- *Corresponding Author:
- Yi Wang
Department of Nutrition, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, China
E-mail: 13587873269@163.com
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “25-32” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Diabetic cardiomyopathy is the complication of diabetes and a serious health risk for diabetics. Pyrroloquinoline quinone, is a food-derived vitamin. The aim of this study was to investigate whether pyrroloquinoline quinone has a protective effect on the myocardium of patients with diabetic cardiomyopathy. We designed five groups of mice; control, diabetic cardiomyopathy, diabetic cardiomyopathy+pyrroloquinoline quinone-L, diabetic cardiomyopathy+pyrroloquinoline quinone-M, and diabetic cardiomyopathy+pyrroloquinoline quinone-H, and determined the changes of diabetic cardiomyopathy in each group based on the echocardiographic results of the mice. In addition, we also observed the myocardial tissue specimens of mice and found myocardial fibrosis was lower in pyrroloquinoline quinone group mice. To further explore the mechanism of pyrroloquinoline quinone affecting myocardial fibrosis, we used reverse transcriptase-polymerase chain reaction to measure the long noncoding RNA associated with myocardial fibrosis in mice, and found the expression of metastasis associated lung adenocarcinoma transcript 1, growth arrestspecific 5, and KCNQ1OT1 were higher in the groups of pyrroloquinoline quinone. This also indicated that pyrroloquinoline quinone improved myocardial fibrosis by affecting metastasis associated lung adenocarcinoma transcript 1, growth arrestspecific 5, and KCNQ1OT1 expressions.
Keywords
Diabetic cardiomyopathy, pyrroloquinoline quinone, myocardial fibrosis, long noncoding RNA, echocardiography
Diabetic Cardiomyopathy (DCM) is a cardiomyopathy and cannot be accounted for by either hypertensive heart disease, coronary atherosclerotic heart attack or other heart lesions. The disease triggers extensive focal myocardial necrosis based on disturbed metabolism and pre-existing microangiopathy, accompanied by subclinical abnormalities of cardiac function, eventually progressing to heart failure, arrhythmias and cardiogenic stunning, and in severe cases, sudden death. DCM was first reported by Rubler et al.[1]. In 1974 Hamby et al. first introduced the concept of DCM[2]. After excluding coronary artery disease, hypertensive heart disease, heart valve disease and other heart diseases, DCM is diagnosed based on the symptoms and signs of diabetic patients combined with laboratory tests. The stages of DCM can be divided into three stages. The appearance of cardiac architectural changes, but no alteration of diastolic function, normal left Ventricular Tachycardia (VTR) ejection fraction, in a subclinical state; aggravation of cardiac architecture changes, the appearance of ventricular hypertrophy, fibrosis of the myocardium, reduced ventricular diastolic function of cardiac function, progressive abnormal systolic function, and left VTR ejection fraction <50 %. Further aggravation of cardiac structural changes, cardiac microvascular changes, further aggravation of ventricular hypertrophy and myocardial fibrosis, and whole heart diastolic and systolic dysfunction. A definitive clinical diagnosis of DCM is sometimes difficult. Diabetic patients with combined hypertension or asymptomatic coronary artery disease are more frequent, especially because long-term hypertension itself can cause left ventricular enlargement and even heart failure, which is clinically difficult to distinguish clearly from cardiomyopathy caused by metabolic disorders of diabetes. The pathogenesis of DCM is diverse. Increased glucose concentrations in diabetic patients induce apoptosis by generating excess Reactive Oxygen Species (ROS) through the chain of electrical transport, and ROS trigger Poly (ADP-Ribose) Polymerase (PARP), which directly mediates the glycosylation and inhibits phosphoglyceraldehyde, ROS activates PARP. Directly mediates glycosylation and inhibits phosphoglyceraldehyde dehydrogenase, shifts transfer of glucose from the glycolytic pathway to other biochemical trains of high-glucose-induced myocardial injury[3-5]. Advanced glycosylation end products can stimulate collagen expression and accumulation, and promote collagen cross-linking, leading to increased myocardial fibrosis and decreased myocardial compliance. The decreased glucose transporter protein-4 activity in the hyperglycemic state reduces the transmembrane transport of glucose into the cardiomyocyte, decreases glucose uptake by the cardiomyocyte, and subsequently affects cardiomyocyte energy metabolism, leading to DCM[6]. In diabetes, myocardial glucose utilization is significantly reduced, fat Beta (β) oxidation is increased, and triglyceride glycerol and free fatty acids accumulate in cardiomyocytes, resulting in increased oxygen demand and mitochondrial uncoupling, impairing myocardial calmodulin and affecting the diastolic contractile function of cardiomyocytes; it also increases ROS production, induces endoplasmic reticulum stress, and promotes cardiomyocyte apoptosis. Insulin Resistance (IR) is an important risk factor for cardiovascular complications of diabetes mellitus[7], and IR can directly cause structural and functional damage to the left ventricle, in addition to exacerbating disorders of myocardial energy mechanisms. IR and hyperinsulinemia may enhance metabolic disorders, promoting the sympathetic nervous system and Renin-Angiotensin-Aldosterone System (RAAS), promote oxidative stress, mitochondrial dysfunction, endoplasmic reticulum stress and calcium homeostasis imbalance, leading to myocardial fibrosis, myocardial hypertrophy, myocardial apoptosis and coronary microcirculation disorders, ultimately causing heart failure[8]. Activation of the RAAS system in diabetic patients is closely associated with myocardial hypertrophy and fibrosis. Angiotensin II stimulates angiotensin receptor-1 to act directly on cardiomyocytes and fibroblasts of the heart, resulting in increased collagen synthesis and decreased collagen breakdown, causing myocardial hypertrophy and fibrosis, leading to reduced ventricular compliance and cardiac systolic and diastolic insufficiency. Cytokines, chemokines and exosomes secreted by inflammatory cells promote cardiomyocyte hypertrophy and extracellular matrix remodeling. In a hyperglycemic environment, an activated myocardial inflammatory response and upregulated expression of pro-inflammatory factors can lead to accumulation and infiltration of pro-inflammatory macrophages and lymphocytes in the myocardial interstitium. Large amounts of inflammatory cytokines can exacerbate cardiac the immune system and DCs are also associated with DCs. In addition, the body's immune system is closely related to the development of DCM[9,10]. In addition, diffuse intramural small vessel lesions are present in the myocardium of diabetic patients. Due to the long duration of diabetes, glycosylated collagen deposition can lead to myocardial interstitial fibrosis. Cardiac autonomic neuropathy is also present in approximately 83 % of diabetic patients.
Pyrroloquinoline Quinone (PQQ) is widely found in a variety of common foods including fruits, vegetables, cereals and beverages of plant and animal origin, with concentrations ranging from 3.65-61.0 ng/g or ng/ml. The total content of PQQ and its derivative in human milk is 140-180 ng/ml, showing that it may play a role in human growth[11]. PQQ has a strong ability to scavenge peroxides and protects mitochondria from oxidative damage; inhibits lipid peroxidation; protects cardiomyocytes from ischemia-reperfusion; protects the redox site of oxidized N-Methyl-D-Aspartate (NMDA) receptors, thus promoting recovery from spinal cord injury, preventing brain ischemia and hypoxia, and avoiding severe shock in animal models[12,13]. In addition, PQQ can protect the heart against myocardial ischemia and myocardial infarction, and these protective effects are attributed to the antioxidant capacity of PQQ. In addition, PQQ was able to scavenge ROS and counteract inflammatory damage caused by oxidative stress. Injecting PQQ before administration of carrageenan to mice reduced the inflammatory response induced by carrageenan in the rat paw. The addition of PQQ to parenteral nutrition increased the number of lymphocytes in lymphatic aggregates, as well as Clusters of Differentiation (CD)-4+ and CD8+ cells in lymphatic aggregates, and restored the integrity of lymphoid organs associated with the intestine to some extent. However, we are unclear about the PQQ in DCM.
Materials and Methods
Animals and DCM models:
PQQ was supplied by Zhucheng Haotian Pharmaceutical Co., Ltd., with a purity of 98 % or higher. 8 w old of C57BL/6 male mice were provided by the Animal Experiment Center of Zhejiang Academy of Medical Sciences. Mice were raised at a temperature of 22°±1° and 55 %±5 % humidity. We divided the animals into five groups in a randomized manner; control group, DCM, low-dose (10 mg/kg body weight/day, PQQL), medium-dose (20 mg/kg body weight/day, PQQM) and high-dose (40 mg/kg body weight/day, PQQH) treated DCM mice. After grouping, mice in the experimental group were injected Intraperitoneally (Ip) with 50 mg/kg/day of Streptozotocin (STZ); (Sigma, St. Louis, Missouri, United States of America (USA)) for 5 d, fasting blood glucose concentrations were measured by a glucometer, and mice with blood glucose concentrations ≥16.7 mmol/l served, and then different doses of PQQ were administered orally to some of the selected hyperglycemic mice, and all mice underwent 12 w of feeding. Finally, we anesthetized the mice with 3 % pentobarbital sodium.
Echocardiogram measurements:
After 12 w of PQQ treatment, we performed transthoracic echocardiographic measurements of left ventricular function in mice using an ultrasound machine equipped with a 10 MHz phased-array transducer, the Vevo2100 high-resolution imaging system (Visual Sonics). M-mode tracings were recorded to measure Left Ventricular Internal Dimensions end-Diastolic (LVIDD), Left Ventricular Internal Dimensions end-Systolic (LVIDS), and Left Ventricular Posterior Wall Thickness end-Diastolic (LVPWD). LVPWD, end-diastolic septal thickness (IVSD), end-systolic septal thickness (IVSs), left ventricular Ejection Fraction (EF), and Fractional Shortening (FS). All measurements and calculations were performed in three consecutive heartbeats.
Trichrome staining:
Mouse heart tissue was immobilized in 4 % paraformaldehyde and embedded in paraffin. Then it was cut into 5 μm slides and colored with Haematoxylin & Eosin (H&E) and Masson trichrome staining. Pieces of the left ventricle were viewed under a light mircroradiometer, and then the pattern of collagen deposition in the interstitial region was determined. Fibrotic areas were identified using Image-Pro Plus imaging software.
Ribonucleic Acid (RNA) separation and Polymerase Chain Reaction (PCR):
We used TRIzol and NanoDrop spectrophotometer to extract total RNA from the left ventricle of mice and determine the RNA concentration. The complementary Deoxyribonucleic Acid (cDNA) was generated using a retrotranscription kit. cDNA was amplified and quantified at 95° for 15 s, 60° for 30 s, and 72° for 45 s. After 5 min, 40 cycles were performed at 95° for 15 s, 60° for 30 s, and 72° for 45 s, respectively. We then localized the expression level of Long Non-Coding RNA (LncRNA) and determined its relative content.
Statistical analysis:
We performed statistical analysis using Statistical Package for the Social Sciences (SPSS) software (version 22.0). The outcome of the trial was stated as mean±standard deviation. Statistical significance of the results was considered if p<0.05 using one-way Analysis of Variance (ANOVA).
Results and Discussion
PQQ has an effect on blood glucose concentration and heart weight/body weight in diabetic mice.
The fasting blood glucose concentration of diabetic mice was (22.19±1.37) mmol/l, which was higher than that of the control group (6.73±0.17) mmol/l; the body weight of diabetic mice was (23.16±0.76) g lower than that of the control group (26.88±0.43) g.
Treatment with PQQH (40 mg/kg/day) was found to significantly reduce the blood glucose concentration in diabetic mice. However, the effect of PQQ on the body weight of mice was not statistically significant. For the heart weight/body mass index, it was significantly increased in the diabetic group. Similarly, treatment with PQQH reduced the increase in heart weight/body weight index.
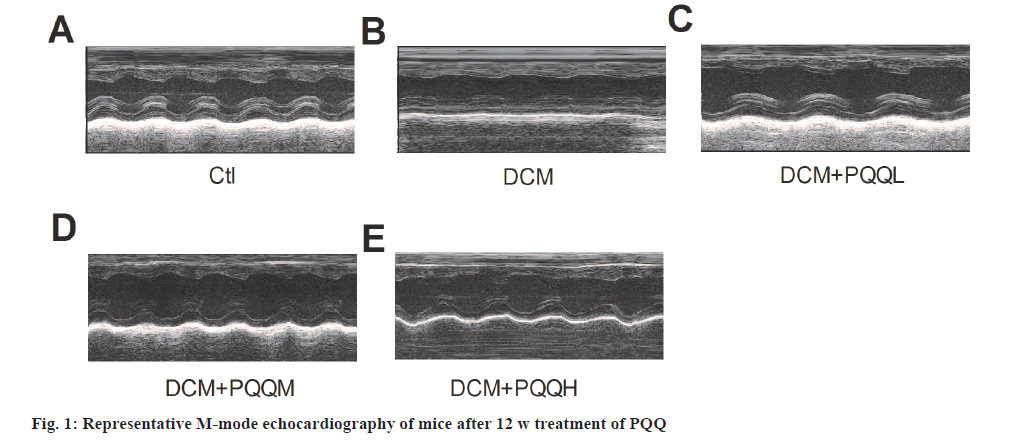
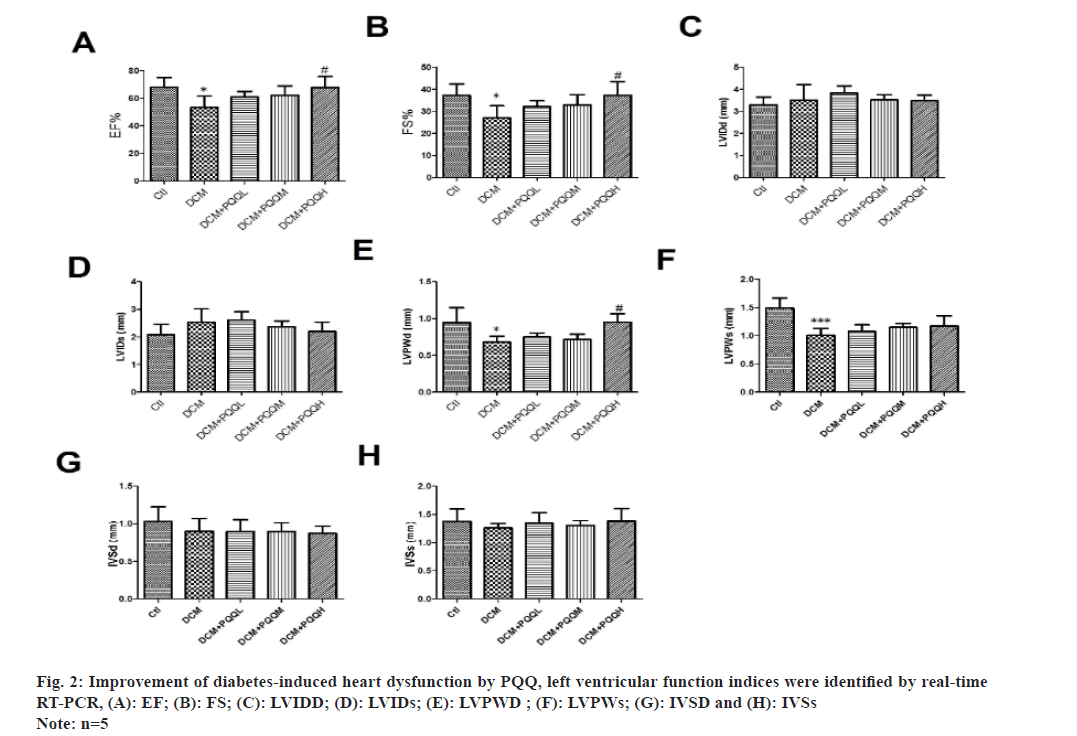
Echocardiography showed that EF, FS, LVPWD and LVPWs were significantly decreased in diabetic mice, indicating that cardiac function had been significantly impaired, which was improved after treatment with PQQ (fig. 1 and fig. 2A-fig. 2F). However, PQQ treatment had no effect on LVIDD, LVIDS, IVSD, and IVSs (fig. 2C-fig. 2H).
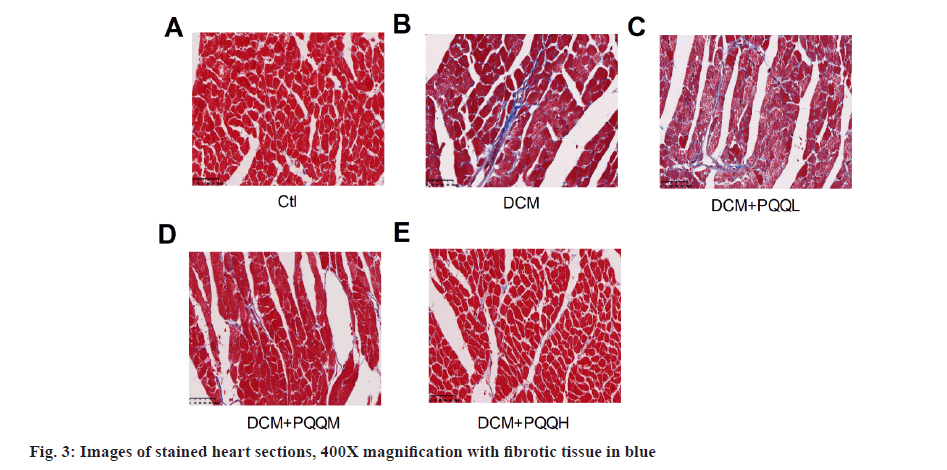
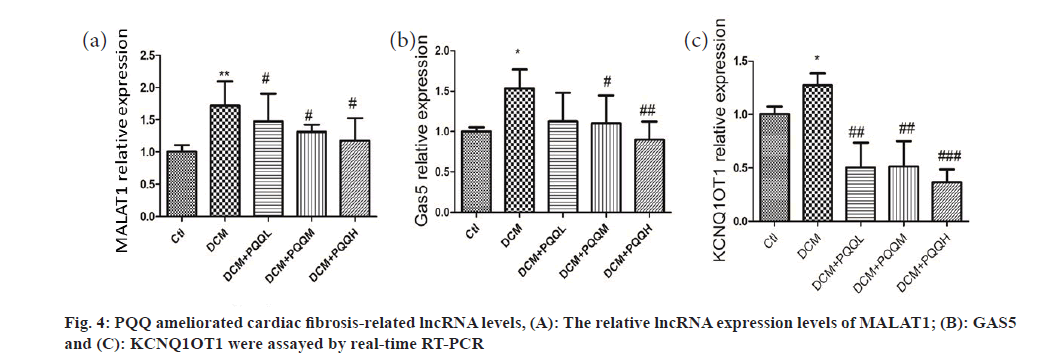
Collagen deposition in the interstitial region of the myocardium of diabetic mice was markedly up-regulated, as illustrated in fig. 3. Yet, PQQ therapy with 20 or 40 mg/kg/day was shown to decrease the size of diabetes-induced fibrillation. To determine further the influence of PQQ on heart fibrosis, we inspected the fibrosis-associated LncRNAs, including Metastasis-Associated Lung Adenocarcinoma Transcript 1 (MALAT1), Growth Arrest-Specific 5 (GAS5), and KCNQ1 Overlap Transcript 1 (KCNQ1OT1). As shown in fig. 4, the LncRNA levels of MALAT1, GAS5, and KCNQ1OT1 were elevated in the mice treated with STZ, but the amounts of these LncRNAs were recovered after PQQ processing. All these results indicated that PQQ can treat heart fibrillation in DCM.
DM is a chronic disease occurring in 9.3 % of the US population[14]. There are also many complications of DB, among which heart failure has a high prevalence in DM patients, accounting for about 19 %-26 % of all DM patients[15]. Our study also focused on a possible therapeutic drug for DCM, PQQ, which we found to have a cardio protective effect as an exotic vitamin. We first constructed a DCM mouse model, and then administered different concentrations of PQQ treatment to observe the ultrasound performance and tissue changes of the myocardium of mice to determine the effect of the drug on the myocardium of DCM mice. Finally, we also observed the expression of LncRNA related to myocardial fibrosis using Reverse Transcription (RT)-PCR, which corroborated the myocardial protection of PQQ on DCM from the mechanism level probably through altering the expression of LncRNA related to myocardial fibrosis.
The most prominent clinical manifestation of the new model of diabetes mellitus is congestive heart failure. In addition, arrhythmias are caused by focal myocardial necrosis and fibrous scar formation, resulting in homogeneity of electrophysiological properties of the myocardium. These include atrial fibrillation, sinus syndrome, atrioventricular block, ventricular preconstruction and ventricular tachycardia, etc., mainly presenting as various ventricular arrhythmias and angina pectoris.
We can make a preliminary diagnosis of diabetes mellitus by testing the significantly higher values of blood glucose and glycated hemoglobin and LVDD using echocardiography. In diabetic patients without clinical manifestations of heart failure, it is characterized by abnormalities in left ventricular diastolic function, which appear earlier and are more pronounced than abnormalities in systolic function. When diabetes is complicated by congestive heart failure, there are echocardiographic manifestations of dilated cardiomyopathy such as cardiac enlargement, left ventricular systolic dyskinesia, and impaired left ventricular systolic function. The heart size is normal in most patients with DCM, but in patients with heart failure or hypertension, the left ventricle is enlarged. Endomyocardial biopsy can be performed in patients with suspected DCM to exclude other causes of cardiomyopathy. Interventional cardiac catheterization in patients with DCM generally has elevated Left Ventricular End-Diastolic Pressure (LVEDP) and normal or increased Left Ventricular End-Diastolic Volume (LVEDV), with an increased ratio of the former to the latter (LVEDP/LVEDV), which reflects left ventricular stiffness and left ventricular diastolic function status. In addition, the patients have reduced expired volume per beat and ejection fraction, and some patients have diffusely reduced left ventricular systolic exercise. Heart Rate Variability (HRV) testing is reduced or absent in approximately 50 % of diabetic patients with 24 h heart rate variability. The disappearance of 24 h blood pressure fluctuations in diabetic patients, i.e. the disappearance of nocturnal blood pressure troughs, a phenomenon mainly attributed to nocturnal sympathetic hyperexcitability, may suggest that diabetic patients, who die due to cardiovascular pathology, are particularly likely to see nocturnal causes. Cardiac autonomic function tests can be used clinically to assess the degree of sympathetic nerve damage in diabetic patients. Cardiac function tests in DCM involve a large portion or all of the ventricular muscle, a general decrease in contractility of the entire ventricle, reduced compliance of the ventricular wall, and uncoordinated myocardial contraction.
There are many traditional treatments for DCM as well[16-18]. Diet and exercise are beneficial in the treatment of DCM, helping to improve glycemic control while improving insulin sensitivity and weight control helps to reduce the burden on the heart. Aggressive glycemic control can improve hyperglycemic toxicity. Patients can take medications to control blood pressure such as Angiotensin-Converting Enzyme (ACE), Angiotensin Receptor Blockers (ARBs), and calcium channel blockers. Statins have anti-inflammatory response and anti-oxidative stress. The treatment of congestive heart failure with systolic dysfunction is the same as that of general heart failure; for diastolic dysfunction, calcium antagonists should be the mainstay, together with other anti-heart failure therapeutic drugs, for example diuretics, angiotensin-converting enzyme inhibitors and nitrates. β-blockers have a blunting effect on the adrenergic response of the body during hypoglycemic response in diabetic patients and should be treated with caution. The prognosis of DCM varies with individual differences in the condition, with some remitting as the diabetic condition improves and others worsening with their condition, with the occurrence of heart failure or sudden cardiac death. Therefore, early detection, early diagnosis, and standardized treatment are necessary to maximize the prognosis.
PQQ is a cofactor for many enzymes and is responsible for the transfer of electrons, protons and chemical groups in enzymatic reactions, as well as stimulating the growth of microorganisms, the germination of plant pollen and promoting plant growth. The biological functions of PQQ are mainly focused on two aspects; firstly, it can support the growth and development of mitochondria and stimulate the rapid growth of human cells; secondly, it has good antioxidant properties, which can help to scavenge free radicals and reduce cell damage. These two functions make it powerful in brain health, cardiovascular health, and metabolic function health. Because the body cannot synthesize PQQ on its own, it can be supplemented with dietary supplements, which are usually in powder form and prepared through microbial fermentation. The antioxidant effect of PQQ was clearly observed in experiments with PQQ-fed animals. It was found that PQQ inhibited the formation of peroxynitrite, prevented SIN-1-induced ATP depletion, and scavenged superoxide anion to avoid nitration of bovine serum albumin. In addition, PQQ prevents the neurotoxin 6-hydroxydopamine from producing reactive oxygen species and reduces the cytotoxicity of 6-hydroxydopamine. Because the body cannot synthesize PQQ on its own, it can be supplemented by ingestion. The recommended daily intake for adults (except pregnant or lactating women) is 20 mg, which is currently available in China in various beverages. In addition, PQQ could protect the liver against alcohol- and carbon tetrachloride-induced liver injury and TAA-induced liver fibrosis, and it could improve neuronal damage caused by the oxygen glucose deprivation model, reduce the content of nisin vesicles caused by D-galactose treatment, inhibit neuronal degeneration, weaken the neurological damage caused by β-amyloid, and help promote the secretion of neurotrophic factors. In addition, PQQ significantly improved the memory function of rats, and had a significant effect on memory retention and dementia prevention. Giving a PQQ-deprived diet to primiparous female rats, it was found that if the female rat’s diet was deficient in PQQ, they showed reproductive impairment, and after mating and giving birth, the newborn pups would show symptoms such as growth retardation and stunting, and in addition, the female rats would become sterile or mutilate the newborn pups. If the appropriate amount of PQQ is supplemented at this time, the growth of young rats, female fertility and behavior gradually become normal. In the case of feeding a sufficient amount of PQQ, the reproductive capacity of female rats, the numbers of litters are higher. PQQ deprivation in the diet of female rats resulted in growth retardation and stunting of newborn pups; lack of PQQ in the diet also cause a large decrease in lysine oxidase content in the skin of rats, which cause a decrease in skin collagen content and damage to elastic protein cross-linking, manifesting as fragile and flaky skin, thinning hair, bent body, bowed back, serious abdominal bleeding and even death. Therefore, PQQ is an essential nutritional factor for animal growth and development. A significant increase in body weight of the pups was observed when the maternal generation was supplemented with sufficient amount of PQQ. In addition, PQQ was also found in human milk, suggesting that PQQ was very important in the growth of young animals.
Myocardial fibrosis is myocardial remodeling. It has been shown that one of the factors that act a key role in DCM is the fibrosis of the interstitial myocardium, with massive deposition of collagen, which increases the stiffness of the ventricular wall, decreases ventricular compliance, and causes ventricular systolic and diastolic insufficiency. The development of myocardial fibrosis in diabetes mellitus follows a progressive course. Pathological results showed that the manifestation of myocardial fibrosis started to be obvious after 3 mo in diabetic rats. There was a significant increase in the amount of intracellular collagen and an increase in myocardial interstitial fibers with a disturbed distribution. Myocardial type I collagen expression appeared significantly increased, resulting in thickening of myocardial fibers and increased myocardial stiffness in diabetic rats. Therefore, interventional treatment of diabetes early in life would be beneficial in delaying the onset of myocardial fibrosis and altered cardiac function. In addition, reduced diabetic Alpha (α)-actin may alter the basal metabolism and gene expression of cardiac energy as a mechanism for the development of DCM. Whereas once it was thought that the transcriptome consisted mainly of messenger RNA (mRNA), the development of high-throughput sequencing technology and the ENCODE project have unveiled a completely different world. Non-coding RNAs are the real protagonists. In addition to those well-known RNAs, many different types of regulatory RNAs have come into our attention. LncRNAs are a class of RNA molecules. LncRNA expression is developmentally regulated and is tissue- and cell-specific. A significant proportion of lncRNAs are located only in the nucleus. They contain many types of transcripts that are structurally similar to mRNA and are sometimes transcribed into antisense transcripts that encode genes. LncRNAs are thought to perform important regulatory functions and are also relevant to disease development. Studies have identified lncRNAs that are differentially expressed in a variety of cancers, including leukemia, breast, liver, colon and prostate cancers. Other diseases in which lncRNAs are dysregulated include cardiovascular disease, neurological disorders and immune-mediated diseases. MALAT1 degrades microRNA (miR)-503 through endogenous sponge action; reduced expression of miR-503 can MALAT1 degrades miR-503 through endogenous sponging; decreased expression of miR-503 can upregulate the expression of its target gene apelin and activate the Transforming growth factor-β (TGF-β) signaling pathway thus promoting myocardial fibrosis[19]. It has been shown that the expression of GAS5 is increased in myocardial fibrosis, and the mechanism is that GAS5 can act together with miR-21 to promote the development of myocardial fibrosis. KCNQ1OT1 has also been shown to be associated with fibrosis[20]. It has been demonstrated that knockdown of KCNQ1OT1 and silencing of SORBS2 inhibited cell fibrosis and induced apoptosis, while overexpression of SORBS2 restored KCNQ1OT1 knockdown-mediated fibrosis in cells. Therefore, upregulation of KCNQ1OT1 expression promoted cell fibrosis, which was achieved by targeting miR-18b-5p.
Although, we have experimentally demonstrated the effect of PQQ on the myocardium of DCM patients, our study has certain shortcomings. Firstly, the sample size of our study was small. Second, we only performed in vivo experiments to examine cardiac ultrasound and myocardial tissue changes in mice, and did not perform a deeper validation of the effects of PQQ in vitro. Finally, although our study demonstrated the regulatory effect of PQQ on lncRNAs associated with myocardial fibrosis, it did not elucidate the upstream and downstream pathways and specific mechanisms, which also needs to be further explored next. PQQ has not received enough attention in previous studies, and we only know that the food with the highest known PQQ content is Japanese Natto. So there is still a long way to go for the application of PQQ in DCM.
Funding:
The project was supported by Public Welfare Project of Zhejiang Provincial SCI And Tech Bureau under the project “Study on the anti-cervical cancer immune function induced by recombinant adenovirus vector expressing human papillomavirus type 16 E6E7 and CD137L” (No: LGF19C080001).
Authors’ contributions:
Yin Wang and Yi Wang have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 1972;30(6):595-602.
[Crossref] [Google Scholar] [PubMed]
- Hamby RI, Zoneraich S, Sherman L. Diabetic cardiomyopathy. JAMA 1974;229(13):1749-54.
[Google Scholar] [PubMed]
- Avagimyan A, Popov S, Shalnova S. The pathophysiological basis of diabetic cardiomyopathy development. Curr Prob Cardiol 2022;47(9):101156.
[Crossref] [Google Scholar] [PubMed]
- El Hayek MS, Ernande L, Benitah JP, Gomez AM, Pereira L. The role of hyperglycaemia in the development of diabetic cardiomyopathy. Arch Cardiovasc Dis 2021;114(11):748-60.
[Crossref] [Google Scholar] [PubMed]
- Zhan J, Chen C, Wang DW, Li H. Hyperglycemic memory in diabetic cardiomyopathy. Front Med 2022;16(1):25-38.
[Crossref] [Google Scholar] [PubMed]
- Salvatore T, Pafundi PC, Galiero R, Albanese G, di Martino A, Caturano A, et al. The diabetic cardiomyopathy: The contributing pathophysiological mechanisms. Front Med 2021;8:695792.
[Crossref] [Google Scholar] [PubMed]
- Jia G, de Marco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol 2016;12(3):144-53.
[Crossref] [Google Scholar] [PubMed]
- Jaquenod GC, Palomeque J, Mattiazzi A. Ca2+ mishandling and mitochondrial dysfunction: A converging road to prediabetic and diabetic cardiomyopathy. Pflugers Arch Eur J Physiol 2022;474(1):33-61.
[Crossref] [Google Scholar] [PubMed]
- Lafuse WP, Wozniak DJ, Rajaram MV. Role of cardiac macrophages on cardiac inflammation, fibrosis and tissue repair. Cells 2020;10(1):51.
[Crossref] [Google Scholar] [PubMed]
- Jin L, Deng Z, Zhang J, Yang C, Liu J, Han W, et al. Mesenchymal stem cells promote type 2 macrophage polarization to ameliorate the myocardial injury caused by diabetic cardiomyopathy. J Transl Med 2019;17(1):251.
[Crossref] [Google Scholar] [PubMed]
- KasaharaT KT. Nutritional biochemistry: A new redox-cofactor vitam in formammals. Nature 2003;422(6934):832.
[Crossref] [Google Scholar] [PubMed]
- Xu F, Yu H, Liu J, Cheng L. Pyrroloquinoline quinone inhibits oxygen/glucose deprivation-induced apoptosis by activating the PI3K/AKT pathway in cardiomyocytes. Mol Cell Biochem 2014;386(1-2):107-15.
[Crossref] [Google Scholar] [PubMed]
- Tao R, Karliner JS, Simonis U, Zheng J, Zhang J, Honbo N, et al. Pyrroloquinoline quinone preserves mitochondrial function and prevents oxidative injury in adult rat cardiac myocytes. Biochem Biophys Res Commun 2007;363(2):257-62.
[Crossref] [Google Scholar] [PubMed]
- Dillmann WH. Diabetic cardiomyopathy. Circ Res 2019;124(8):1160-2.
- Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Circ Res 2018;122(4):624-38.
[Crossref] [Google Scholar] [PubMed]
- Murtaza G, Virk HU, Khalid M, Lavie CJ, Ventura H, Mukherjee D, et al. Diabetic cardiomyopathy-A comprehensive updated review. Prog Cardiovasc Dis 2019;62(4):315-26.
[Crossref] [Google Scholar] [PubMed]
- Paolillo S, Marsico F, Prastaro M, Renga F, Esposito L, de Martino F, et al. Diabetic cardiomyopathy: Definition, diagnosis and therapeutic implications. Heart Fail Clin 2019;15(3):341-7.
[Crossref] [Google Scholar] [PubMed]
- Alonso N, Moliner P, Mauricio D. Pathogenesis, clinical features and treatment of diabetic cardiomyopathy. Adv Exp Med Biol 2018;1067:197-217.
- Zhou Y, Deng L, Zhao D, Chen L, Yao Z, Guo X, et al. Micro RNA-503 promotes angiotensin II-induced cardiac fibrosis by targeting Apelin-13. J Cell Mol Med 2016;20(3):495-505.
[Crossref] [Google Scholar] [PubMed]
- Jie R, Zhu P, Zhong J, Zhang Y, Wu H. LncRNA KCNQ1OT1 affects cell proliferation, apoptosis and fibrosis through regulating miR-18b-5p/SORBS2 axis and NF-ĸB pathway in diabetic nephropathy. Diabetol Metab Syndr 2020;12:77.
[Crossref] [Google Scholar] [PubMed]