- *Corresponding Author:
- W. Zhao
Department of Orthopaedics, Affiliated Hospital of Shaanxi University of Traditional Chinese Medicine, Xianyang, Shaanxi Province 712000, China
E-mail: yougu838665495@163.com
| Date of Received | 14 August 2021 |
| Date of Revision | 09 February 2022 |
| Date of Acceptance | 04 December 2022 |
| Indian J Pharm Sci 2022;84(6):1597-1601 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effect and mechanism of Tripterygium wilfordii polyglycosides on rheumatoid arthritis rats by regulating the signal transducer and activator of transcription 3 pathways. 45 specific-pathogen free male Sprague-Dawley rats were randomly divided into normal control group, model group and Tripterygium wilfordii polyglycosides group with 15 rats in each group. The histopathological changes of the synovium of the ankle joint of rats in each group, on the 12th, 20th and 28th d were compared. On the 12th d, the degree of tissue swelling in the model group was significantly higher than that in the normal control group (p<0.05); there was no significant difference in the degree of toe swelling between the Tripterygium wilfordii polyglycosides group and the model group (p>0.05). At 20 d and 28 d, the degree of toe swelling in the model group was significantly higher than that in the normal control group (p<0.05); the swelling degree of toes in Tripterygium wilfordii polyglycosides group was significantly lower than that in model group (p<0.05). The serum interleukin-6 level in model group was significantly higher than that in normal control group and interleukin-10 level was significantly lower than that in normal control group (p<0.05). The level of interleukin-6 in serum of rats in Tripterygium wilfordii polyglycosides group was significantly lower than that in model group and the level of interleukin-10 was significantly higher than that in model group (p<0.05). The expression levels of phosphorylated Janus kinase 2 and phosphorylated signal transducer and activator of transcription 3 protein in model group were significantly higher than those in normal control group; the expression levels of phosphorylated Janus kinase 2 and phosphorylated signal transducer and activator of transcription 3 protein in Tripterygium wilfordii polyglycosides group were significantly lower than those in the model group (p<0.05). Tripterygium wilfordii polyglycosides has the effect of anti-rheumatoid arthritis and its mechanism may be through regulating the Janus kinase 2/ signal transducer and activator of transcription 3 signal pathways by reducing the expression level of phosphorylated Janus kinase 2 and phosphorylated signal transducer and activator of transcription 3 proteins.
Keywords
Tripterygium wilfordii polyglycosides, Janus kinases 2, signal transducer, transcription 3, rheumatoid arthritis
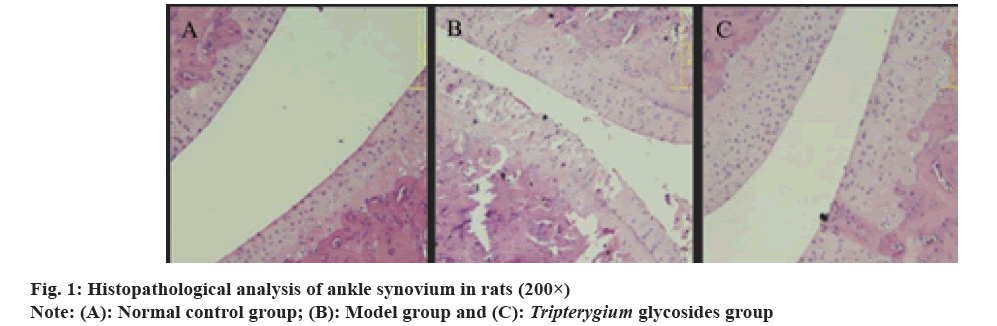
Rheumatoid Arthritis (RA) is a chronic disease caused by the interaction of heredity, environment and other factors, whose incidence is about 0.35 % but the disability rate is up to 20 % within 1 y after onset, causing a great influence on the daily life of patients[1]. At present, there are no reliable and effective intervention drugs for the disease at home and abroad. Hormone and non-steroidal anti- inflammatory drugs are primarily applied in the treatment of RA. However, many experiments have proved that the drugs pose a threat to the health of patients to a certain extent. Thus, it is of great value to investigate new and safe drugs to improve the clinical efficacy and prognosis of patients with RA[2,3]. Tripterygium glycosides has been proved to be useful in the treatment of the disease, it shows ideal effects of effectively relieving pain and improving clinical symptoms[4]. The clinical literature reveals that Tripterygium glycosides contains some diterpenoids and alkaloids which are widely used in clinical practice since they can reduce the incidence of side effects and maintain the clinical effect at the same time[5]. Animal experiments have suggested that the expression disturbance of Signal Transducer and Activator of Transcription 3 (STAT3) may be associated with the inflammatory response of arthritis[6]. Numerous studies have indicated that STAT3, a key pathogen of RA, blocks apoptosis of fibroblast-like synovial cells and improve T cell survival[7]. Animal RA related studies have shown that abnormal activation of the Janus Kinase 2 (JAK2) or STAT3 signaling pathway can cause inflammatory diseases in joints[8]. The role and mechanism of Tripterygium glycosides and JAK2-STAT3 signaling pathway in RA are clinically few, so some analysis will be made from the aspects in this study. 45 specific-pathogen free-grade male Sprague-Dawley (SD) rats obtained from Beijing Xinshengyuan Biomedical Technology Co., Ltd. License key-SCXK (Beijing) 2019-0015, all rats were separately raised in cages, drank and fed freely, at room temperature of 20°-25° with the humidity of 50 %-55 % and they were fed adaptively for 1 w. Tripterygium glycosides tablets obtained from Hunan Qianjin Xieli Pharmaceutical Co., Ltd.; complete Freund’s adjuvant obtained from Shanghai WeGene Biotechnology Co., Ltd. 10 % polyformaldehyde obtained from Guangzhou Pythonbio Products Co., Ltd.; Enzyme-Linked Immunosorbent Assay (ELISA) kit obtained from Guangzhou Jinde Biotech Co., Ltd.; rabbit anti-phosphorylated (p)-JAK2, JAK2, p-STAT3, STAT3 obtained from Wuhan Yipu Biotechnology Co., Ltd.; mice anti-beta (β)-actin obtained from Beijing Dingguo Changsheng Biotechnology Co., Ltd.; concentrated Diaminobenzidine (DAB) kit obtained from Beijing Jiehui Bogo Biotechnology Co., Ltd. Toes volume meter obtained from Nanjing; ultraviolet gel imaging system obtained from Shenzhen coastal trading Co., Ltd.; low-speed desk centrifuge obtained from Beijing Taize Jiaye technology development Co., Ltd.; microscope obtained from Olympus (Beijing) sales and service Co., Ltd.; electrophoretic apparatus obtained from Hangzhou Big Fish Bio-tech Co., Ltd. 45 SD rats were randomized into several groups; normal, control, model and Tripterygium glycosides, with 15 rats in each group, 0.1 ml of complete Freund’s adjuvant were intracutaneously injected in the left rear toes to cause inflammation in the model and Tripterygium glycosides groups, after 12 d, rats in the Tripterygium glycosides group were given 10 mg/kg-1 Tripterygium glycosides for intragastric administration and in the control and model groups, 0.9 % normal saline was given for the administration, once per day for 16 successive days. At 28 d, the rats were anesthetized and killed by abdominal aorta extraction and their blood was centrifuged and stored in the ultra-cold freezer at -80°, the synovial tissue of right posterior ankle joint was collected and fixed in 10 % polyformaldehyde for detection. Using the toe volume meter, the right rear toes volume of rats in each group was measured before inflammation and at 12 d, 20 d, 28 d and the value and mean volume were recorded, swelling degree=(post inflammation-pre inflammation) mean volume value. The effect of Tripterygium glycosides on histopathological morphology of ankle synovium in rats was determined by Hematoxylin and Eosin (H&E) staining. The levels of serum inflammatory related factors (Interleukin (IL)-6, IL-10) and the expression of JAK2-STAT3 pathway related proteins (p-JAK2, JAK2, p-STAT3, STAT3) were measured by ELISA and Western blot respectively. The research data were analyzed by Statistical Package for the Social Sciences (SPSS) 22.0. The measurement data of toe swelling, IL-6 and IL-10 levels were expressed as (x±s). T-test and Analysis of Variance (ANOVA) were employed to make pairwise comparison and multiple comparisons for the groups respectively. p<0.05 means the existence of significant difference. At 12 d, the tissue in the model group experienced more severe swelling vs. the normal control group (p<0.05). The Tripterygium glycosides group showed no significant difference in toe swelling vs. the model group (p>0.05). At 20 d, 28 d, the tissue in the model group experienced more severe swelling vs. the normal control group (p<0.05). The swelling of toes in the Tripterygium glycosides group was evidently milder vs. the model group (p<0.05) as shown in Table 1. In normal control group, the cells were arranged neatly, the cartilage surface of ankle joint was smooth, the synovial structure was intact, without inflammatory cell infiltration and angiogenesis. In the model group, the cells were disordered, cartilage of ankle joint was damaged, synovial cells proliferated, a large number of inflammatory cells infiltrated and pannus occurred. The pathology in Tripterygium glycosides group significantly improved vs. the model group, which is similar to that of normal control group as shown in fig. 1. In contrast with the normal control group, serum IL-6 levels were remarkably higher and IL-10 levels were apparently lower in the model group (p<0.05). In the model group, serum IL-6 levels were markedly lower and IL-10 levels were significantly higher in the Tripterygium glycosides group (p<0.05) as shown in Table 2. The expression levels of p-JAK2 and p-STAT3 in the model group were significantly higher than those in control group. The expression levels of p-JAK2 and p-STAT3 in Tripterygium glycosides group were prominently lower vs. the model group (p<0.05) as shown in Table 3. RA is a widespread disease globally which is often seen in people aged 25-55 and is more common in female than male, the incidence in female approximately triples that in male[9]. RA is a key factor leading to the weakening of labor force and high disability rate in our country, so the research on the mechanism of the disease has always been a hot topic clinically. Clinical studies have demonstrated that Tripterygium glycosides are effective and have become the second-line drug of choice in the treatment of RA[10]. It has also been reported that Tripterygium glycosides can effectively improve the clinical manifestation of patients with RA and turn rheumatoid factors negative. Additionally, the drug can also reduce immunoglobulin levels and alleviate the damage to articular cartilage[11]. Tripterygium glycosides have been reported to effectively and selectively group the activity of nuclear factor kappa B and thus inhibit the expression of cyclooxygenase-2 and nitric oxide synthase. Some researchers have found that Tripterygium glycosides can block the expression of matrix metalloproteinase and inflammation-related factors and inhibit the synthesis of nitric oxide and prostaglandin E2 so as to fight against RA[12]. This study found that the swelling of toes in the Tripterygium glycosides group was markedly milder vs. the model group (p<0.05), the histopathology of ankle synovium in the Tripterygium glycosides group was significantly improved vs. the model group. IL-6 is a multifunctional proinflammatory factor and its expression level is closely related to RA[13]. Clinical studies have shown that a mutation in the Y759F site of IL-6 receptor leads to the occurrence of RA like lesions[14]. IL-10 is an anti-inflammatory factor and it has been reported that increasing IL-10 levels can further regulate cell-related factors to resist inflammatory reactions in the body[15]. IL-10 has promoter gene polymorphism and there are numerous studies on its mechanism. After intervention with related drugs, IL-10 related genotype will alter, suggesting that IL-10 may be used as an important index to evaluate the clinical effect of disease-related intervention drug. It was found in this study that the proinflammatory cytokine IL-6 and IL-10 level in the serum of RA rats suffered a significant decrease and increase respectively, following the intervention with Tripterygium glycosides, suggesting that the drug has good anti-inflammatory effect. The JAK-STAT signaling pathway has been activated during suffering from RA. It has been reported that many types of cytokines can be involved in signal transduction through the mediation of JAK that can also be activated by epidermal growth factor receptor and fibroblast growth factor receptor. Clinical studies have revealed that cytokine signal transduction can be mediated and cytokine binding to its surface receptors can promote receptor dimerization, activate JAKs by tyrosine phosphorylation and then control the expression of related genes by hyper accelerated phosphorylation of JAK and STAT, ultimately involving in cytokine-mediated signal pathway. It has been clinically confirmed that cytokines can transmit information mediated by JAK/STAT signaling pathways, so it is of great significance to study cytokines and their JAK2-STAT3 signaling pathways. The clinical experiments have demonstrated that the level of synovial cytokine in RA patients increased significantly and the cytokine can bind to JAK2 to activate JAK-STAT signaling pathway, thereby involving in the development of RA and the occurrence of joint damage, so effective and selective blocking of JAK2 expression plays a critical role in fighting against RA. This study found that Tripterygium glycosides could protect RA rats by blocking the expressions of p-JAK2 and p-STAT3 proteins in synovial tissues. In conclusion, Tripterygium glycosides shows anti-RA effect, which may be achieved by reducing the expressions of p-JAK2 and p-STAT3 proteins and regulating the JAK2-STAT3 signaling pathway.
| Grouping | n | 12 d | 20 d | 28 d |
|---|---|---|---|---|
| Normal control group | 15 | 0.07±0.02 | 0.10±0.13 | 0.14±0.06 |
| Model group | 15 | 0.49±0.17* | 1.21±0.25* | 1.25±0.32* |
| Tripterygium glycosides group | 15 | 0.45±0.03 | 0.73±0.15# | 0.23±0.14# |
Note: Compared with the control group, *p<0.05 and compared with the model group, #p<0.05
Table 1: Comparison of TOE Swelling in Different Groups (x±s)
| Grouping | n | IL-6 (pg/ml) | IL-10 (pg/ml) |
|---|---|---|---|
| Normal control group | 15 | 56.53±17.73 | 57.66±9.04 |
| Model group | 15 | 76.81±18.32* | 36.17±8.83* |
| Tripterygium glycosides group | 15 | 61.04±12.87# | 55.97±6.90# |
Note: Compared with the control group, *p<0.05 and compared with the model group, #p<0.05
Table 2: The Concentration of IL-6 and IL-10 in the Serum of Each Group of RATS (x±s)
| Grouping | n | p-JAK2/JAK2 | p-STAT3/STAT3 |
|---|---|---|---|
| Normal control group | 15 | 0.09±0.05 | 0.15±0.06 |
| Model group | 15 | 0.75±0.29* | 0.89±0.31* |
| Tripterygium glycosides group | 15 | 0.31±0.15# | 0.24±0.11# |
Note: Compared with the control group, *p<0.05 and compared with the model group, #p<0.05
Table 3: Comparison of p-JAK2/JAK2 and p-STAT3/STAT3 of RATS in Each Group
Conflict of interests:
The authors declared no conflict of interests
References
- Mota LM, Kakehasi AM, Gomides AP, Duarte AL, Cruz BA, Brenol CV, et al. 2017 recommendations of the Brazilian Society of Rheumatology for the pharmacological treatment of rheumatoid arthritis. Adv Rheumatol 2019;58(1):2-6.
[Crossref] [Google Scholar] [PubMed]
- Baker KF, Isaacs JD. Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann Rheum Dis 2018;77(2):175-87.
[Crossref] [Google Scholar] [PubMed]
- Krüger K, Burmester GR, Wassenberg S, Bohl-Bühler M, Thomas MH. Effectiveness and safety of golimumab in patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis under real-life clinical conditions: Non-interventional GO-NICE study in Germany. BMJ Open 2018;8(6):e021082.
[Google Scholar] [PubMed]
- Wang XY, Li TX, Xue ZP, Lyu C, Li HZ, Fan YF, et al. Clinical symptoms effect of Tripterygium glycosides tablets alone or combined with methotrexate in treatment of rheumatoid arthritis: A meta-analysis. Zhongguo Zhong Yao Za Zhi 2019;44(16):3533-41.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Sun W, Xia Q. Effects of Tripterygium glycosides combined with recombinant human tumor necrosis factor receptor type II antibody fusion protein on serum vascular endothelial growth factor, rheumatoid factor and human nuclear factor κB receptor activating factor ligand in patients with rheumatoid arthritis. Clin Med Chin 2019;35(3):250-4.
- Chen JF, Wu P, Xia R, Yang J, Huo XY, Gu DY, et al. STAT3-induced lncRNA HAGLROS overexpression contributes to the malignant progression of gastric cancer cells via mTOR signal-mediated inhibition of autophagy. Mol Cancer 2018;17(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Cui X, Jing X, Yi Q, Long C, Tan B, Li X, et al. Systematic analysis of gene expression alterations and clinical outcomes of STAT3 in cancer. Oncotarget 2018;9(3):3198.
[Crossref] [Google Scholar] [PubMed]
- Tang Y, Tong X, Li Y, Jiang G, Yu M, Chen Y, et al. JAK2/STAT3 pathway is involved in the protective effects of epidermal growth factor receptor activation against cerebral ischemia/reperfusion injury in rats. Neurosci Lett 2018;662:219-26.
[Crossref] [Google Scholar] [PubMed]
- Nordgren B, Fridén C, Demmelmaier I, Bergström G, Lundberg IE, Nessen T, et al. An outsourced health-enhancing physical activity program for people with rheumatoid arthritis: Study of the maintenance phase. J Rheumatol 2018;45(8):1093-100.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Xue Z. Short-term efficacy of Tripterygium glycosides combined with methotrexate in the treatment of rheumatoid arthritis. Jilin J Chin Med 2018;38(6):660-3.
- Chanjuan J, Jieying J, Pharmacy DO. Effects of Tripterygium glycosides combined with compound α-keto acid tablets on inflammatory response, oxidative stress and urinary TGF-β1, IV-C levels in patients with chronic renal failure. J Hainan Med Univ 2019;3:78-9.
- Chen J. Effects of Tripterygium glycosides combined with methotrexate in the treatment of rheumatoid arthritis. Prac Clin J Int Tradit Chin Western Med 2019;19(7):145-6.
- Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med 2018;24(6):739-48.
[Crossref] [Google Scholar] [PubMed]
- Zhang X, Hu F, Li G, Li G, Yang X, Liu L, et al. Human colorectal cancer-derived mesenchymal stem cells promote colorectal cancer progression through IL-6/JAK2/STAT3 signaling. Cell Death Dis 2018;9(2):1-3.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Wu Y, Jiao J, Huang Q. Mycobacterium tuberculosis infection induces IL-10 gene expression by disturbing histone deacetylase 6 and histonedeacetylase 11 equilibrium in macrophages. Tuberculosis 2018;108:118-23.
[Crossref] [Google Scholar] [PubMed]