- Corresponding Author:
- M. Indira
Department of Biochemistry, University of Kerala, Kariavattom, Thiruvananthapuram-695 581, India
E-mail: indiramadambath@gmail.com
| Date of Submission | 20 February 2009 |
| Date of Revision | 10 August 2009 |
| Date of Acceptance | 31 August 2009 |
| Indian J. Pharm. Sci., 2009, 71 (5): 527-532 |
Abstract
This study examined the protective effects of quercetin on chronic ethanol-induced liver injury. Rats were treated with ethanol at a dose of 4 g/100 g/day for 90 days. After ethanol intoxication, levels of serum amino transferases were significantly elevated. Decreased activity of superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase was also observed on ethanol administration. Increased amounts of lipid peroxidation products viz. hydroperoxides, conjugated dienes and malodialdehyde were observed on ethanol intoxication. Ethanol administration resulted in significant decrease in liver glutathione content. After 90 days, the control animals were divided into two groups, the control group and the control+quercetin group. Ethanol-treated group was divided into two groups, abstention group and quercetin-supplemented group. After 30 days, the animals were sacrificed and various biochemical parameters were analyzed. The changes in enzyme activities as well as levels of lipid peroxidation products were reversed to a certain extent by quercetin. Quercetin supplementation resulted in increase of glutathione content to a significant level compared to normal abstention group. Quercetin supplemented group showed a faster recovery than abstention group. This shows the protective effect of quercetin against chronic ethanol induced hepatotoxicity. Histopathological study is also in line with these results.
Keywords
Quercetin, ethanol, hepatotoxicity, serum amino transferases, lipid peroxidation
Alcohol abuse and its medical and social consequences are a major health problem in many areas of the world. Ethanol exerts its effect either directly or through derrangements in metabolic, hormonal and nutritional mechanisms[1]. Excessive generation of free radicals play an important role in alcohol induced cellular damage[2]. Ethanol abuse affects many organ systems most notably the liver causing both acute and chronic liver disease and the central nervous system[3]. A large number of studies are in progress aiming to identify substances that would be effective in reducing the severity of alcoholic liver disease. Although removal of alcohol still represents the most effective intervention to prevent the manifestation of alcoholic liver disease, trials are underway aiming a faster recovery.
It was established that generation of reactive oxygen species is enhanced after ethanol intoxication, and that they play a major role in the creation of oxidative stress, which may additionally be enhanced by the depletion in the antioxidant defense system and, in consequence, by an imbalance between prooxidants and antioxidants[4]. Aim of our study was to induce hepatotoxicity using ethanol and then to assess the reversal of damage once ethanol consumption is stopped.
Flavanoids are a group of naturally occuring compounds widely distributed as secondary metabolites in plant kingdom. One of these ß avanoids, quercetin (3,5,7,3,4-pentahydroxy ß avon), is one of the most prominent dietary antioxidant. The ability of quercetin to scavenge highly reactive species such as peroxynitrite and the hydroxy radical is suggested to be involved in these possible beneficial health effects[5]. Tieppo et al.[6] has stated that quercetin increased the genomic stability in the cirrhotic rats, suggesting beneficial effects, probably by its antioxidant properties[7]. Quercetin is also known to act as an effective antioxidant by chelating metal ions and/or scavenging free radicals[8]. Several studies have demonstrated that quercetin enhanced the antioxidative defense system by upregulating antioxidant enzymes[9].
One of the most important problems faced by those who have stopped alcohol consumption after chronic intake, is the recovery of damaged hepatocytes to normal level. Liver regeneration occurs normally in damaged liver. But the time required varies depending upon the severity of damage, which inturn is determined by the dose and duration of alcohol intake.
There are hardly any studies reported in the literature regarding the protective effect of quercetin in ethanolinduced toxicity. It has been proved that oxidative stress is the mechanism behind ethanol-induced toxicity and quercetin is shown to have beneficial effect on hepatic oxidative stress. Hence, we have studied the effect of quercetin in the regression of ethanol-induced hepatotoxicity.
Materials and Methods
Male Sprague Dawley rats weighing between 150 and 200 g were used. Animals were housed in polypropylene cages. Cages were kept in a room that was maintained between 28 and 32° and a 12 h dark and light cycle was maintained. The study protocol was approved by the Institutional Animal Ethics Committee. Animals were handled using the laboratory animal welfare guidelines. Rats were fed with rat feed (Lipton India Ltd). Water was given ad libitum. Quercetin was purchased from M/s SRL Ltd., Mumbai, India and ethanol from M/s Merck Ltd, Mumbai, India.
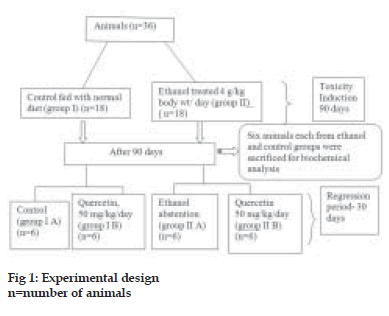
A total of 36 animals were divided into two groups, group I was the control group fed with normal diet and group II was the ethanol group (4 g ethanol/kg/ day). Ethanol diluted with distilled water (1:1) was given orally by gastric tube. After 90 days, ethanol administration was stopped and 6 animals each from control group and ethanol group were sacrificed after overnight fasting and liver was collected for biochemical analysis. The rest of animals in control group were divided into two groups, group I A, control group fed with normal diet and group I B, quercetin-supplemented group (50 mg quercetin/kg/ day). Quercetin was freshly dissolved in distilled water during treatment and given orally by gastric tube. The animals in the ethanol group were also divided into two groups, group II A, abstention group and group II B, quercetin-supplemented group (50 mg quercetin/kg/day) for 30 days. Group I was given isocaloric glucose solution. This is schematically represented in the fig. 1. At the end of the experimental period, animals were sacrificed after overnight fasting. The liver was dissected out and cleaned with ice-cold saline, blotted dry and immediately transferred to ice-cold container for various evaluations.
Biochemical methods
Activities of super oxide dismutase (SOD, EC.1.15.1.1) and catalase (EC.1.11.1.6) were determined by the method of Kakkar et al.[10] and Maehly and Chance[11], respectively. The activity of glutathione peroxidase (GPx, EC.1.11.1.9) was determined by Lawerence and Burk as modified by Agergurd and Jence[12]. The activity of glutathione reductase (GR) was determined by the procedure of David and Richard[13]. Protein was estimated by the method of Lowry et al.[14]. The activities of aspartate amino transferase (AST) and alanine amino transferase (ALT) were determined by the method of Reitman and Frankel as described by Wooten[15]. Malondialdehyde (MDA) was estimated by the method of Ohkava[16]. Hydroperoxides were estimated by the method of Mair and Hall[17] and conjugated dienes (CD) were estimated by the method of Recknagel and Ghoshal[18]. Glutathione content in tissue was estimated by the method of Beutler et al[19].
Statistical analysis
The results were analysed using a statistical programme SPSS/PC+, version 10 (SPSS Inc. Chicago, IL, USA). A one way ANOVA was employed for comparison among the six groups. Duncan’s post hoc multiple comparison test of significant difference among groups were determined. p≤ 0.05 was considered significant.
Histopathological studies
For histopathological studies, liver was fixed in Bauins’ fixative and sections were taken in the microtome. Sections were stained using haematoxylin and eosin. Pathological changes were examined using a sensitive light microscope.
Results and Discussion
Activities of AST, ALT and GGT in serum and liver significantly increased in rats given ethanol (Table 1). Activities of these enzymes were decreased in group II A in comparison with group II. Supplementation of quercetin in group II B further reduced the activities of these enzymes. The activities of antioxidant enzymes SOD and catalase were found to be significantly decreased in tissues of rats exposed to ethanol (Table 2). The activities of these enzymes were considerably increased in quercetin supplemented group in comparison with those fed ethanol alone.
The activity of glutathione peroxidase was reduced in ethanol-treated group (Table 2). The activity of this enzyme was significantly enhanced in quercetin supplemented group. The activity of glutathione reductase was lowered in ethanol treated group (Table 2). The activity of this enzyme was significantly enhanced in the liver of quercetin supplemented group when compared to other groups.
Alcohol induced significant lipid peroxidation (Table 3). Level of malondialdehyde was increased significantly in ethanol treated group. This was significantly reduced by quercetin supplementation. Upon alcohol administration; there was significant increase in tissue hydroperoxide level. This was reduced significantly by quercetin supplementation. Conjugated diene level was also found to be higher in ethanol treated group. Quercetin supplementation caused a reduction in conjugated diene level compared to abstention group. Ethanol administration resulted in significant decrease in liver glutathione content. Quercetin supplementation resulted in increase of glutathione content to a significant level compared to normal abstention group (Table 4).
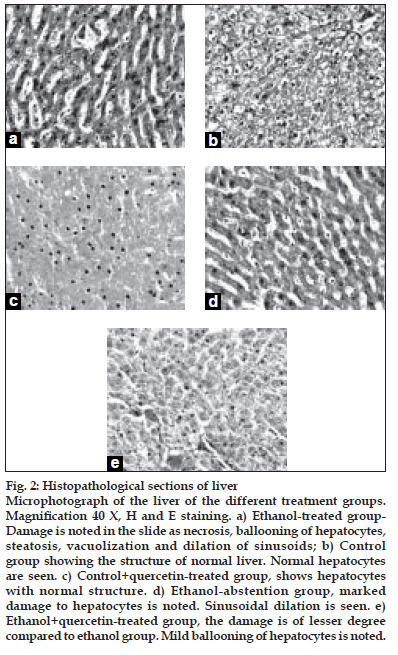
After ethanol administration liver sections showed extensive hepatocellular damage as evidenced by ballooning of hepatocytes, steatosis, vacuolization and dilation of sinusoids (fig. 2a). The histological features of the liver in the control group showed a normal liver architecture and cell structure (fig. 2b). Quercetin supplementation to control rats did not alter normal liver structure (fig. 2c). Abstention from ethanol resulted in reduction in hepatic damage as evidenced by mild steatosis (fig. 2d). The histopathological changes were ameliorated by quercetin treatment. Damage is of lesser degree in quercetin-treated group (fig. 2e).
| Group | ALT (µmoles of | pyruvate | AST (µmoles of OAA | |
|---|---|---|---|---|
| liberated /min/mg protein) | liberated/min/mg protein) | |||
| Liver | serum | Liver | serum | |
| I | 16.38±1.84 | 193.13±18.52 | 15.42±1.47 | 52.51± 4.6 |
| II | 96.85±8.41a | 534.11±48.64a | 61.12±5.57a | 144.83±12.8a |
| I A | 16.87±1.86 | 201.32±19.1 | 16.12±1.56 | 54.82±4.8 |
| I B | 21.44±1.96 | 191.35±17.39 | 16.29±1.55 | 48.42±3.1 |
| II A | 60.77±5.54b | 499.67±48.62b | 49.31±4.71b | 112.09±9.2b |
| II B | 49.22±4.86c | 342.88±32.88c | 34.48±3.26c | 78.74±5.9c |
Table 1: Toxicity Marker Enzymes.
| Group | SOD | Catalase | GPx (µmole NADPH | GR (µmole NADPH |
|---|---|---|---|---|
| (#units/ mg protein) | (*units /mg protein) | oxidised/min) | oxidised/min) | |
| I | 82.67±8.34 | 64.21±6.15 | 12.30±1.17 | 20.98±2.02 |
| II | 20.11 ±1.83a | 37.57±3.60a | 4.48±0.42a | 3.80±0.41a |
| I A | 84.56±8.36 | 62.41± 5.9 | 11.92±1.06 | 19.88±1.86 |
| I B | 74.42±6.76b | 51.56±4.94b | 9.53±0.86b | 6.06±0.58b |
| II A | 31.93±3.07c | 45.60±4.61c | 5.82±0.62c | 8.83±0.80c |
| II B | 42.11±3.83d | 58.09±5.57d | 7.21±0.65d | 15.02±1.36d |
Table 2: Activity Of Antioxidant Enzymes In Liver.
| Group | MDA (mM/100g wet tissue) | Hydroperoxides dienes (mM/100g wet tissue) | Conjugated (mM/ 100g wet tissue) |
|---|---|---|---|
| I | 0.70±0.067 | 8.83±0.85 | 69.01±6.23 |
| II | 1.67±0.16a | 23.64±2.09a | 213.78±19.53a |
| I A | 0.68±0.064 | 8.86±0.87 | 70.42±6.81 |
| I B | 0.63±0.06 | 7.80±0.68 | 66.31±6.36 |
| II A | 1.37±0.12b | 18.10±1.32b | 196.25±17.72b |
| II B | 1.10±0.11c | 12.82±1.17c | 134.59±14.47c |
Table 3: Concentration Of Lipid Peroxidation Products In Liver.
| Group | Glutathione content (mg/100g wet tissue) |
|---|---|
| I | 572.46±52.51 |
| II | 391.06± 35.68a |
| I A | 576.36±52.68 |
| I B | 542.25±47.92 |
| II A | 412.01±37.99b |
| II B | 482.16± 41.34c |
Table 4: Glutathione Content Level In Liver.
Ethanol treatment caused significant increase in AST and ALT activities, an indication of hepatocellular damage in rats. Treatment with quercetin reduced ethanol-induced toxicity as indicated by drop in activities of marker enzymes. In alcohol intoxication, as a result of structural changes, an increase in membrane permeability to ions has been demonstrated in different animal models[20]. The increase in membrane permeability causes translocation of ALT and AST into blood circulation as shown by abnormally high level of serum hepatic markers.
Antioxidant enzymes such as SOD, CAT and GPX comprise a major supportive group of protection against free radicals. Chronic ethanol treatment caused significant decrease in SOD, CAT and GPX. There are reports to indicate that there is significant decrease in glutathione peroxidase in chronic ethanol abuse[21]. This is in agreement with our results. However, supplementation of quercetin increased GPx activity significantly.
Figure 2:Histopathological sections of liver.
Microphotograph of the liver of the different treatment groups. MagniÞ cation 40 X, H and E staining. a) Ethanol-treated group- Damage is noted in the slide as necrosis, ballooning of hepatocytes, steatosis, vacuolization and dilation of sinusoids; b) Control group showing the structure of normal liver. Normal hepatocytes are seen. c) Control+quercetin-treated group, shows hepatocytes with normal structure. d) Ethanol-abstention group, marked damage to hepatocytes is noted. Sinusoidal dilation is seen. e) Ethanol+quercetin-treated group, the damage is of lesser degree compared to ethanol group. Mild ballooning of hepatocytes is noted.
SOD prevents the inhibition of GPX and CAT by scavenging superoxide radicals and GPx and CAT in turn prevent the inhibition of SOD by scavenging H2O2 [22] . In quercetin treated animals, SOD activity is increased, which could be due to the increase in glutathione peroxidase activity that lowered the levels of H2O2, thereby preventing the retro-inhibition on SOD. This is in accordance with earlier reports[23]. Quercetin supplementation attenuated all alterations in antioxidant enzymes- SOD, CAT, GP, GR, reduced glutathione in ethanol treated animals. Quercetin shows beneficial effects on liver damage by enhancing antioxidant enzyme activity and decreasing prooxidant effect[24]. This is due to the ability of quercetin to interact with hydroxyl, superoxide, alkoxyl and peroxyl radicals thereby subsequently scavenging them. Quercetin supplementation led to a slight decrease in antioxidant defense in normal controls. This may be due to prooxidant effect of quercetin in normal cells. Choi et al. had observed earlier that quercetin acts as a prooxidant in normal rats[25].
Glutathione constitutes the first line of defense against free radical and is a determinant of tissue susceptibility to oxidative damage. Quercetin significantly increased the reduced glutathione levels in alcohol intoxication. It has been reported that quercetin corrects the reduction in glutathione concentration and partially prevented the increase in collagen concentration, TBARS and GSSG/GSH ratio[26].
In the present study we observed that there was significant increase in lipid peroxidation products in ethanol consumption as shown by earlier studies[27]. The increased concentration of MDA, hydroperoxides and conjugated dienes, an index of lipid peroxidation may be due to increased production of free fatty acids which may serve as substrates for lipid peroxidation[28]. Quercetin also increased serum albumin and hepatic glutathione levels and reduced the hepatic level of malondialdehyde[29].
Previous studies have demonstrated that quercetin intake prevented and protected the liver from the oxidative damage induced by the ingestion of ethanol[30]. Effects and diminished liver injury by quercetin may be partially related to preservation of antioxidant defenses. The ß avonoid has been shown to scavenge highly reactive species implicated in the peroxidation process such as oxygen and hydroxyl radicals.
Hepatotoxicity induced by ethanol is further confirmed by abnormal histological findings. Toxicity manifestations by ethanol in the liver tissue are revealed by morphological changes such as inflammation around portal triad (Triaditis) with severe fatty degeneration. Quercetin supplementation resulted in a faster recovery of normal cell structure compared to abstention group.
From these observations it can be concluded that quercetin offers protective effect against ethanol induced hepatotoxicity by attenuating lipid peroxidation, by scavenging free radicals and by enhancing the activity of antioxidants, which in turn detoxify free radicals. It can be developed as a drug for the reversal of chronic ethanol-induced hepatotoxicity.
References
- Amanvermez R, Demir S, Tunçel OK, Alvur M, Agar E. Alcohol-induced oxidative stress and reduction in oxidation by ascorbate/L-cys/L-met in the testis, ovary, kidney, and lung of rat. AdvTher 2005;22:548-58.
- Zima T, Kalousova M. Oxidative stress and signal transduction pathways in alcoholic liver disease. Alcohol ClinExp Res 2005;29:110S-5S.
- Lieber CS. Pathogenesis and treatment of alcoholic liver disease: progress over the last 50 years. RoczAkad Med Bialymst 2005;50:7-20.
- Halliwel B, Gutteridge JM. Free radicals in biology and medicine, Oxford: Oxford University Press; 2001.
- Boots AW, Haenen GR, Bast A. Health effects of quercetin: From antioxidant to nutraceutical. Eur J Pharmacol 2008;585:325-37.
- Tieppo J, Vercelino R, Dias AS, Silva Vaz MF, Silveira TR, Marroni CA, et al. Evaluation of the protective effects of quercetin in the hepatopulmonary syndrome. Food ChemToxicol 2007;45:1140-6.
- Vicente-Sánchez C. Effect of the flavonoid quercetin on cadmium-induced hepatotoxicity. Food ChemToxicol 2008;46:2279-87.
- Terao JJ. Dietary flavonoids as antioxidants in vivo: Conjugated metabolites of (-)-epicatechin and quercetin participate in antioxidative defense in blood plasma. Med Invest 1999;46:159-68.
- Alia M, Mateos R, Ramos S, Lecumberri E, Bravo L, Goya L. Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line HepG2. Eur J Nutr 2006;45:19-28.
- Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J BiochemBiophys 1984;21: 130-2.
- Maehly AC, Chance B. The assay of catalase and peroxide. In: Glick D, editors. Methods of Biochemical Analysis, Vol. 1. New York: Interscience; 1954. p. 357-424.
- Agergurd N, Jense PJ. Procedure for blood glutathione peroxidase determination in cattle and swine. Acta Vet Scand 1982;23:515-29.
- David M, Richard JS. Glutathione reductase. In: Bergmeyer H, Ulrich Jr, editors. Methods of enzymatic analysis. Vol. 3. New York: Academic Press; 1983. p. 258-65.
- Lowry OH, Rosebrough NJ, Farr AL, Randell RJ. Protein measurement with the folin phenol reagent. J BiolChem 1951;193:265-75.
- Wooten ID. Microanalysis in medical biochemistry, 4th ed. London: Churchill; 1964. p. 140.
- Ohkawa H, Ohishi N, Yagi K. Assay of lipid peroxide in animal tissue by TBA reaction. Anal Biochem 1979;95:351-8.
- Mair RD, Hall T. In: Swern D, Willey CD, editors. Inorganic peroxides 2nd ed. New York: Intersciences; 1971. p. 535-8.
- Reckangel RO, Ghoshal AK. Quantitative estimation of peroxidative degeneration of rat liver microsomal and mitochondrial lipids after carbon tetrachloride poisoning. ExpMolPathol 1966;5:413-8.
- Beutler E, Duran O, Kelley BM. Improved method for the determination of blood glutathione. J Lab Clin Med 1963;61:882-5.
- Saravanan N, Nalini N. Effect of 2-hydroxy-4-methoxy benzoic acid on an experimental model of hyperlipidaemia, induced by chronic ethanol treatment. J Pharm Pharmacol 2007;59:1537-42.
- Zhou Z, Wang L, Song Z, Saari JT, McClain CJ, Kang YJ. Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. Am J Pathol 2005;166:1681-90.
- Pigeolet E, Corbisier P, Houbion A, Lambert D, Michiels C, Raes M, et al. Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech Ageing Dev 1990;51:283-97.
- Kamaraj S, Vinodhkumar R, Anandakumar P, Jagan S, Ramakrishnan G, Devaki T. The effects of quercetin on antioxidant status and tumor markers in the lung and serum of mice treated with benzo(a)pyrene. Biol Pharm Bull 2007;12:2268-73.
- Amalia PM, Possa MN, Augusto MC, Francisca LS. Quercetin prevents oxidative stress in cirrhotic rats. Dig Dis Sci 2007;52:2616-21.
- Choi EJ, Chee EM, Lee BH. Anti- and prooxidant effects of chronic quercetin administration in rats. Eur J Pharmacol 2003;482:281-5.
- Peres W, Tunon MJ, Collado PS, Hermann S, Marroni, N, Gonzalvez-Gallego J. The ßavanoidquercetin ameliorates liver damage in rats with biliary obstruction. J Hepatol 2000;33:742-50.
- Das SK, Vasudevan DM. Protective effects of silymarin, a milk thistle (Silybiummarianum) derivative on ethanol-induced oxidative stress inliver. Indian J BiochemBiophys 2006;43:306-11.
- Nanji AA, French SW. Animal models of alcoholic liver disease focus on the intragastric feeding model. Alcohol Res Health 2003;27:325-30.
- Lee ES, Lee HE, Shin JY, Yoon S, Moon JO. The ßavonoidquercetin inhibits dimethylnitrosamine-induced liver damage in rats. J Pharm Pharmacol 2003;55:1169-74.
- Molina MF, Sanchez-Reus I, Iglesia I, Bendi J. Quercetin, a ßavanoid antioxidant, prevents and protects against ethanol induced oxidative stress in mouse liver. Biol Pharm bull 2003;26:1398-402.