- *Corresponding Author:
- Dan Wang
Department of Histology and Embryology, College of Basic Medical Science, Jinzhou Medical University, Key Laboratory of Follicular Development and Reproduction Regulation of Liaoning Province Liaoning Jinzhou 121001, China
E-mail: wanganan1997@163.com
| Date of Submission | 27 May 2017 |
| Date of Revision | 03 April 2018 |
| Date of Acceptance | 10 October 2018 |
| Indian J Pharm Sci 2018;80(6):1100-1107 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Effects of melatonin against carbon tetrachloride-induced acute liver and testicular toxicity in rats as well as the possible mechanisms were investigated in the present study. Rats were acclimatized and the serum levels of alanine aminotransferase, aspartate aminotransferase and reduced glutathione were measured. The malondialdehyde, superoxide dismutase, and reduced glutathione concentrations in the hepatic homogenate, and histopathological changes in rat liver and testes were also determined. Membrane potential was analysed with flow cytometry. The expression level of telomerase and caspase-3 were determined by reverse transcriptase polymerase chain reaction. Melatonin increased the content of serum reduced glutathione in rats with acute liver injury induced by carbon tetrachloride. The extent of malondialdehyde formation was reduced; superoxide dismutase activity and the reduced glutathione contents were increased in the hepatic homogenate in melatonin groups. Histopathological examination of rat liver and testicular sections confirmed the biochemical observations. Melatonin significantly restored the plasma membrane potential and decreased mRNA level of telomerase and caspase-3. These results indicated that melatonin exerted a significant protective effect against acute hepatotoxicity induced by carbon tetrachloride in rats, which might be due to free radical scavenging, inhibition of lipid peroxidation, increasing antioxidant activity as well as restoration plasma membrane potential. The expression of telomerase and caspase-3 might partially contribute to the protective effects on carbon tetrachloride-induced acute liver damage. Reduced glutathione content might also partially contribute to the protective effects of melatonin on the oxidative damage caused by carbon tetrachloride in rat testis.
Keywords
Melatonin, protective effects, CCl4, testicular toxicity, lipid peroxidation
Excessive production of free radicals and oxidative stress can be induced by a variety of factors such as ionizing radiation or exposure to drugs and xenobiotics (e.g. carbon tetrachloride, CCl4). CCl4 is a wellestablished hepatotoxin, which induces free radical damage in tissues. Studies have demonstrated that the metabolites of CCl4-induced lipid peroxidation, increased the amount of glutathione, glutathione reductase and heme oxygenase-1 leading to oxidative damage in tissues such as the liver, lungs, testis and kidney [1,2].
Administration of antioxidants could conceivably protect the human body from the effects of free radicals and lipid peroxidation, thereby retard the progress of many chronic diseases [3]. As it is well-known, melatonin, the chief secretory product of the pineal gland, participates in many important physiological functions, including antiapoptosis and as an antioxidant [4-6]. In addition, melatonin also decreases free radical levels by stimulating the activities of enzymes involved in antioxidative defence [7].
Many studies showed that melatonin exerted protective role in different types of liver injury and fibrosis [8] and that melatonin ameliorated ischemia-reperfusion toxicity in mouse testicular tissue [9]. Further studies are needed to clarify the direct protective mechanism of melatonin in the pathogenesis of CCl4 injury. The mechanism of melatonin’s protective effects on liver and testicular injury would be of great interest, therefore, the present study was designed to evaluate the effect of melatonin on CCl4-induced acute damage of liver and testicular tissue of rats.
Materials and Methods
Melatonin (Sigma, USA) was kept at −20° in the dark, it was dissolved in normal saline (NS). CCl4 (Chongqing Chuandong Chemical Co., Ltd.), bean oil and diagnostic kits for alanine aminotransferase (ALT, Beijing BHKT Clinical Reagent Co., Ltd.); aspartate aminotransferase (AST, Changchun Huili Biotech Co., Ltd.), malondialdehyde (MDA), superoxide dismutase (SOD), reduced glutathione (GSH, Nanjing Jiancheng Bioengineering Institute) and all other chemicals were purchased from commercial sources. Six week old Sprague Dawley rats of both sexes were purchased from the animal facility at the China Medical University, P. R. China (certificate SCXK(LIAO)2015-0001). The study was approved by the local Animal Ethics Committee. Rats were reared under standard laboratory conditions (22±2°, 60±10 % relative humidity and a 12-h light-dark cycle) and had free access to food and water throughout the experiment.
Experimental protocol
In the pre-treatment studies, rats were randomly divided into 3 groups with each group containing 8 animals, including a control group, CCl4 group, and CCl4 group pretreated with melatonin (CCl4+melatonin). Melatonin was diluted in NS to a final concentration of 10 %. CCl4 was injected at 8 g/kg (1:1 in bean oil, intraperitoneal, ip). The control group and CCl4 group were given NS (1 ml/100 g, ip) daily. The CCl4+melatonin group animals were given melatonin (10 mg/kg, ip). All administrations were conducted once daily for two consecutive days. On the second day, all rats except those in the control group were given simultaneously a CCl4 (ip) 1 h after the last administration, while the control group received bean oil alone. All animals were sacrificed following deep chloral hydrate anaesthesia 24 h after CCl4 administration.
Liver function monitoring
Blood was taken from abdominal aorta for analysis of the serum ALT, AST and GSH levels, which were determined with commercially available diagnostic kits [10,11]. Livers were excised immediately after animals were sacrificed. Levels of MDA, GSH and SOD in the liver homogenate were estimated using commercially available diagnostic kits. The level of MDA, a measure of lipid peroxidation, was measured by the thiobarbituric acid reaction method [12]. GSH measurements were taken using a modification of the Boyne and Ellman procedure [13]. The activity of SOD was measured by the method of Fridovich [14]. The protein concentration was measured by the method of Lowry [15].
Light microscopy assay
A portion of the left lobe of the liver and testes were fixed in 4 % paraformaldehyde for 48 h. The samples were incubated in 50 % ethanol for 1 h, routinely dehydrated and embedded, and sections of 5 μm thick were obtained, deparaffinised, dehydrated in ethanol (50-100 %), and cleared with xylene. The slices were dewaxed, stained with haematoxylin and eosin (H and E) for histological examination. H and E sections were examined under the optical microscopy (OLYMPUS, BX60 Japan) at 100-fold magnification.
Measurement of plasma membrane potential
DiBAC4(3) belongs to a class of anionic slow potential-sensitive dyes and has been shown to respond to membrane depolarization with an increase in fluorescence resulting from the increased intracellular concentration and accumulation in intracellular lipidrich compartments [16]. The membrane-potentialsensitive dye DiBAC4(3) is widely used in determining the changes in membrane potential. The distribution of this probe within the membrane and the resulting fluorescence is dependent on the transmembrane potential of the cell. A linear relationship between membrane potential and the DiBAC4(3) fluorescence in various cell types has been established [17]. Liver tissues were removed and placed in a 5-ml conical tube in 2 ml PBS, washed twice and blew out using a Transferpettor. After centrifugation at 1000 rpm for 5 min, liver cells were loaded with 4 μM DiBAC4(3) for 30 min at 37°, then, washed and filtered through a 200 μm mesh filter before being resuspended in 500 μl of PBS, ready to be analysed by flow cytometry.
Reverse transcription-polymerase chain reaction (RT-PCR) analysis of telomerase
Total RNA from liver was isolated using reagent Trizol (Gibco, Inc., Grand Island, NY) according to manufacturer’s instructions [18]. cDNA was amplified for 25 cycles so that the PCR product remained in the linear range. β-actin was used as an internal control to confirm mRNA integrity. The identity of all PCR products was confirmed by size based on the known length of the DNA sequence on 1 % agarose gel stained by ethidium bromide. The optical density of the bands was analysed on the GeneSnap system (Syngene, Synoptics Ltd. Frederick, MD).
Statistical analyses
Values are expressed as mean±standard error of the mean (SEM). One-way ANOVA was used followed by Fisher’s least-significant difference for the homogeneity testing of variance (Levene’s test), and the data were analysed by Dunnett’s T3 for the heteroscedasticity of variance test. All statistical procedures were performed using SPSS 17.0 software for Windows (SPSS Inc., USA).
Results and Discussion
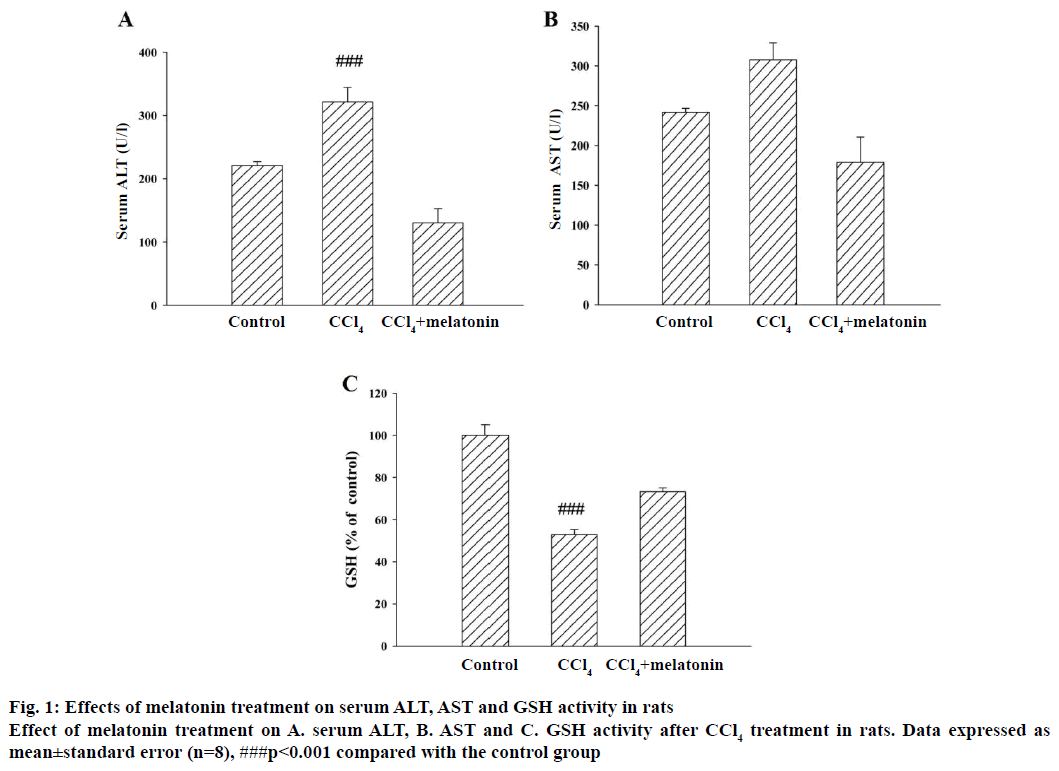
The results of the hepatoprotective effect of melatonin on the serum ALT and AST activity are shown in Figure 1A and B. In the CCl4 group, serum ALT and AST activities were 321.6 and 307.5 units/l, respectively, whereas these values were only 220.6 and 179.0 units/l, respectively, in the control group. These data indicated that CCl4 significantly increased both of these two enzyme activities. Moreover, the elevated levels of serum ALT and AST were significantly reduced in the groups pre-treated with melatonin (10 mg/kg). The level of GSH was substantially decreased in the CCl4 group compared to the control group. Pre-treatment with melatonin significantly prevented the decrease in GSH level, but not up to normal levels (Figure 1C).
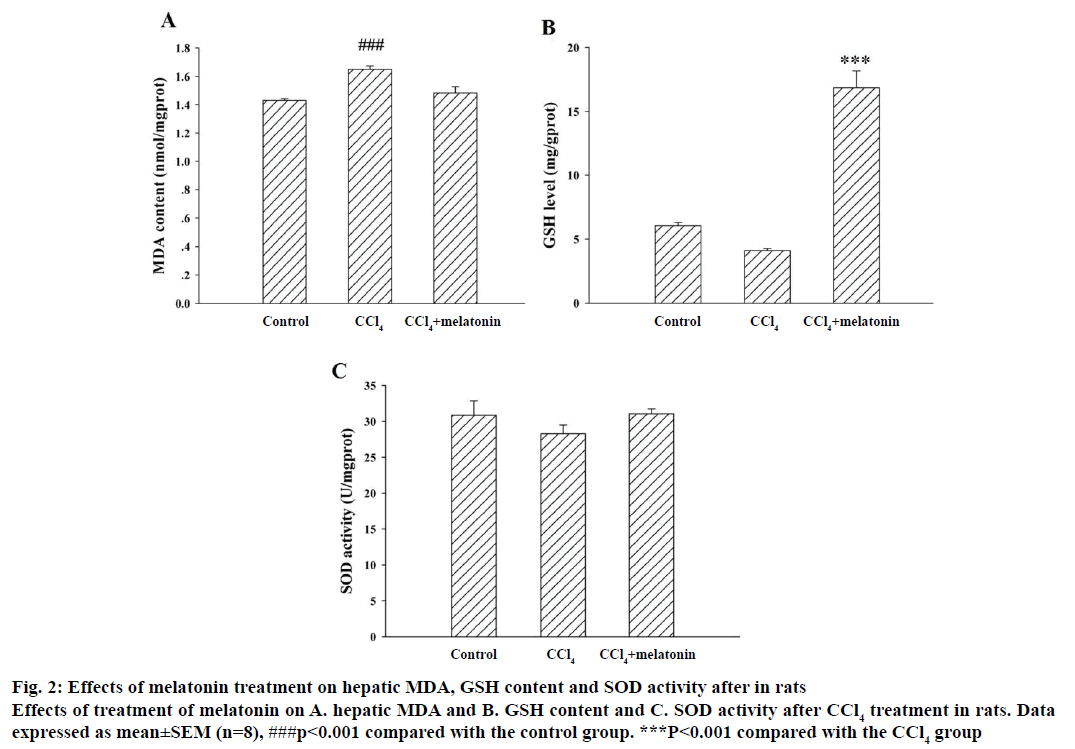
Lipid peroxidation is considered to be one of the principal causes of CCl4-induced liver injury. Pretreatment of rat with melatonin effectively inhibited CCl4-induced hepatotoxicity, as shown by the reduced level of hepatic MDA formation, an index of the chain reaction of lipid peroxidation (Figure 2A). SOD is an extremely effective antioxidant enzyme. The increased production of free radicals caused by administration of CCl4 was a major cause of the reduced SOD activity. GSH, a non-enzymatic antioxidant, is an important biomolecule for combating chemically-induced toxicity. The data showed that the SOD activity significantly decreased and the GSH stores were markedly depleted in rats treated with CCl4 compared with the control group. Pre-treatment with melatonin significantly reduced the extent of CCl4-induced hepatic GSH depletion (Figure 2B) and prevented the decrease in SOD activity (Figure 2C).
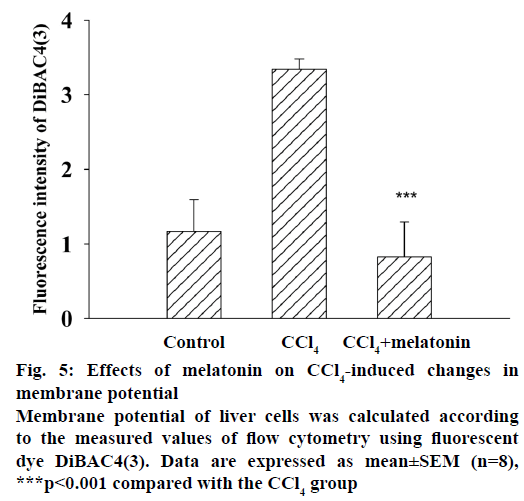
Figure 2: Effects of melatonin treatment on hepatic MDA, GSH content and SOD activity after in rats
Effects of treatment of melatonin on A. hepatic MDA and B. GSH content and C. SOD activity after CCl4 treatment in rats. Data expressed as mean±SEM (n=8), ###p<0.001 compared with the control group. ***P<0.001 compared with the CCl4 group
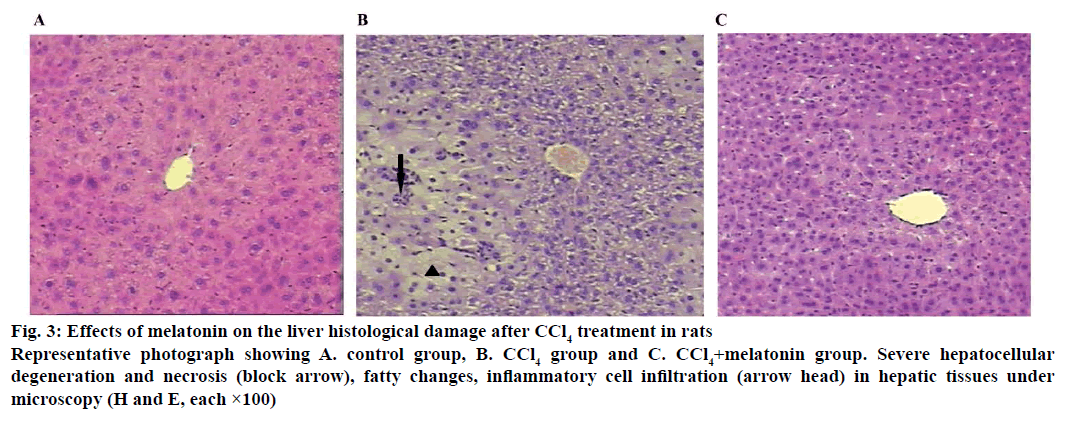
Histological assessment was used to complete the study of the hepatoprotective effects of melatonin on CCl4-induced acute liver damage (Figure 3). The histology of the liver sections of control animals showed normal hepatic cells with well-preserved cytoplasm, prominent nucleus and nucleolus, and visible central veins. The liver sections of CCl4-intoxicated rats revealed extensive liver injuries, characterized by moderate to severe hepatocellular degeneration and necrosis, fatty changes, inflammatory cell infiltration and ballooning degeneration. However, the histopathological hepatic lesions were markedly reduced by pre-treatment with melatonin. This was in good agreement with the results of serum aminotransferase activity (Figure 1) and hepatic oxidative stress levels (Figure 2).
Figure 3: Effects of melatonin on the liver histological damage after CCl4 treatment in rats
Representative photograph showing A. control group, B. CCl4 group and C. CCl4+melatonin group. Severe hepatocellular degeneration and necrosis (block arrow), fatty changes, inflammatory cell infiltration (arrow head) in hepatic tissues under microscopy (H and E, each ×100)
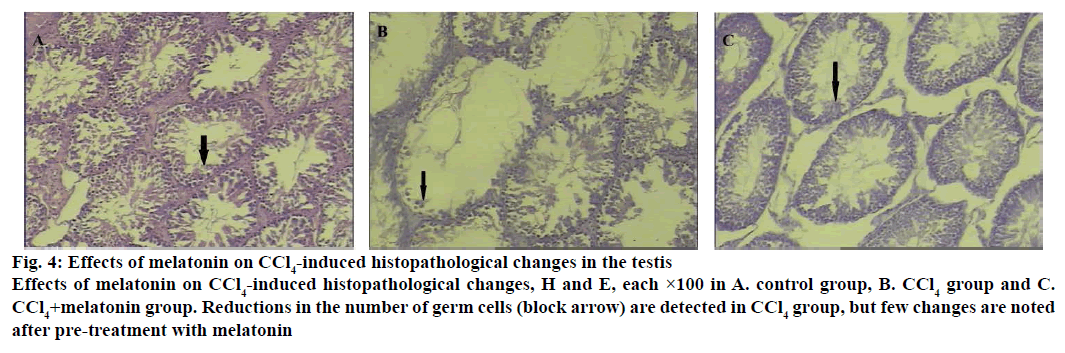
Histopathology of testis sections show (Figure 4) reductions in the number of germ cells in the CCl4-treated group compared with the control group. The CCl4-induced testicular lesions were reduced by pre-treatment with melatonin. Cells exposed to CCl4 showed an increased DiBAC4(3) fluorescence indicating depolarization of the membrane (Figure 5). Treatment with melatonin (10 mg/kg) reduced the depolarization and restored the normal level.
Figure 4: Effects of melatonin on CCl4-induced histopathological changes in the testis
Effects of melatonin on CCl4-induced histopathological changes, H and E, each ×100 in A. control group, B. CCl4 group and C. CCl4+melatonin group. Reductions in the number of germ cells (block arrow) are detected in CCl4 group, but few changes are noted after pre-treatment with melatonin
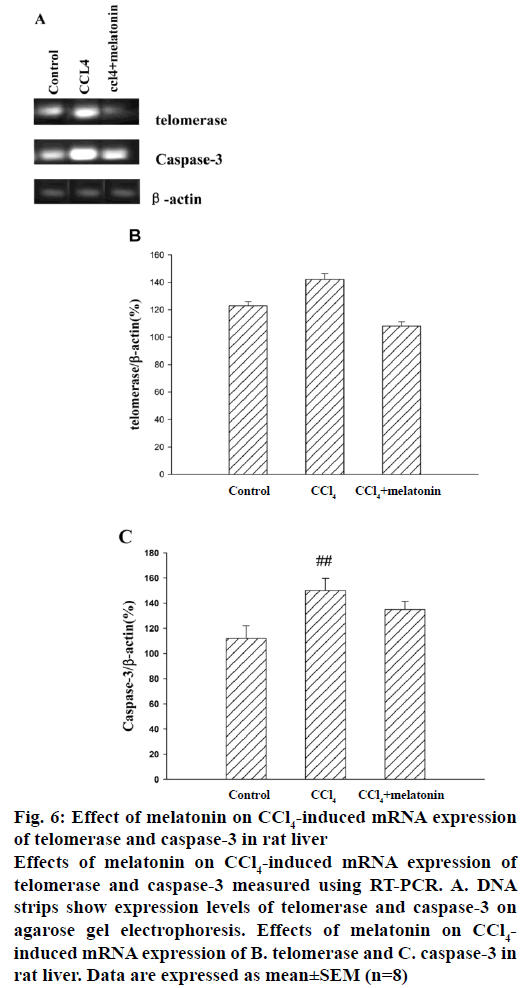
In this investigation whether melatonin could modulate hepatic cell survival after CCl4 injection was tested by analysing the transcriptional changes of related genes (telomerase and caspase-3). Representative RT-PCR results for telomerase and caspase-3 mRNA expression are shown in Figure 6A, and quantitative data are summarized in Figure 6B and C. Telomerase and caspase-3 were significantly unregulated at 24 h after the CCl4 injection compared with the control group. In the melatonin pre-treatment group, telomerase and caspase-3 mRNA expression was significantly lower than in the CCl4 group.
Figure 6: Effect of melatonin on CCl4-induced mRNA expression of telomerase and caspase-3 in rat liver
Effects of melatonin on CCl4-induced mRNA expression of telomerase and caspase-3 measured using RT-PCR. A. DNA strips show expression levels of telomerase and caspase-3 on agarose gel electrophoresis. Effects of melatonin on CCl4-induced mRNA expression of B. telomerase and C. caspase-3 in rat liver. Data are expressed as mean±SEM (n=8)
In the present study, a rat model of CCl4-induced liver injury was adopted to investigate the possible hepatoprotective effects of melatonin. In this study, a significant increase in the level of AST and ALT in the serum was observed after administration of CCl4, as reported previously [19]. However, the increased levels of these enzymes were significantly decreased by pre-treatment with melatonin, implying that the drug reduced liver damage, which is further confirmed by the alleviated amount of histopathological injury. Many researchers reported the effects of melatonin on CCl4-induced liver toxicity. However, more research studied the influence of melatonin on CCl4-induced fibrosis [20]. For acute toxicity, the dose of CCl4 used in this investigation was different from what was reported in previous literature [19]. Lower dose of melatonin was used compared to what was reported in earlier literature. In addition, the effects of melatonin on testicular toxicity of the model were investigated.
Lipid peroxidation has been implicated in the pathogenesis of hepatic injury by the free radical derivatives of CCl4 and is responsible for cell membrane damage and the consequent release of marker enzymes of hepatotoxicity. In this study, significantly elevated levels of MDA, products of membrane lipid peroxidation observed in CCl4-treated rats indicated hepatic damage. Pre-treatment with melatonin reduced lipid peroxidation, which could be attributed to the radical scavenging antioxidant constituents. GSH is the major non-enzymatic antioxidant and regulator of intracellular redox homeostasis, ubiquitously present in all cell types. CCl4 administration leads to a significant decrease in the GSH level, which could be an important factor in CCl4 toxicity. The mechanism of hepatoprotection by melatonin against CCl4 toxicity might be due to restoration of the GSH level at least. The coordinate actions of various cellular antioxidants in mammalian cells are critical for the effective detoxification of free radicals. Among the cellular antioxidants, SOD has been studied extensively. SOD catalyses the dismutation of the superoxide anion to H2O2 and O2. Administration of CCl4 to rats decreased the antioxidant capacity of rat liver as evidenced by the decreased activity of the antioxidant enzymes. Melatonin pre-treatment prevented the reduction in the antioxidant enzyme activities and the consequent oxidative damage to the CCl4. The influence of SOD was similar with reported in other study [21]. There was a significant improvement in the testicular morphology detected by melatonin.
In the present study, systemic administration of melatonin morphologically demonstrated a beneficial effect on liver and testis damage caused by CCl4 in rats. This was in good agreement with the results of serum aminotransferase activity and hepatic oxidative stress levels [7,9].
Plasma membrane potential depolarization has been recognized as a primary signal that conveys multiple biological messages within cells [20,22]. It is believed that plasma membrane potential depolarization may play a role in predisposing factor of cell apoptosis. The change of membrane potential will disturb ion channels in the plasma membrane, which can be important for physiological functions of cells. In the melatonin pre-treatment group, the DiBAC4(3) fluorescence decreases, implying the recovery of the membrane potential.
Numerous studies on melatonin have shown its indirect antioxidant role in stimulating antioxidant enzymes, its ability to augment the activities of other antioxidants, its protective role for antioxidant enzymes against oxidative damage and its ability to protect against liver fibrosis via upregulation of mitophagy and mitochondrial biogenesis [20,21]. Present experiments showed that the short-term low dose of melatonin has protective effect on acute injury in rats, which can not only change biochemical indicators but also improve cell membrane potential damage.
On the other hand, recent studies have implicated telomerase in the cellular response to oxidative damage, suggesting that telomerase has a telomerelength independent function that promotes survival. Human telomerase reverse transcriptase (hTERT) enhancement confers resistance to genomic damage due to the amelioration of the cell's basic antioxidant machinery [23]. CCl4 treatment activated the telomerase activity in rat hepatic samples [24]. In this present study, RT-PCR analyses revealed that telomerase and caspase-3 mRNA expression was significantly lower in the melatonin group than in the CCl4 group. These results indicated that melatonin treatment may modulate expression of telomerase and caspase-3 to inhibit CCl4-induced damage. Previous results showed that melatonin not only inhibited breast cancer cell growth, but also was capable of inhibiting angiogenesis, cancer cell invasion, and telomerase activity [25]. Although the subjects and objectives were different, the effects of melatonin on telomerase were consistent.
In conclusion, it was observed that pre-treatment with melatonin exerted a protective effect against acute liver damage and testicular toxicity induced by the administration of CCl4 in rat and that the protective effects of melatonin may be due to the free radical scavenging effect, inhibition of lipid peroxidation, and increased antioxidant activity, as well as restored plasma membrane potential in liver. Further studies are needed to clarify the possible mechanisms of melatonin. This study suggested that downregulation of telomerase and caspase-3 mRNA might play an important role in the protective effect of melatonin against the CCl4-induced damage. Considering the large number of studies documenting the ameliorative effects of melatonin against injury in organs other than the liver [8,9,21], along with the current findings, it seems likely that melatonin might have therapeutic utility in conditions where the liver and testicles were subjected to damage.
Conflict of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Abraham P, Wilfred G, Cathrine. Oxidative damage to the lipids and proteins of the lungs, testis and kidney of rats during carbon tetrachloride intoxication. Clin Chim Acta 1999;289:177-9.
- Aayadi H, Mittal SPK, Deshpande A, Gore M, Ghaskadbi SS. Protective effect of geraniin against carbon tetrachloride induced acute hepatotoxicity in Swiss albino mice. Biochem Biophys Res Commun 2017;487(1):62-7.
- Lai LS, Chou ST, Chao WW. Studies on the antioxidative activities of Hsian-tsao (Mesona procumbens Hemsl) leaf gum. J Agric Food Chem 2001;49:963-8.
- Lanoix D, Lacasse AA, Reiter RJ, Vaillancourt C. Melatonin: The watchdog of villous trophoblast homeostasis against hypoxia/reoxygenation-induced oxidative stress and apoptosis. Mol Cell Endocrinol 2013;381(1-2):35-45.
- Li B, He X, Zhuang M, Niu B, Wu C, Mu H, et al. Melatonin ameliorates busulfan-induced spermatogonial stem cell oxidative apoptosis in mouse testes. Antioxid Redox Signal 2018;28(5):385-400.
- Yang F, Li Y, Yan G, Liu T, Feng C, Gong R, et al. Inhibition of iron overload-induced apoptosis and necrosis of bone marrow mesenchymal stem cells by melatonin. Oncotarget 2017;8(19):31626-37 .
- Saki G, Mirhoseini M, Hemadi M, Khodadadi A,Amiri FBT. The effect of the melatonin on cryopreserved mouse testicular cells. Int J Reprod Biomed 2016;14(1):23-8.
- Mortezaee K, Khanlarkhani N, Sabbaghziarani F, Nekoonam S, Majidpoor J, Hosseini A, et al. Preconditioning with melatonin improves therapeutic outcomes of bone marrow-derived mesenchymal stem cells in targeting liver fibrosis induced by CCl4. Cell Tissue Res 2017;369(2):303-12.
- Sekmenli T, Gunduz M, Öztürk B, Karabağlı P, Ciftci I, Tekin G, et al. The effects of melatonin and colchicine on ischemia-reperfusion injury in experimental rat testicular torsion model. J Pediatr Surg 2017;52(4):582-6.
- Miesel R, Sanocka D, Kurpisz M, Kröger H. Antiinflammatory effects of NADPH oxidase inhibitors. Inflammation 1995;19:347-62.
- Singelton VR, Orthifer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 1999;299:152-78.
- Andersen HJ, Chen H, Pellett LJ, Tappel AL. Ferousiron-induced oxidation in chicken liver slices as measured by hemichrome formation and thiobarbituric acid reactive substances: effect of dietary vitamin E and beta-carotene. Free Radic Biol Med 1993;15:35-48.
- Boyne AF, Ellman GL. A methodology for analysis of tissue sulfhydryl components. Anal Biochem 1972;46:639-53.
- Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol 1983;23:239-57.
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265-75.
- Postma FR, Jalink K, Hengeveld T, Bot AG, Alblas J, de Jonge HR, et al. Serum-induced membrane depolarization in quiescent fibroblasts: activation of a chloride conductance through the G protein-coupled LPA receptor. EMBO J 1996;15:63-72.
- Epps DE, Wolfe ML, Groppi V. Characterization of the steady-state and dynamic fluorescence properties of the potential-sensitive dye bis-(1,3-dibutylbarbituric acid)trimethine oxonol (Dibac4(3)) in model systems and cells. Chem Phys Lipids 1994;69:137-50.
- Gao Y, Deng XG, Sun QN, Zhong ZQ. Ganoderma spore lipid inhibits N-methyl-N-nitrosourea-induced retinal photoreceptor apoptosis in vivo. Exp Eye Res 2010;90:397-404.
- Lee GH, Lee HY, Choi MK, Chung HW, Kim SW, Chae HJ. Protective effect of Curcuma longa L. extract on CCl4-induced acute hepatic stress. BMC Res Notes 2017;10:77.
- Kang JW, Hong JM, Lee SM. Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride-induced liver fibrosis. J Pineal Res 2016;60(4):383-93.
- Goc Z, Szaroma W, Kapusta E, Dziubek K. Protective effects of melatonin on the activity of SOD, CAT, GSH-Px and GSH content in organs of mice after administration of SNP. Chin J Physiol 2017;60(1):1-10.
- Sallmann S, Jüttler E, Prinz S, Petersen N, Knopf U, Weiser T, et al. Induction of interleukin-6 by depolarization of neurons. J Neurosci 2000;20:8637-42.
- Trachana V, Petrakis S, Fotiadis Z, Siska EK, Balis V, Gonos ES, et al. Human mesenchymal stem cells with enhanced telomerase activity acquire resistance against oxidative stress-induced genomic damage. Cytotherapy 2017;19(7):808-20.
- Khan RA, Khan MR, Sahreen S. Attenuation of CCl4-induced hepatic oxidative stress in rat by Launaea procumbens. Exp Toxicol Pathol 2013;65:319-26.
- Nooshinfar E, Safaroghli-Azar A, Bashash D, Akbari ME. Melatonin, an inhibitory agent in breast cancer. Breast Cancer 2017;24(1):42-51.