- *Corresponding Author:
- A. Mahesh
Institute of Plant Sciences, ARO, The Volcani Center, Bet-Dagan 50250, Israel
E-mail: a.mahesh05@gmail.com
| Date of Submission | February 25, 2011 |
| Date of Revision | February 03, 2012 |
| Date of Acceptance | February 15, 2012 |
| Indian J Pharm Sci, 2012, 74 (2): 157-160 |
Abstract
The chemopreventive effect of ethanol root extract of Indigofera aspalathoides was evaluated in Nâ??nitrosodiethylamineinduced experimental liver tumor in mice. Pretreatment with ethanol root extract (100 mg/kg, p.o.) for three weeks significantly reduced the impact of Nâ??nitrosodiethylamine toxicity (50 mg/kg, i.p.) on the serum markers of liver damage, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase and total bilirubin. Protective effect was reconfirmed the elevated serum total protein levels were significantly restored towards normalization by the extracts, and this was confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Histological examination of the liver of animals treated with ethanol root extract of Indigofera aspalathoides showed the reduction of necrosis. These results suggest that ethanol root extract of Indigofera aspalathoides possess protective effect against Nâ??nitrosodiethylamineâ??induced hepatocellular carcinogenesis.
Keywords
Ethanol extract, histopathology, Indigofera aspalathoides, N?nitrosodiethylamine, SDS?PAGE
The liver plays a crucial role in the hepatobiliary excretion of many endogenous and exogenous substances from the body. Various liver diseases have an influence on this excretion [1]. The risk of liver damage has recently increased due to greater exposure to environmental toxins, pesticides and frequent use of chemotherapeutics. Liver damage commonly results from viral and protozoal infection, toxicity due to drugs, food additives and fungal toxins. Several investigations have provided convincing evidence that N?nitrosamines cause a wide range of tumors in all animal species. These compounds are considered to be potential health hazards to man [2]. Exposures of man to preformed N?nitrosamines occur through the diet, in certain occupational settings and also due to the use of tobacco products, cosmetics, pharmaceutical products and agricultural chemicals [3].
N?Nitrosodiethylamine (DEN), one of the most important environmental carcinogens of this class, primarily induces liver tumor. DEN is considered to be a genotoxic carcinogen [4]. It is often assumed that DEN initiates and propagates tumor development primary by inducing DNA alterations that lead to mutations [5]. Indeed, indicative mutations in the ras gene have been observed in mouse liver tumors arising in response to DEN treatment [6].
Indigofera aspalathoides Vahl ex DC. (Papilionaceae) commonly known as Shivanar vembu is distributed throughout the South India and Sri Lanka. The whole plant has been traditionally used for cooling, demulcent and odematous tumors; used in the form of decoction for leprosy and cancerous affections [7]. The ashes are used in preparations against dandruff’s. The stem is traditionally used for various skin disorders and cancer [8]. Methanol extract of Indigofera aspalathoides stem also possess hepatoprotective activity [9].
Indigofera aspalathoides Vahl ex DC. roots were collected from Bharathidasan University campus, and the identity of plant was verified at Rapinat Herbarium, St. Joseph’s College, Tiruchirappalli, South India. Shade?dried and powdered root (50 kg) was extracted at room temperature with ethanol (1:6 w/v) by using Soxhlet extractor for 48 h. The extracts were concentrated in vacuum at 40° using a rotary evaporator and stored in glass vials at 4° until use.
Male albino Wistar mice (25?30 g) were used as experimental animals. They were housed in polypropylene cages with sterile, inert husk materials as bedding. The experimental animals were maintained under controlled environment conditions of light and dark cycle (light 12 h: Dark 12 h, temperature 23±2º and relative humidity 55±10%). Animals were allowed to take standard pellet diet and water ad libitum. The whole experiment was conducted according to guidelines and approval of the Animal Ethical Committee of Sri Krishnadevaraya University at Anantapur, India (Reg. No. 25/1/99/AWD).
Animals were divided into five groups of each having 6 mice. Group I served as placebo control, received an injection of the vehicle (Saline 1 ml/kg i.p.) only. Carcinogenesis was induced in the groups II and III by administrating intraperitoneally 50 mg/kg of DEN (in saline) once in a week for a period of three week. Soon after DEN administration, ethanol root extract of Indigofera aspalathoides (EIA, 100 mg/kg) were orally given to group III, whereas group IV was administrated only EIA.
At the end of the treatment schedule, blood samples were collected by cardiac puncture and allowed to clot. Serum was then separated by centrifugation at 3000 g for 10 min. Serum samples stored at ?70° were later used for biochemical assays such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and serum bilirubin levels using commercially available kits according to manufacturer’s protocols (Randox Laboratories Co, UK). Serum total protein levels were determined by Lowry’s method [10], using bovine serum albumin as a standard.
Animals were sacrificed by cervical dislocation, and the liver perfused with ice?cold saline. Ten percent of homogenate was prepared in 50 mM phosphate buffer (pH 7) using a polytron homogenizer at 4° and the homogenates were centrifuged at 12,000 g for 15 min at 4°. The liver total protein levels were determined by the method of Lowry et al. [10], maintaining bovine serum albumin as a standard.
Serum and liver protein profiles were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE). Protein samples were supplemented with reducing SDS loading buffer (10% (v/v) glycerol, 50 mM Tris/HCl, 20 g/l sodium dodecylsulfate, 2 g/l bromphenolblue, 10% (v/v) b?mercaptoethanol, pH 6.8), boiled for 5 min and subsequently separated loaded on 10% acrylamide gel for separation. Equal amounts of total protein were loaded per lane. Silver staining method was used to visualize the protein bands on the gel after running.
A portion of the median lobe of the liver was dissected and fixed in 10% neutral buffered formalin solution for 24 h. The washed tissue was dehydrated in descending grades of isopropanol and finally cleared in xylene. The tissue was then embedded in molten paraffin wax. Sections were cut at 5 μm thicknesses, deparaffinized, rehydrated and stained with haematoxylin and eosin. The sections were then viewed under light microscope for analyzing the histopathological changes. The extent of DEN-induced necrosis was evaluated by comparing with the normal control samples.
The statistical differences among different groups were analyzed using one?way analysis of variance (ANOVA) and Tukey’s post hoc test using SPSS version 13 software (SPSS Inc., Chicago, USA). The results are expressed as mean±SD. Statistical significance was considered at P<0.05.
The protective effect of EIA on the DEN-induced hepatocarcinogenesis was evaluated in male Swiss albino mice. A significant increase in the activities of the serum enzymes, AST, ALT and ALP and total bilirubin were observed in mice receiving DEN when compared to placebo control. The pretreatment with EIA significantly (P<0.05) protected the elevation of AST, ALT, ALP and total bilirubin activities in DEN-induced liver cancer (Table 1). Elevation in the activities of serum transaminases and ALP has been considered as sensitive indicators of hepatic injury. The increased levels of serum transaminases and ALP have been attributed to the damaged structural integrity of the liver, since they are cytoplasmic and are released into circulatory system after cellular damage [11]. The level of ALT is an indicator of cell membrane damage. Whereas, the level of AST is an indicator of mitochondrial damage, since mitochondria carries 80% of this enzyme [12].
| Group | AST (IU/l) | ALT (IU/l) | ALP (IU/l) | Total bilirubin (mg/dl) |
|---|---|---|---|---|
| Placebo control | 110.37 ± 0.72 | 50.84 ± 0.63 | 146.57 ± 0.79 | 1.34 ± 0.04 |
| DEN treated | 170.98 ± 1.50# | 80.41 ± 0.60# | 205.46 ± 1.12# | 3.92 ± 0.13# |
| DEN+EIA | 127.16 ± 0.92* | 59.35 ± 0.44* | 176.04 ± 1.67* | 2.06 ± 0.07* |
| EIA alone | 109.79 ± 1.18* | 50.91 ± 0.53* | 153.08 ± 0.96* | 1.35 ± 0.03* |
| F- value | 657.30 | 622.44 | 503.78 | 221.23 |
Each value represents the mean±SE, #significant difference at P<0.05 (Tukey’s test) compared with the placebo control, *significant difference at P<0.05 (Tukey’s test) compared with the DEN treated group
Table 1: Effect Of Eia On Serum Enzymes In Den-Induced Hepatocarcinogenesis In Mice
Serum bilirubin is one of the most reliable tests employed in the diagnosis of hepatic diseases. It provides useful information on liver functioning [13]. Bilirubin, a chemical breakdown product of hemoglobin, gets conjugated with glucuronic acid in hepatocytes to increase its water solubility. Bilirubin concentration has been used as a reliable method to evaluate chemically induced hepatic injury. Besides various normal functions liver excretes the breakdown product of hemoglobin namely bilirubin into bile [14].
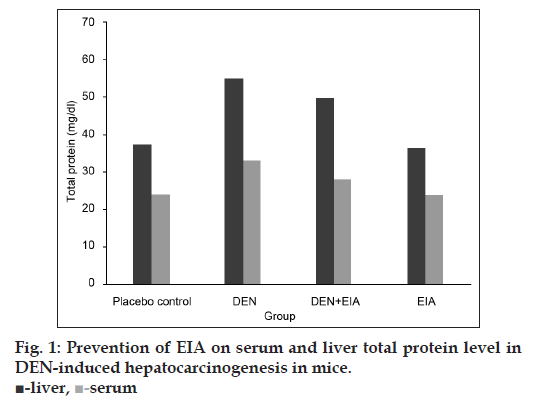
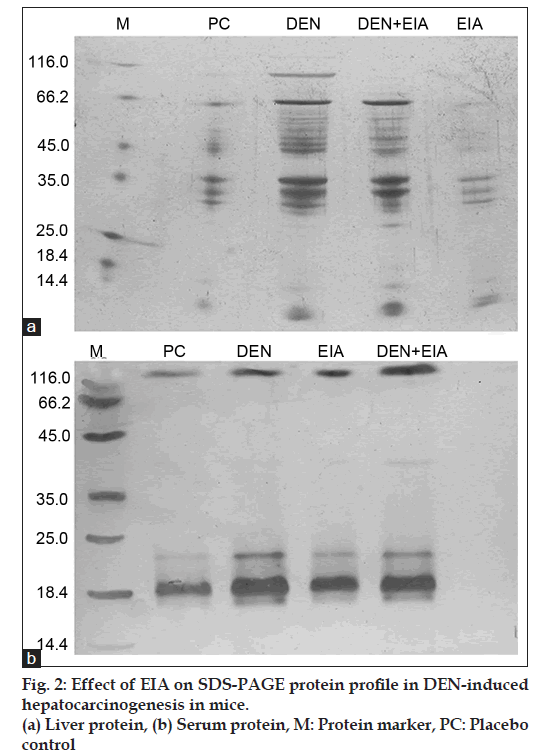
Fig. 1 shows the levels of liver and serum total protein in mice for each group. DEN-induced cancerous mice, significantly elevate the level of liver and serum total protein when compared to placebo control. DEN treated mice were found to accumulate 32 and 18.4 Kda proteins in liver and serum, respectively visualized by SDS?PAGE (fig. 2). Whereas, the mice treated with EIA exhibited significant reduction in total protein levels and also suppressed the 32 and 18.4 Kda proteins in liver and serum. Increased level of liver and serum total protein observed in DEN-induced cancerous mice demonstrate the decreased functional ability of intoxicated mice liver as reported earlier [15]. Stabilization of serum protein levels achieved through the supplementation of EIA is a clear indication of its protective effect stabilizing the functional status of the liver cells.
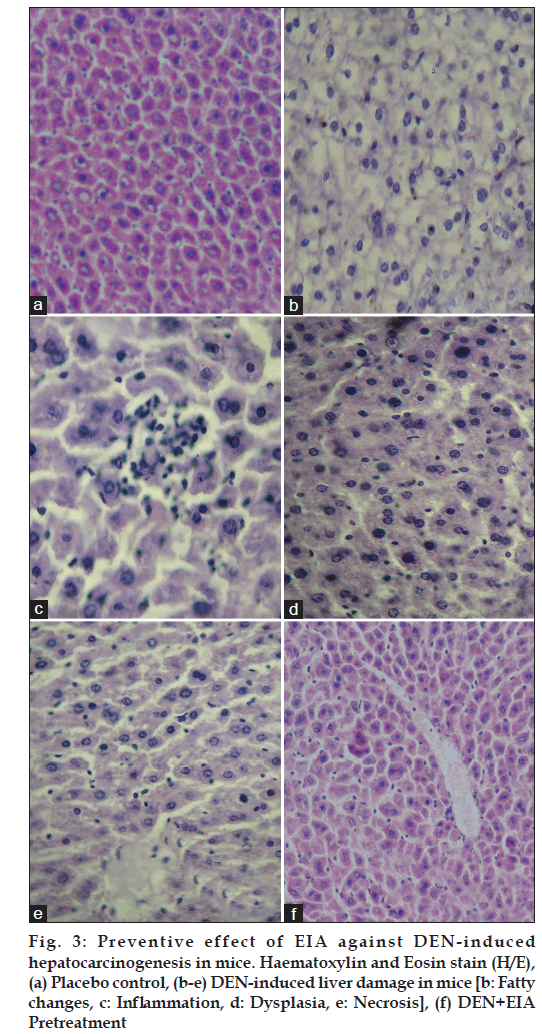
The liver of placebo control animals showed normal hepatic architecture (fig. 3a). Group II mice, exposed to DEN revealed extensive liver damage, characterized by the disruption of the lattice nature of the hepatocyte, damaged cell membranes, fatty changes, necrosis, dysplasia fibrosis, inflammation, disintegrated central vein and damaged hepatic sinusoids (fig. 3b?e). Pretreatment with EIA at 100 mg/kg dose showed significant reduction in the area of necrosis and inflammatory infiltrates in the centrizonal area with disappearance of inflammatory infiltrate around portal triad (fig. 3f). Centrizonal necrosis, which involves the cells around the central hepatic vein, occurs in viral hepatitis, DEN, carbon tetrachloride and chloroform toxicity, and anoxic states such as cardiac failure and shock [15,16]. In the present experiment, it indicates the damage caused by DEN to the hepatocytes. The decrease in the area of necrosis as well as in the infiltration of the inflammatory cells in the liver lobules demonstrated by the extracts is indicates the therapeutic efficacy of the plant.
In conclusion, the mechanism of chemopreventive effects of EIA mainly regulates the activity of serum enzymes and protein levels and thereby protecting the integrity of the liver. The results of this investigation may improve our understanding in usage of this plant as an alternative medicine for hepatic?cancer therapy. However, further studies are needed to characterize the active principles and to elucidate the molecular mechanism and action of EIA.
Acknowledgements
This article is dedicated to the memory of Mr. A. Ayyavu, to whom we are also thankful for his encouragements. Authors would like to thank Dr. S. Soosairaj for plant identification, helpful discussion and critical reading of the manuscript.
References
- Meijer DK, Smit JW, Muller M. Hepatobiliary elimination of cationic drugs: The role of P-glycoproteins and other ATP dependent transporters. Adv Drug Deliv Rev 1997;25:159-200.
- Loeppky RN. Nitrosamine and Nitroso Compound Chemistry and Biochemistry. In: Loeppky RN, editor. ACS Symposium series. Washington, DC: American Chemical Society; 1994. p. 1-18.
- Hecht SS. Approaches to cancer prevention based on an understanding of N-nitrosamine carcinogenesis. ProcSocExpBiol Med 1997;216:181-91.
- Lewis DFV, Brantom PG, Ioannides C, Walker R, Parke DV. Nitrosamine carcinogenesis: Rodent assays, quantitative structure activity relationships, and human risk assessment. Drug Metab Rev 1997;29:1055-78.
- Peto R, Gray R, Brantom P, Grasso P. Effects on 4080 rats of chronic ingestion of N-nitrosodiethylamine or N-nitrosodimethylamine: A detailed dose-response study. Cancer Res 1991;51:6415-51.
- Stowers SJ, Wiseman R, Ward J, Miller E, Miller J, Anderson M, et al. Detection of activated proto-oncogenes in N-nitrosodiethylamine-induced liver tumors: A comparison betweenB6C3F1 mice and Fischer 344 rats. Carcinogenesis 1988;9:271-6.
- Kirtikar KR, Basu BD. Glossary of Indian Medicinal plants. Vol. 1. New Delhi: Periodical Experts; 1975. p. 338.
- The Wealth of India. A Dictionary of Indian Raw Materials and industrial Products, Raw Materials. Vol. 5. New Delhi: Council of Scientific and Industrial Research; 2001. p. 176.
- Gupta M, Mazumder UK, Haldar PK, Manikandan L, Senthilkumar GP, Kander CC. Hepatoprotective activity of Indigoferaaspalathoides against carbon tetrachloride induced liver damage in rats. Orient Pharm Exp Med 2004;4:100-3.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J BiolChem 1951;193:265-75.
- Recknagel RO, Glende EA Jr, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. PharmacolTher 1989;43:139-54.
- Tang X, Gao J, Wang Y, Fan YM, Xu LZ, Zhao XN, et al. Effective protection of Terminaliacatappa L. leaves from damage induced by carbon tetrachloride in liver mitochondria. J NutrBiochem 2006;17:177-82.
- Harper HA. The functions and tests of the liver. In: Review of Physiological Chemistry. Los Atlos, CA: Lange Medical Publishers; 1961. p. 271-83.
- Mahesh A, Jeyachandran R, Cindrella L, Thangadurai D, Veerapur VP, MuralidharaRao D. Hepatocurative potential of sesquiterpene lactones of Taraxacum officinale on carbon tetrachloride induced liver toxicity in mice. ActaBiol Hung 2010;61:175-90.
- Jeyachandran R, Mahesh A, Cindrella L. DEN-induced cancer and its alleviation by Anisomele smalabarica (L.) R. Br. ethanolic leaf extract in male albino mice. Int J Cancer Res 2007;3:174-9.
- Chandrasoma P, Taylor CR. Concise Pathology. Stamford, CT: Appleton and Lange; 1998. p. 450.