlation analysis of immune infiltration of PCDHB2, (A and B): PCDHB2 with stromal and immune scores; (C): PCDHB2
with immune cells; D: Differential expression of immune cells in the high and low expression groups of PCDHB2; (E): PCDHB2
with leukocyte antigens and (F): PCDHB2 with immune monitoring sites.
 .
.
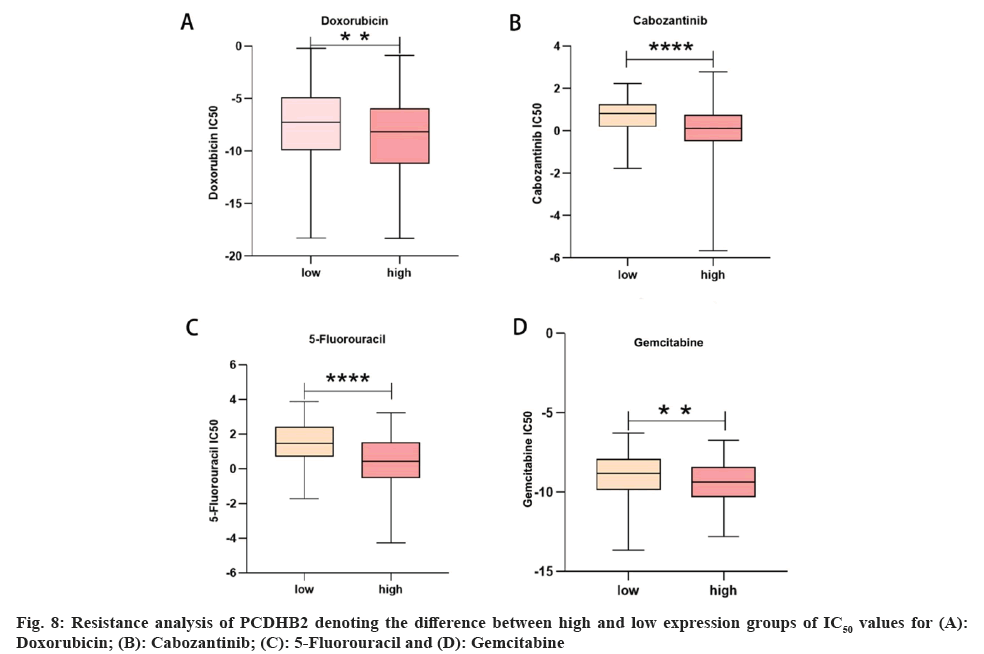
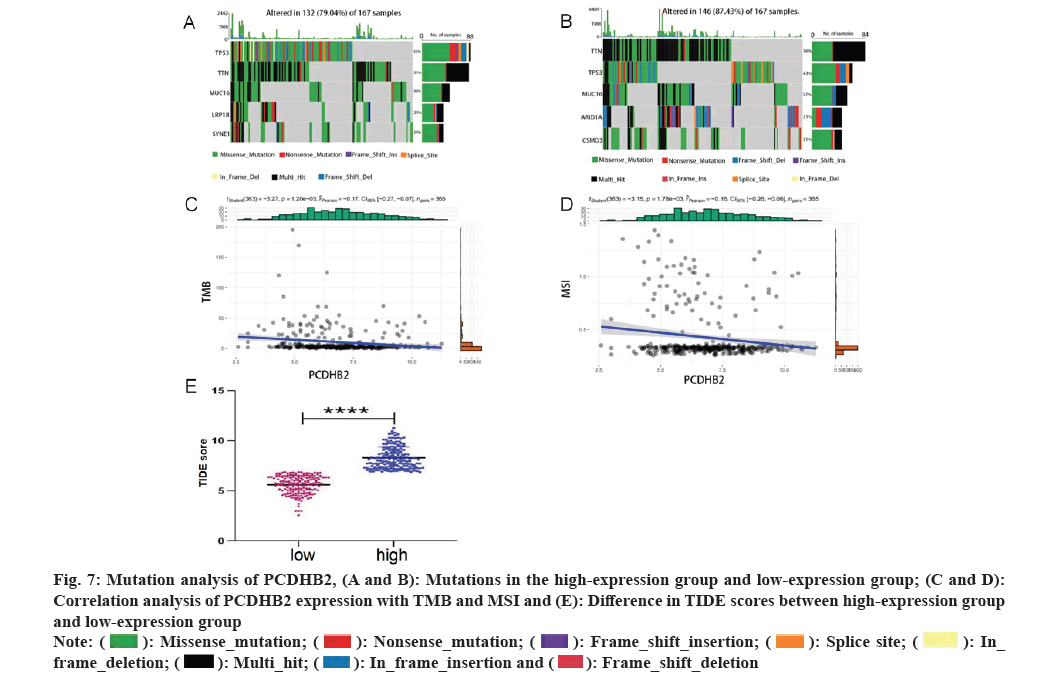
Mutation analysis and drug resistance analysis of PCDHB2 was studied. Various studies have indicated that immunotherapy is a significant asset in the management of gastric cancer. Gene mutations have a strong correlation with the formation of the majority of tumors and serve as a crucial factor in determining the effectiveness of tumor immunotherapy. This study revealed that the PCDHB2 high-expression group had a prevalence of gene mutations including Tumor Protein (TP) 53, Titin (TTN), Mucin 16 Cell surface associated (MUC16), Low-density lipoprotein receptor-Related Protein 1B (LRP1B), and Spectrin repeat containing Nuclear Envelope protein 1 (SYNE1), while the PCDHB2 low-expression group had a prevalence of gene mutations including TTN, TP53, MUC16, AT-Rich Interaction Domain 1A (ARID1A), and CUB and Sushi Multiple Domains 3 (CSMD3) (fig. 7A and fig. 7B). Furthermore, the expression of PCDHB2 exhibited a strong negative correlation with both the Tumor Mutation Burden (TMB) index (fig. 7C) and the Microsatellite Instability (MSI) index (fig. 7D). Using the TIDE database, we assessed the effectiveness of PCDHB2 immunotherapy by comparing the disparity in TIDE scores between the high-expression and low-expression groups. The results revealed a significant increase in TIDE scores for the high-expression group compared to the low-expression group (fig. 7E). In conclusion, we utilized the R language oncoPredict software to compute the variance in IC50 measurements of anticancer medications among the high-expression and low-expression groups (fig. 8A-8D). The findings indicated that the high-expression group exhibited superior effectiveness against gemcitabine, 5-fluorouracil, adriamycin, cabozantinib and various other anti-cancer medications.
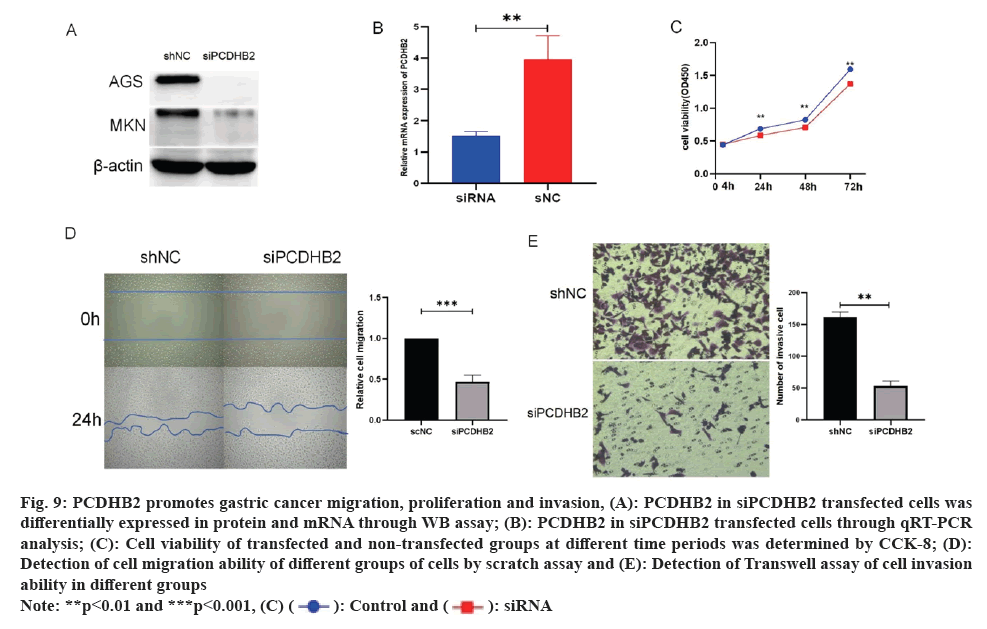
Gastric cancer proliferation, migration, and invasion are suppressed by the downregulation of PCDHB2 expression. Based on the GSEA analysis mentioned above, it was suggested that the growth and movement of gastric cancer could be influenced by PCDHB2. To confirm this, we employed siPCDHB2 to suppress AGS and MKN cells. This was confirmed through WB assay (fig. 9A) and qRT-PCR analysis (fig. 9B). Following a successful transfection, CCK-8 experiments were conducted (fig. 9C), revealing a significant decrease in cell viability in the transfected group compared to the untransfected group after 24 h, 48 h, and 72 h. These findings suggest that PCDHB2 enhances the proliferative capacity of gastric cancer. Additionally, the Transwell invasion assay exhibited a decrease in AGS cell invasion upon transfection with siPCDHB2 and statistically significant (fig. 9D-9E) invasive power compared to controls. The aforementioned findings indicated that PCDHB2 greatly promoted the migration and invasion of gastric cancer.
Fig. 9: PCDHB2 promotes gastric cancer migration, proliferation and invasion, (A): PCDHB2 in siPCDHB2 transfected cells was
differentially expressed in protein and mRNA through WB assay; (B): PCDHB2 in siPCDHB2 transfected cells through qRT-PCR
analysis; (C): Cell viability of transfected and non-transfected groups at different time periods was determined by CCK-8; (D):
Detection of cell migration ability of different groups of cells by scratch assay and (E): Detection of Transwell assay of cell invasion
ability in different groups.
 .
.
As society has progressed, people's ways of life have undergone changes, leading to a notable rise in the incidence of gastric cancer (STAD). Neoplasm remains the main killer worldwide[11-15], among which gastric cancer is acknowledged as the 3rd primary reason for cancer-related fatalities and the 5th prevalent malignant growth[16,17]. Despite the availability of conventional treatments like surgery, radiotherapy, and chemotherapy, gastric cancer (STAD) continues to present significant complications and limitations in recent years. In this form, we hope to break its disadvantages in new approaches such as immunotherapy and targeted therapy[18]. Different immune checkpoint inhibitors have been found to exhibit significant variations in the objective remission rates of gastric cancer patients, as demonstrated by previous research[17,19-21]. These differences could potentially be attributed to the distinctive TME of gastric cancer. Therefore, we hope to use the PCDHB2 gene as a predictive biomarker for immunotherapy based on bioinformatics-related studies.
Protocadherins (Pcdhs) consist of 58 proteins associated with calcitonin, which must contain 3 gene clusters: Pcdh-α, Pcdh-β and Pcdh-Gamma (γ) (also known as PcdhA, PcdhB and PcdhG, respectively)[22,23]. PCDHB2 is a key member of the Pcdh-β family. Various studies have shown that PCDHB2 may play a significant role in the progression of several types of malignancies, such as breast cancer[24], pancreatic cancer[25] and colon cancer. Nonetheless, there has been limited investigation into gastric cancer. This study examined and contrasted the distinct expression of PCDHB2 in gastric cancer using TCGA, GEO and other databases. It has also elucidated the significance of PCDHB2 in relation to the survival, prognosis and TME of gastric cancer. These findings offer a fresh perspective for future research on gastric cancer.
The cell ratio and cell morphology in tumor patients' TME differ greatly from those in healthy tissues[26]. Gastric cancer typically consists of cancerous and normal tissues, which encompass stromal cells and immune cells, forming the TME. Among these, stromal cells primarily facilitate the growth of tumors and impact the progression of tumors. Conversely, immune cells predominantly exert inhibitory effects[27-29]. In the TME of gastric cancer, macrophages and T cells play a crucial role as pivotal components of the immune cell population[30]. T cells exhibit significant heterogeneity, with CD8+ T cells primarily responsible for eliminating cancer cells. The reduction in the quantity and impaired function of CD8+ T cells plays a crucial role in the immune evasion observed in gastric cancer. Tumor cells that evade detection by CD8+ T cells are frequently targeted by NK cells, and the prognosis of patients with gastric cancer is positively associated with the extent of NK cell infiltration in both the tumor and peripheral blood[31]. Tumor-Associated Macrophages (TAM), consisting of M1 macrophages with oncogenic properties and M2 macrophages with tumor-promoting properties, infiltrate the tumor microenvironment. In gastric cancer, the induction of M2 activation in TAM and the suppression of M1 activation play crucial roles in the development of immune tolerance[32]. Molecules known as immune checkpoint molecules are found on immune cells and can hinder the activity of immune cells, thus preventing the body from producing an immune response and resulting in the evasion of tumor immunity. In gastric cancer, the immune response is inhibited when Cytotoxic T Lymphocyte Antigen-4 (CTLA-4) binds to B7-2 (CD86)[33-35]. The number of CD8+ T cells in the tumor decreases as the expression of B7-H3 (CD276) increases, which is closely associated with the immune detection point in gastric cancer[36]. This study suggested that the expression of the PCDHB2 gene showed a positive correlation with the stromal score and a negative correlation with the immune score. Additionally, the expression of the PCDHB2 gene exhibited a negative correlation with CD8+ T cells and NK cells, while showing a positive correlation with M0 macrophages. Furthermore, the expression of PCDHB2 was found to be positively correlated with immune detection markers like CD86 and CD276. These findings suggest that high expression of PCDHB2 leads to an immune-suppressed state in gastric cancer cells, making them more susceptible to immune evasion. With the rapid advancement of oncology in recent times, there has been an increasing focus on the investigation of leukocyte antigens in tumors. We anticipate that leukocyte antigens hold great potential as a novel target for tumor therapy[37]. This study also examined the association between PCDHB2 and leukocyte antigens, revealing a strong correlation between PCDHB2 and the majority of leukocyte antigens. The immune microenvironment's value of PCDHB2 is further validated.
The presence of tumor mutations is regarded as a significant factor influencing the effectiveness of tumor immunotherapy, with patients exhibiting higher mutation rates being more suitable candidates for this treatment approach. TMB and MSI are frequently used indices to indicate the mutation status of tumors. Previous research has demonstrated that patients with gastric cancer who have high TMB and MSI indices are more likely to benefit from immunotherapy[38-40]. The research revealed that the presence of mutations in TP53 and TTN was linked to the expression of the PCDHB2 gene. Furthermore, the study found a negative correlation between the expression of PCDHB2 and the TMB and MSI scores in gastric cancer. This suggests that individuals with elevated levels of PCDHB2 expression might not be ideal candidates for conventional immunotherapy. Following this, we used the TIDE algorithm to anticipate the responsiveness of the PCDHB2 gene to immunotherapy. The findings indicated that patients in the high-expression category were less suitable for immunotherapy, thus indirectly confirming the hypothesis. In the end, we examined and contrasted the resilience of the high-expression and low-expression groups using various commonly employed chemotherapy medications for stomach cancer. The findings suggest that individuals in the high-expression group are better suited for chemotherapeutic drugs like doxorubicin, cabozantinib, 5-fluorouracil, and gemcitabine.
To summarize, we initially identified the fundamental components in the TME of gastric cancer by utilizing public databases and diverse algorithms. We commenced their investigation by focusing on the PCDHB2 gene, examining its differential expression in gastric cancer, its impact on survival and prognosis, and its association with clinical phenotypes. Through GSEA analysis and cellular experiments, it was revealed that PCDHB2 is linked to the migration and proliferation of gastric cancer. Subsequent examination revealed that PCDHB2 was linked to the infiltration of the immune system in gastric cancer, potentially leading to immune evasion. Studies on the treatment of gastric cancer indicated that individuals with elevated levels of PCDHB2 expression may have reduced tolerance towards immunotherapy while being better suited for conventional chemotherapy. This study proposes the PCDHB2 gene as a possible indicator for identifying and forecasting survival and immunotherapy targets in individuals with gastric cancer, according to our group. The study yielded remarkable results, but there are still some limitations that need to be further explored. For instance, there is still room for improvement in the diversity of research methods and sample size. This aspect can be further explored in the future.
Acknowledgements:
Yuzhu Tang, Xin Liu and Hongqi Li performed the study design. Yuzhu Tang, Xin Liu, Hanwen Luo, Jianchun Fan, and Tian Li extracted the data and analyzed the data. Yuzhu Tang and Xin Liu performed the data validation. Yuzhu Tang and Xin Liu were involved in writing the original manuscript draft. Hongqi Li contributed to the manuscript review and editing. All the authors read and approved the final manuscript.
Conflict of interests:
The authors declared no conflict of interests.
References
- Lopez MJ, Carbajal J, Alfaro AL, Saravia LG, Zanabria D, Araujo JM, et al. Characteristics of gastric cancer around the world. Crit Rev Oncol Hematol 2023;181:103841.
[Crossref] [Google Scholar] [PubMed]
- Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Okumura H, Matsumoto M, et al. Tumor-Associated Macrophage (TAM) infiltration in gastric cancer. Anticancer Res 2003;23(5A):4079-83.
[Google Scholar] [PubMed]
- Zhang H, Wang X, Shen Z, Xu J, Qin J, Sun Y. Infiltration of diametrically polarized macrophages predicts overall survival of patients with gastric cancer after surgical resection. Gastric Cancer 2015;18(4):740-50.
[Crossref] [Google Scholar] [PubMed]
- Ren N, Liang B, Li Y. Identification of prognosis-related genes in the tumor microenvironment of stomach adenocarcinoma by TCGA and GEO datasets. Biosci Rep 2020;40(10):1-13.
[Crossref] [Google Scholar] [PubMed]
- Chang YS, Chou YP, Chung CC, Lee YT, Yen JC, Jeng LB, et al. Molecular classification of hepatocellular carcinoma using Wnt-hippo signaling pathway-related genes. Cancers 2022;14(19):1-15.
[Crossref] [Google Scholar] [PubMed]
- Xia L, Liu Y, Wang Y. PD‐1/PD‐L1 blockade therapy in advanced non‐small‐cell lung cancer: Current status and future directions. Oncologist 2019;24:S31-41.
[Crossref] [Google Scholar] [PubMed]
- Langfelder P, Horvath S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinformatics 2008;9:1-13.
[Crossref] [Google Scholar] [PubMed]
- Zhai X, Xue Q, Liu Q, Guo Y, Chen Z. Colon cancer recurrence-associated genes revealed by WGCNA co-expression network analysis. Mol Med Rep 2017;16(5):6499-505.
[Crossref] [Google Scholar] [PubMed]
- Ding M, Li F, Wang B, Chi G, Liu H. A comprehensive analysis of WGCNA and serum metabolomics manifests the lung cancer-associated disordered glucose metabolism. J Cell Biochem 2019;120(6):10855-63.
[Crossref] [Google Scholar] [PubMed]
- Di Y, Chen D, Yu W, Yan L. Bladder cancer stage-associated hub genes revealed by WGCNA co-expression network analysis. Hereditas 2019;156:1-11.
[Crossref] [Google Scholar] [PubMed]
- Li T, Yang Z, Jiang S, Di W, Ma Z, Hu W, et al. Melatonin: Does it have utility in the treatment of haematological neoplasms? Br J Pharmacol 2018;175(16):3251-62.
[Crossref] [Google Scholar] [PubMed]
- Ma Z, Fan C, Yang Y, Di S, Hu W, Li T, et al. Thapsigargin sensitizes human esophageal cancer to TRAIL-induced apoptosis via AMPK activation. Sci Rep 2016;6(1):1-17.
[Crossref] [Google Scholar] [PubMed]
- Sun M, Liu X, Xia L, Chen Y, Kuang L, Gu X, et al. A nine-lncRNA signature predicts distant relapse-free survival of HER2-negative breast cancer patients receiving taxane and anthracycline-based neoadjuvant chemotherapy. Biochem Pharmacol 2021;189:1-39.
[Crossref] [Google Scholar] [PubMed]
- Yang Z, Jiang S, Lu C, Ji T, Yang W, Li T, et al. SOX11: Friend or foe in tumor prevention and carcinogenesis? Ther Adv Med Oncol 2019;11:1-25.
[Crossref] [Google Scholar] [PubMed]
- Li T, Qiao T. Unraveling tumor microenvironment of small-cell lung cancer: Implications for immunotherapy. Semin Cancer Biol 2022;86:117-25.
[Crossref] [Google Scholar] [PubMed]
- Jun KH, Kim JH, Jung JH, Choi HJ, Chin HM. Expression of claudin-7 and loss of claudin-18 correlate with poor prognosis in gastric cancer. Int J Surg 2014;12(2):156-62.
[Crossref] [Google Scholar] [PubMed]
- Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390(10111):2461-71.
[Crossref] [Google Scholar] [PubMed]
- Fang X, Xu J, Jin K, Qian J. Combining of immunotherapeutic approaches with chemotherapy for treatment of gastric cancer: Achievements and limitations. Int Immunopharmacol 2023;118:110062.
[Crossref] [Google Scholar] [PubMed]
- Shitara K, Ozguroglu M, Bang YJ, di Bartolomeo M, Mandala M, Ryu MH, et al. Pembrolizumab vs. paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial. Lancet 2018;392(10142):123-33.
[Crossref] [Google Scholar] [PubMed]
- Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib KEYNOTE-012 study. J Clin Oncol 2016;34(21):2460-7.
[Crossref] [Google Scholar] [PubMed]
- Zeng D, Wu J, Luo H, Li Y, Xiao J, Peng J, et al. Tumor microenvironment evaluation promotes precise checkpoint immunotherapy of advanced gastric cancer. J Immunother Cancer 2021;9(8):1-15.
[Crossref] [Google Scholar] [PubMed]
- Wu Q, Maniatis T. A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell 1999;97(6):779-90.
[Crossref] [Google Scholar] [PubMed]
- Ing-Esteves S, Kostadinov D, Marocha J, Sing AD, Joseph KS, Laboulaye MA, et al. Combinatorial effects of alpha-and gamma-protocadherins on neuronal survival and dendritic self-avoidance. J Neurosci 2018;38(11):2713-29.
[Crossref] [Google Scholar] [PubMed]
- Du J, Dong Y, Li Y. Identification and prognostic value exploration of cyclophosphamide (cytoxan)-centered chemotherapy response-associated genes in breast cancer. DNA Cell Biol 2021;40(11):1356-68.
[Crossref] [Google Scholar] [PubMed]
- Carter H, Samayoa J, Hruban RH, Karchin R. Prioritization of driver mutations in pancreatic cancer using Cancer-specific High-throughput Annotation of Somatic Mutations (CHASM). Cancer Biol Ther 2010;10(6):582-7.
[Crossref] [Google Scholar] [PubMed]
- Sonugür FG, Akbulut H. The role of tumor microenvironment in genomic instability of malignant tumors. Front Genet 2019;10:1-7.
[Crossref] [Google Scholar] [PubMed]
- Ishimoto T, Miyake K, Nandi T, Yashiro M, Onishi N, Huang KK, et al. Activation of transforming growth factor beta 1 signaling in gastric cancer-associated fibroblasts increases their motility, via expression of rhomboid 5 homolog 2, and ability to induce invasiveness of gastric cancer cells. Gastroenterology 2017;153(1):191-204.e116.
[Crossref] [Google Scholar] [PubMed]
- Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013;501(7467):346-54.
[Crossref] [Google Scholar] [PubMed]
- Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: A prognostic factor that should not be ignored. Oncogene 2010;29(8):1093-102.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Li C, Lu Y, Liu C, Yang W. Tumor microenvironment-mediated immune tolerance in development and treatment of gastric cancer. Front Immunol 2022;13:1-17.
[Crossref] [Google Scholar] [PubMed]
- Du Y, Wei Y. Therapeutic potential of natural killer cells in gastric cancer. Front Immunol 2018;9:1-12.
[Crossref] [Google Scholar] [PubMed]
- Chen D, Zhang X, Li Z, Zhu B. Metabolic regulatory crosstalk between tumor microenvironment and tumor-associated macrophages. Theranostics 2021;11(3):1016-30.
[Crossref] [Google Scholar] [PubMed]
- Bolandi N, Derakhshani A, Hemmat N, Baghbanzadeh A, Asadzadeh Z, Afrashteh NM, et al. The positive and negative immunoregulatory role of B7 family: Promising novel targets in gastric cancer treatment. Int J Mol Sci 2021;22(19):10719.
[Crossref] [Google Scholar] [PubMed]
- Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 1995;182(2):459-65.
[Crossref] [Google Scholar] [PubMed]
- Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med 2012;209(6):1201-17.
[Crossref] [Google Scholar] [PubMed]
- Ulase D, Behrens HM, Kruger S, Zeissig S, Rocken C. Gastric carcinomas with stromal B7-H3 expression have lower intratumoural CD8+ T cell density. Int J Mol Sci 2021;22(4):2129.
[Crossref] [Google Scholar] [PubMed]
- Chen QY, Zhou WJ, Zhang JG, Zhang X, Han QY, Lin A, et al. Prognostic significance of the immune checkpoint HLA-G/ILT-4 in the survival of patients with gastric cancer. Int Immunopharmacol 2022;109:108798.
[Crossref] [Google Scholar] [PubMed]
- Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019;51(2):202-6.
[Crossref] [Google Scholar] [PubMed]
- Greally M, Chou JF, Chatila WK, Margolis M, Capanu M, Hechtman JF, et al. Clinical and molecular predictors of response to immune checkpoint inhibitors in patients with advanced esophagogastric cancer. Clin Cancer Res 2019;25(20):6160-9.
[Crossref] [Google Scholar] [PubMed]
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372(26):2509-20.
[Crossref] [Google Scholar] [PubMed]
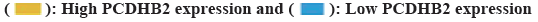 .
.

 .
.
 .
.