- Corresponding Author:
- V. Kumar
Department of Pharmacology, KIET School of Pharmacy, Ghaziabad-201 206
E-mail: vinaykumarpatel@gmail.com
| Date of Submission | 24 July 2013 |
| Date of Revision | 07 June 2014 |
| Date of Acceptance | 15 June 2014 |
| Indian J Pharm Sci 2014;76(4): 315-322 |
Abstract
Obesity is associated with numerous co-morbidities such as cardiovascular diseases, type 2 diabetes, hypertension and others. Therefore, the present study was planned to investigate the effect of water- soluble fraction of Gymnema sylvestre ethanol extract on biochemical and molecular alterations in obese diabetic rats. Diabetes was induced by single i.v. injection of streptozotocin (45 mg/kg) via tail vein. Obesity was induced by oral feeding of high fat diet for a period of 28 days in diabetic rats. Body weight gain, food intake, water intake, hemodynamic parameters (systolic, diastolic, mean arterial blood pressures and heart rate), serum biochemical parameters (leptin, insulin, lipid levels, apolipoprotein B and glucose), cardiomyocyte apoptosis (cardiac caspase-3, Na + /K + ATPase activity and DNA fragmentation) organs and visceral fat pad weight and oxidative stress parameters were measured. Oral treatment with water soluble fraction of Gymnema sylvestre ethanol extracts (120 mg/kg/p.o.) for a period of 21 days, resulted in significant reduction in heart rate, mean arterial pressure, serum leptin, insulin, apolipoprotein B, lipids, glucose, cardiac caspase-3 levels, Na + /K + ATPase activity and DNA laddering, visceral fat pad and organ's weight and improved the antioxidant enzymes levels in the high fat diet induced obesity in diabetic rats. The results of present study reveal that water soluble fraction of Gymnema sylvestre ethanol extract could be useful intervention in the treatment of obesity and type-2 diabetes mellitus.
Keywords
Gymnema sylvestre, leptin, insulin, blood pressure, caspase-3, dyslipidemia
Obesity has been a common feature in Western Societies, although recent evidence suggests that it becomes also a major problem in Asia and almost all over the world. Obesity is associated with diabetes mellitus, hypertension, dyslipidemia and cardiovascular disease [1]. There has been a tragic increase in diabetes across the world, paralleling the overweight and obesity epidemic. Type 2 diabetes mellitus is an increasingly common disorder of carbohydrate and lipid metabolism [2]. The high risk of both diabetes and cardiovascular disease associated with obesity in Asians may be due to a predisposition to abdominal obesity, which can lead to the metabolic syndrome and impaired glucose tolerance. Furthermore, approximately 197 million people worldwide have impaired glucose tolerance, most commonly because of obesity and the associated metabolic syndrome [3].
A number of studies have reported that rats treated with streptozotocin (STZ)-induced diabetes show severe hyperlipidemia after exogenous fat loading [4]. One important pathologic phenomenon associated with obesity and its related conditions is cardiomyocyte cell death, and in particular, cardiomyocyte apoptosis [5]. It has been reported that high fat diet induces apoptosis [6]. During apoptosis, various proteases are activated that lead to the destruction of diverse intracellular proteins and the fragmentation of nuclear chromatin. Caspase-3 is a key player involved in the caspase-dependant apoptotic pathway.
The sodium (Na) and potassium (K)-activated adenosine-triphosphatase (Na+/K+ ATPase) is a membrane enzyme that energizes the Na-pump by hydrolysing adenosine triphosphate and wasting energy as heat, so playing a role in thermogenesis and energy balance. Animal and human obesity is associated with reduction of tissue Na+/K+ ATPase, linked to hyperinsulinemia [7].
Gymnema sylvestre R. Br. (family: Asclepidaceae) is a native plant in the south west of India, Australia and tropical Africa. From ancient times, G. sylvestre has been used in Indian traditional medicine (Ayurvedic medicine) and is considered to be effective in improving urination, and diabetes [8]. In an animal study, extract of Gymnema sylvestre R. Br leaves improved serum cholesterol and triglyceride levels through influence of lipid metabolism. Bishayee and Chaterjee reported the hypolipidemic and antiatherosclerotic effect of Gymnema sylvestre extract in albino rats fed on high fat diet [9]. The present study was designed to evaluate the protective effect of water soluble fraction of G. sylvestre ethanol extract on blood pressure, hyperleptinemia, hyperinsulinemia, hyperglycemia, dyslipidemia, caspase-3 levels, organs and visceral fat pad weight and oxidative stress on HFD-induced obesity in diabetic albino rats.
Materials and Methods
The present study was approved by the Institutional Animal Ethics Committee (IAEC) of Hamdard University, New Delhi, which is registered with Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India, India, (Registration No. 173/ CPCSEA, dated Jan 28, 2000). Male Wistar albino rats, weighing 150-200g, were procured from the Central Animal House Facility, Hamdard University, New Delhi and acclimatized under standard laboratory conditions (12 h light and 12 h dark:day:night cycle) at 25±2°, relative humidity (50±15%) and had a free access to water ad libitum.
Diet and chemicals
High fat diet was purchased from National Centre for Laboratory Animal Sciences (NCLAS), National Institute of Nutrition (NIN), Hyderabad, Andhra Pradesh, India. HFD contains (g/kg): Casein-342 g; cystine-3 g; starch-172 g; sucrose-172; cellulose-50 g; G. N. oil-25 g; tallow-90 g; mineral oil-37 g; vitamin mix-10 g. All other chemicals used were of analytical grade.
Preparation of water-soluble fraction of the Gymnema sylvestre extract
The leaves of Gymnema sylvestre are purchased locally, air dried and authenticated by Raw Materials Herbarium and Museum, NISCAIR, New Delhi, India. The specimen voucher (Ref. NISCAIR/RHMD/Consult/2008-09/980/11) was retained to the Department of Pharmacology, Faculty of Pharmacy Jamia Hamdard, New Delhi-110 062. Air-dried leaves of G. sylvestre extracted with ethanol (70%) in Soxhlet?s apparatus. The solvent was removed under reduced pressure. The ethanol extract yield was 10.8% (w/w) in terms of starting material. The ethanol extract was standardized by determination of Ash value, pH, foam index, phytochemical constituent and microbial load according to WHO guidelines [10]. Further, the ethanol extract was sub fractionated into water-soluble (W-S) fraction and waterinsoluble (W-INS) fractions [11]. The yield obtained of W-S and W-INS were 60% and 40% in terms of the total ethanol extract, respectively.
Experimental design
After acclimatization, except rats of Group I (normal healthy control), all rats were treated with streptozotocin (STZ, 45 mg/kg, i.v. single dose), those rats having fasting blood glucose level more than 200 mg/dl were included in this study and divided in following groups of 8 animals each and treated as follows: Group I?rats fed with standard rat diet for a period of 28 days; Group II (pathogenic control) ?rats fed with HFD for a period of 28 days; Group III ?rats fed with HFD for a period of 28 days + from 8th day 120 mg/kg/p.o. water soluble fraction of G. Sylvestre ethanol extract.
Measurement of anthropometric, hemodynamic parameters
Body weight gain, daily food intake, daily water intake were measured. Hemodynamic parameters (systolic, diastolic, mean arterial blood pressure and heart rate) were measured using non invasive Blood Pressure Recorder using rat tail-cuff method (Kent Scientific Corporation, USA) on 29th day.
Measurement of serum biochemical parameters
Blood was collected from the retro-orbital plexus of overnight fasted rats using microcapillary tubes on 29th day. Serum was separated by centrifugation (4000 rpm for 10 min) and transferred to Eppendorf tubes. The concentrations of lactate dehydrogenase (Reckon Diagnostics Pvt. Ltd., Baroda, India) glucose, total cholesterol, triglycerides (all three from Span diagnostics Ltd., Surat, Gujarat, India), HDLcholesterol (Reckon Diagnostics Pvt. Ltd., vadodara, India), and apolipoprotein- B by immunoturbidimetric immunoassay kit (Randox Laboratories Ltd., Antrim, UK) in serum were measured with commercial kits. Glycohemoglobin was estimated in blood with a commercial available kit (Asritha In vitro Diagnostic Reagents, Hyderabad, India). The concentrations of insulin and leptin in the serum were measured respectively with rat insulin ELISA kit (Alpco Diagnostics, Salem, USA), rat leptin ELISA kit (BioVendor, Brno, Czech Republic). On the final day of experiment the rats were fasted overnight and sacrificed by cervical dislocation and then different organs (heart, liver, Kidney and pancreas) and fat pads (epididymal, perirenal and mesentric) were removed, washed with normal saline and weighed [12].
Cardiomyocyte apoptosis measurement
Caspase-3 activity was measured using Caspase-3/ CPP 32 colorimetric assay kit. 50 μl supernatant from homogenized tissue with cooled lysis buffer was taken from each sample and 50 μl of 2X Reaction Buffer (containing 10 mM DTT) was added to each sample. Then 5 μl of the 4 mM DEVD-pNA substrate (200 μM final conc.) was added and incubated at 37º for 1-2 h to allow a dissociation of p-nitroanilide (pNA) from the conjugate DEVD-pNA to form a blue colour. CPP-32 activity was measured spectrophotometrically at 405 nm. Caspase-3 activity was calculated as nmol/h/mg protein [13]. Na+/K+ ATPase activity was measured in heart tissue, method reported by Bonting [14].
Apoptosis was evaluated by examining the characteristic pattern of DNA laddering generated in the apoptotic myocardium using gel electrophoresis. Myocardial samples were homogenized in solution containing 50 mmol/l Tris-HCl (pH 8.0), 100 mmol/l EDTA, 100 mmol/l NaCl, and 1% sodium dodecyl sulphate. The tissue homogenate was digested with 5 μl of proteinase K (stock solution 20 mg/ml)) at 56° for 2 h and incubated with RNase A (1 μl/ml) at 37° for 1 h. After that phenol/chloroform (1:1) extraction was performed twice. The tubes were shaken and kept at room temperature (5-10 min. Then tissues were precipitated and centrifuged in cooling centrifuge at 4°; 10 000 rpm for 10 min. Supernatant containing DNA were precipitated with 600 μl isopropanol and the resulting DNA pellets after centrifugation were washed with 75% chilled ethanol and dissolved in 100 μl of TE buffer solution (10 mmol/l Tris HCl (pH 8.0), 1 mmol/l EDTA). DNA samples (5 μl DNA+1 μl gel loading dye) were subjected to electrophoresis on 2% agarose gel, stained with ethidium bromide. DNA laddering, an indicator of tissue apoptotic nucleosomal DNA fragmentation was visualized and photographed under ultraviolet transilluminator [15].
Determination of antioxidant enzymes and oxidative stress in heart tissue
All antioxidant enzyme activities were determined after heart tissue was homogenized with phosphate buffered saline (PBS) at a pH of 7·0. Glutathione was measured according to Ellman method [16]. Glutathione peroxidase (GPx) activity was determined according to the method of Mohandas et al [17]. Glutathione reductase (GR) was determined according to the method of Carlberg and Mannerviek [18]. GST activity was measured by the method of Habig et al [19]. Superoxide dismutase (SOD) and catalase activities were estimated according to Marklund and Marklund [20] and Claiborne [21] methods.
Lipid peroxidation was determined with spectrophotometric measurement of the amount of malondialdehyde equivalents with thiobarbituric acid and was expressed as thiobarbituric acid-reactive substances (TBARS; nmoles malondialdehyde/mg protein), according to the method of Ohkawa et al [22].
Histopathological examination
The studied animals were killed and heart tissue samples collected, fixed in 10% formalin buffered solution, cut into 5 µm sections and stained with haematoxylin/eosin. The sections of heart tissues were studied to determine the protective effect of water soluble fraction of G. sylvstre extract on tissue damage by high fat diet and STZ.
Statistical analysis
All values are expressed as mean±standard error of mean (SEM). Comparisons between the treatment groups and pathogenic control group were performed by analysis of variance (ANOVA) followed by Dunnett?s t- test. P<0.05 was assumed to be statistical significant.
Results
Effect of water-soluble fraction of G. sylvestre ethanol extract on anthropometric and hemodynamic parameters
As shown in Table 1, after STZ (45 mg/kg single i.v.) administration rats fed on HFD for 28 days, water intake and food intake were significantly (P<0.05) increased while body weight gain decreased in Group II (i.e. Pathogenic control group) as compared to the Group I (normal healthy control group). These parameters were reversed in Group III (i.e. STZ 45 mg/kg, i.v. single dose+HFD for 28 days+water soluble fraction of G. sylvestre extract 120 mg/kg/p.o. for 21 days) as compared to the Group II.
| Group I | Group II | Group III | |
|---|---|---|---|
| Food Intake (g/100 g day) | 12.78±0.31 | 17.27±0.36* | 12.07±0.51ns# |
| Water Intake (ml/100 g/day) |
40.34±3.81 | 49.58±3.41* | 42.57±1.26# |
| Body weight gain (g) | 75.13±5.02 | 46.46±11.61* | 59.33±8.44ns# |
| Systolic BP (mm Hg) | 127.83±6.86 | 161±1.43* | 106±1.52# |
| Diastolic BP (mm Hg) | 96±4.65 | 126.66±1.80* | 79.16±2.65# |
| Mean BP (mm Hg) | 106.16±5.00 | 137.83±0.94* | 90.83±1.07# |
| Heart Rate (BPM) | 421.83±15.77 | 3463.66±23.65* | 350.33±9.65# |
| Total cholesterol (mg/dl) | 101.69±2.00 | 373.22±17.51* | 132.08±8.61# |
| HDL-C (mg/dl) | 32.26±1.01 | 28.54±0.86* | 48.45±1.04# |
| Triglycerides (mg/dl) | 64.97±2.19 | 346.57±18.07* | 113.23±7.30# |
| LDL-C (mg/dl) | 56.64±0.31 | 275.37±18.74* | 60.99±9.27# |
| VLDL-C (mg/dl) | 12.99±0.43 | 69.31±3.61* | 22.64±1.46# |
| Atherogenic index | 3.25±0.09 | 13.06±0.38* | 2.73±0.19# |
All values were expressed as mean±SEM, (n=8 rats/group), *P<0.05 as compared to the Group I; #P<0.05, ns#: non significant as compared to the Group II (ANOVA followed by Dunnett?s test), BP: blood pressure, HDL: highdensity lipoprotein, LDL: low-density lipoprotein VLDL: very low-density lipoprotein, GSE: G. sylvestre ethanol extract
Table 1: Effect of W-S Fraction of Gse on Anthropometric, Hemodynamic and Serum Lipid Parametres
There was significant (P<0.05) increase in hemodynamic parameters (systolic, diastolic, mean BP and heart rate) in Group II as compared to the normal control group (i.e. Group I) while in Group III, these parameters were significantly reduced as compared to the Group II.
Effect of Water-soluble fraction of G. sylvestre ethanol extract on hyperlipidemia, hyperleptinemia, hyperinsulinemia and hyperglycemia
As shown in Tables 1 and 2, Group II showed significant (P<0.05) increase in TC (3.7 fold), TGs (5.4 fold), LDL-C (5 fold), VLDL-C (5 fold) and atherogenic index as compared to the Group I, while serum HDL-C (1.2 fold) level was significantly reduced in Group II. These parameters were significantly decreased in the Group III (TC- 2.8 fold, TGs- 3 fold, LDL-C- 4.5 fold and VLDL-C- 3 fold) and Group IV (TC- 3.1 fold, TGs- 3.3 fold, LDL-C- 4.8 fold and VLDL-C- 3.2 fold), while HDL-C (1.7 fold in Group III) was increased as compared to the Group II. Serum leptin (3.2 fold), insulin (7.5 fold), apolipoprotein-B (apo-B) (7.8 fold), lactate dehydrogenase (LDH) (12 fold), glucose (4.9 fold) and glycosylated hemoglobin (1.5 fold) were significantly (P<0.05) increased in Group II as compared to the Group I. These parameters were significantly (P<0.05) decreased in Group III (leptin- 3.3 fold, insulin- 11 fold, apo-B- 5 fold, LDH- 3.5 fold, blood glucose- 4.8 fold and glycosylated hemoglobin- 1.4 fold) as compared to the Group II.
Effect of water-soluble fraction of G. sylvestre ethanol extract on cardiomyocyte apoptosis
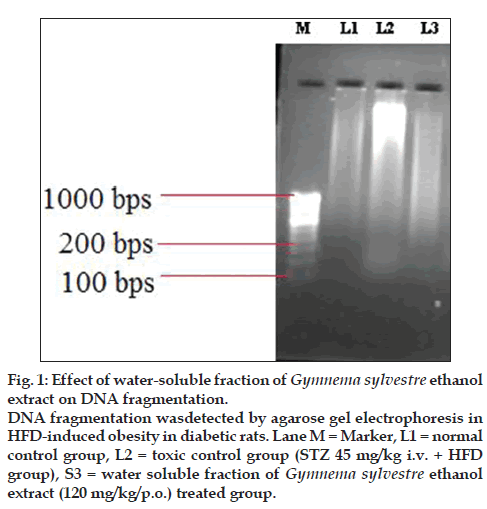
The mean cardiac caspase -3 levels (4.5 fold) were significantly (P<0.05) increased in Group II as compared to the Group I while in Group III (2.3 fold), mean caspase-3 levels were significantly decreased as compared to the Group II. The Na+/K+ ATPase activity was significantly (1.1 fold) decreased in Group II as compared to the Group I while in Group III (1.4 fold) the activity was significantly increased in heart tissue as compared to the Group II. (Table 2) DNA laddering was more significant in the group II, while DNA laddering was preserved in the group III (fig. 1).
| Parameters | Group I | Group II | Group III |
|---|---|---|---|
| Serum leptin (pg/ml) | 159.37±6.26 | 513.5±64.01* | 155.5±8.19# |
| Serum insulin (ng/ml) | 0.116±0.02 | 1.832±0.076* | 0.175±0.02# |
| LDH (IU/L) | 23.48±0.64 | 285.26±11.49* | 79.89±9.61# |
| Apolipoprotein-B (mg/dl) | 4.65±0.22 | 32.78±2.01* | 6.18±0.26# |
| Glucose (mg/dl) | 94.14±2.16 | 430.62±8.78* | 95.72±3.30# |
| Glycated hemoglobin (in %) | 7.94±0.11 | 13.88±0.19* | 8.82±0.18# |
| Caspase-3 activity (nmole/hr/mg protein) |
62.34±9.28 | 288.80±8.36* | 124.45±3.77# |
| Na-K ATPase activity (µmol of Pi liberated/ min/mg protein) |
0.725±0.04 | 0.654±0.03* | 0.909±0.02# |
| Organ’s weight (g/100gm body weight) |
9.60±0.28 | 12.14±0.42* | 12.28±0.39ns# |
| Visceral fat pad weight ((g/100 gm body weight) |
1.64±0.04 | 2.14±0.08* | 1.85±0.05# |
All values were expressed as mean±SEM, (n=8 rats/group), *P<0.05 as compared to the Group I; #P<0.05, ns#: non significant as compared to the Group II (ANOVA followed by Dunnett?s test), LDH: low-density lipoprotein, GSE: G. sylvestre ethanol extract
Table 2: Effect of W-S Fraction of Gse on Biochemical Parameters In Serum and Heart Tissue
Figure 1: Effect of water-soluble fraction of Gymnema sylvestre ethanol
extract on DNA fragmentation.
DNA fragmentation wasdetected by agarose gel electrophoresis in
HFD-induced obesity in diabetic rats. Lane M = Marker, L1 = normal
control group, L2 = toxic control group (STZ 45 mg/kg i.v. + HFD
group), S3 = water soluble fraction of Gymnema sylvestre ethanol
extract (120 mg/kg/p.o.) treated group.
Effect on organs and visceral fat pad weight
Organs weight (heart, liver, kidney and pancreas) and visceral fat pad weights (mesenteric, perirenal and epididymal) were significantly (P<0.05) increased in group II as compared to the group I. The visceral fat pad weights decreased in Group III, while there was no change in organ?s weight as compared to the Group II (Table 2).
Effect of water-soluble fraction of G. sylvestre ethanol extract on oxidative stress
Lipid peroxide levels (i.e. nmol MDA/mg protein) were significantly (5.5 fold) increased in Group II as compared to the Group I, while the levels of lipid peroxides were significantly decreased in Group III (2 fold). Superoxide dismutase levels were significantly decreased in Group II (1.7 fold) as compared to the Group I, while these levels were significantly increased in Group III as compared to the Group II. Catalase levels (5 fold) decreased in Group II as compared to the Group I, while catalase levels in Group III (2.5 fold) were increased as compared to the Group II. Reduced glutathione levels (1.3 fold) were decreased in Group II as compared to the Group I, while these levels were in Group III (1.6 fold) increased as compared to the Group II (Table 3).
| Parameters | Group I | Group II | Group III |
|---|---|---|---|
| TBARS (nmol MDA/mg protein) |
0.200±0.003 | 1.149±0.02* | 0.583±0.02# |
| SOD (IU/mg protein) CAT (nmol H2O2 ? consumed/min/mg protein) |
1.771±0.01 | 1.123±0.015* | 1.308±0.011# |
| 55.75±1.88 | 10.64±0.34* | 26.34±0.35# | |
| GSH (µmol of phosphorus liberated/min/mg protein) |
26.75±0.97 | 15.86±0.48* | 26.26±0.45# |
| Glutathione peroxidase (nmol NADPH oxidized/ min/mg of protein) |
184.81±7.54 | 121.28±6.46* | 173.47±10.46# |
| Glutathione reductase (nmol NADPH oxidized/ min/mg of protein) |
29.90±0.43 | 17.39±1.22* | 33.70±1.09# |
| Glutathione S transferase (nmol CDNB conjugate formed/min/mg protein) |
492.31±9.01 | 155.80±9.71* | 399.79±5.68# |
All values were expressed as mean±SEM, (n=8 rats/group), *P<0.05 as compared to the Group I; #P<0.05 as compared to the Group II (ANOVA followed by Dunnett?s test), TBARS: thiobarbituric acid-reactive substances, SOD: superoxide dismutase, NADPH: nicotinamide adenine dinucleotide phosphate, CDNB: 1-chloro-2,4-dinitrobenzene, GSH: glutathione, CAT: catalase
Table 3: Effect of W-S Fraction of Gse on Oxidative Stress and antioxidant Enzymes
Glutathione peroxidase levels (1.5 fold) decreased in Group II as compared to the Group I while these levels were in Group III (1.4 fold) increased as compared to the Group II. Glutathione reductase levels (1.7 fold) were decreased in Group II as compared to the Group I, while in Group III (1.8 fold) increased as compared to the Group II. Glutathione-S-transferase levels (3.1 fold) were decreased in Group II as compared to the Group I; while in Group III (2.2 fold) were increased as compared to the Group II shown in Table 3.
Effect of water-soluble fraction of G. sylvestre ethanol extract on histopathological changes
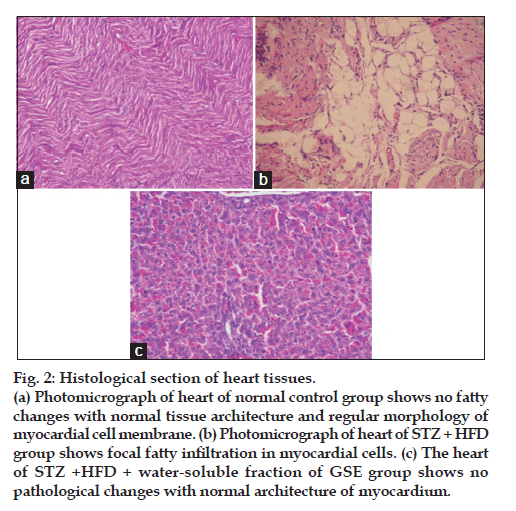
Water soluble fraction of G. sylvestre ethanol extract improved histopathological changes as demonstrated in fig. 2. Photomicrograph of heart of normal control group shows no fatty changes with normal tissue architecture and regular morphology of myocardial cell membrane (fig. 2a). Photomicrograph of heart of STZ+HFD group shows focal fatty infiltration in myocardial cells (fig. 2b). The heart of STZ+HFD+water-soluble fraction of GSE group shows no pathological changes with normal architecture of myocardium (fig. 2c).
Figure 2: Histological section of heart tissues.
(a) Photomicrograph of heart of normal control group shows no fatty
changes with normal tissue architecture and regular morphology of
myocardial cell membrane. (b) Photomicrograph of heart of STZ + HFD
group shows focal fatty infiltration in myocardial cells. (c) The heart
of STZ +HFD + water-soluble fraction of GSE group shows no
pathological changes with normal architecture of myocardium.
Discussion
There is an increasing amount of data showing that being overweight during childhood and adolescence significantly associated with insulin resistance, dyslipidemia, and elevated blood pressure in young adulthood. Type-2 diabetes is strongly associated with obesity and cardiovascular risk. Diabetic rats when fed with high fat diet produce a metabolic syndrome characterized by insulin resistance, dyslipidemia, type-2 diabetes and central obesity, which is similar with the metabolic syndrome caused by obesity. As carbohydrate and lipid metabolisms are closely linked processes, derangement in the carbohydrate metabolism produces dyslipidemia, hence, STZ+HFD model is one of the ideal model for screening of antiobesity activity in diabetic rats.
This statement is supported by the findings of Ding et al. who reported that Wistar rats injected intraperitoneally with low dose of STZ (30 mg/kg) and fed with a high sucrose-fat diet for 8 weeks, develop significant insulin resistance and obesity [23]. Similarly, Srinivasan et al. reported that high fat diet?fed and low dose of STZ (35 mg/kg, i.p.) treated rats simulate natural disease progression and metabolic characteristics typical of individuals at increased risk of developing type 2 diabetes because of insulin resistance and obesity [24]. Further, Zhang et al. demonstrated that a combination of HFD and low dose of STZ (45 mg/kg) injection effectively used to generate a rat model that mimics the natural history and metabolic characteristics of type-2 diabetes in humans [25].
In the present study, the protective effect of watersoluble fraction of G. sylvestre ethanol extract was determined on STZ diabetic rats in the presence of high fat diet. Previous reports suggested that diabetic animals maintained on a HFD were normophagic relative to diabetic rats maintained on high cholesterol diet [26]. In the present study, after STZ administration, there was no significant change in food intake of rats maintained on the high fat diet, as compared to the other groups.
In the present study, blood pressures and heart rate were significantly increased in Group II as compared to the Group I, while these levels decreased significantly by water soluble fraction of G. sylvestre extract treatment. HFD induces BP elevation, could derange the neurohumoral control of the kidney [27]. At the same time, diabetes is also associated with alterations in resting heart rate and blood pressure [28].
The serum lipid levels were increased in HFD fed diabetic rats, while the lipid levels were significantly decreased in water-soluble fraction of G. sylvestre ethanol extract treated group. Fat-fed, diabetic animals showed abnormalities in lipid metabolism as evidenced from increased serum TC, LDL-C, VLDL-C and TGs levels, as in case of human type 2 diabetic patients, which might contribute to various cardiovascular complications. The hypertriglyceridemia observed in fat-fed/STZ rats may be due to increased absorption and formation of triglycerides in the form of chylomicrons following exogenous consumption of diet rich in fat or through increased endogenous production of TG-enriched hepatic very low density lipoprotein (VLDL) and decreased TG uptake in peripheral tissues [29].
In the present study, serum leptin levels were significantly increased in HFD fed diabetic rats. An increase in leptin levels after feeding of high fat diet to diabetic rats has been reported [30]. Zhao et al. have reported that the HFD feeding rats; develop insulin resistance [31]. At the same time, low-dose STZ has been known to induce a mild impairment of insulin secretion which is similar to the feature of the later stage of type-2 diabetes characterized by a progressive decline in insulin action (insulin resistance), followed by hyperinsulinemia [24].
Serum glucose concentrations were elevated in the diabetic rats maintained on the HFD relative to the normal control group, these concentrations were significantly decreased by water soluble fraction of G. sylvestre extract treated group. Serum apolipoprotein B levels were increased in the diabetic rats maintained on HFD as compared to the normal control group as apolipoprotein B is associated with low density lipoprotein, intermediate-density lipoprotein (IDL), very low-density lipoprotein (VLDL) and chylomicrons [32].
The treatment with water soluble fraction of G. sylvestre extract for 3 weeks suppressed the increase in caspase-3 levels, Na+/K+ ATPase activity, organ?s weight (liver, heart, kidney and pancreas) and weights of perirenal, mesentric and epididymal fat in HFD fed diabetic rats. DNA electrophoresis demonstrates the presence of small DNA fragments in the form of a DNA ladder in HFD fed group, while in the G. sylvestre ethanol extract treatment group, DNA laddering was preserved.
In the present study, antioxidant enzymes levels were significantly increased by water soluble fraction of G. sylvestre extract treated group as compared to the HFD fed diabetic group. This may be due to the high levels of fat increase fat-mediated oxidative stress and decrease antioxidative enzyme activity [33], as well as hyperglycemia was found to increase the production of free radicals that is associated with increased production of reactive oxygen species (ROS), resulting in tissue damage that is assessed by the measurement of lipid peroxides [34].
The results of the present study confirm that water soluble fraction of G. sylvestre ethanol extract effectively alleviates the deleterious effects produced by HFD in diabetic rats. The possible mechanism of water soluble fraction of Gymnema sylvestre ethanol extract for antiobesity activity is due to the reduction of serum leptin, insulin, hyperlidemia and oxidative stress in HFD fed diabetic rats. Further, G. sylvestre ethanol extract offers cardiac protection by decreasing cardiac caspase-3 levels, Na+/K+ ATPase activity, DNA laddering, oxidative stress, and maintaining normal architecture of myocardium. The present study supports the potential of G. sylvestre in diaobesity disorder.
Acknowledgments
The study was supported by major research project by University Grants Commission, New Delhi, India. Authors are thankful to Dr. H. B. Singh, Scientist F and Head, Raw Materials Herbarium and Museum, NISCAIR, New Delhi, India for authentication of Gymnema sylvestre extract leaves.
References
- Marinou K, Tousoulis D, Antonopoulos AS, Stefanadi E, Stefanadis C. Obesity and cardiovascular disease: From pathophysiology to risk stratification. Int J Cardiol 2010;138:3-8.
- Nisoli E, Carruba MO, Tonello C, Macor C, Federspil G, Vettor R. Induction of fatty acid translocase/CD36 peroxisome proliferator-activated receptor-γ2, leptin, uncoupling proteins 2 and 3, and tumor necrosis factor-a gene expression in human subcutaneous fat by lipid infusion. Diabetes 2000;49:319-24.
- Hossain P, Kawar B, El Nahas M. Obesity and Diabetes in the Developing World- A Growing Challenge. N Engl J Med 2007;356:213-5.
- Young NL, Lopez DR, McNamara DJ, Benavides G. Evaluation of the contribution of dietary cholesterol to hypercholesterolemia in diabetic rats and sitosterol as a recovery standard for cholesterol absorption. J Lipid Res 1985;26:62-9.
- Trivedi PS, Barouch LA. Cardiomyocyte apoptosis in animal models of obesity. Curr Hypertens Rep 2008;10:454-60.
- Wang Y, Ausman LM, Russell RM, Greenberg AS, Wang XD. Increased apoptosis in high-fat diet?induced nonalcoholic steatohepatitis in rats is associated with c-Jun NH2-terminal kinase activation and elevated proapoptotic Bax. J Nutr 2008;138:1866-71.
- Iannello S, Milazzo P, Belfiore F. Animal and human tissue Na K-ATPase in normal and insulin-resistant states: Regulation, behaviour and interpretative hypothesis on NEFA effects. Obes Rev 2000;8:231-51.
- Nadkarni AK. Indian Material Medica. 3rd ed. Mumbai: Popular Prakashan Pvt, Ltd; 1982.
- Bishayee A, Chaterjee M. Hypolipidemic and antiatherosclerotic effect of oral Gymnema sylvestre leaf extract in albino rats fed on high fat diet. Phytotherapy Res 1994;8:118-20.
- World Health Organization. Quality control methods for medicinal plant material. Geneva: WHO; 1998. p. 28-36.
- Alam MM, Javed K, Jafri MA. Effect of Rheum emodi (Revand Hindi) on renal functions in rats. J Ethnopharmacol 2005;96:121-5.
- Kaur G, Kulkarni SK. Antiobesity effect of a polyherbal formulation, OB-200G in female rats fed on cafeteria and atherogenic diets. Indian J Pharmacol 2000;32:294-9.
- Gurtu V, Kain SR, Zhang G. Fluorometric and colorimetric detection of caspase activity associated with apoptosis. Anal Biochem 1997;251:98-102.
- Bonting SL. Sodium potassium activated adenosine triphosphatase and cation transport. In: Bitler EE, editor. Membrane and ion transport. Vol. 1. London: Interscience Wiley; 1970. p. 257-63.
- Ling H, Lou Y. Total flavones from Elsholtzia blanda reduce infarct size during acute myocardial ischemia by inhibiting myocardial apoptosis in rats. J Ethnopharmacol 2005;101:169-75.
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82:70-7.
- Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller DJ. Low activities of glutathione-related enzymes as factors in the genesis of urinary bladder cancer. Cancer Res 1984;44:5086-91.
- Calberg I, Mannerviek B. Glutathione reductase levels in rat brain. J Biol Chem 1975;250:5475-80.
- Habig WH, Pabst MJ, Jakoby WB. Glutthione-S-transferase. The first enzymatic step in mercaptouric acid formation. J Biol Chem 1974;249:7130-9.
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the auto-oxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 1974;47:469-74.
- Claiborne A. Catalase activity. In: Greenwald RA. editors. CRC Handbook of Methods for Oxygen Radical Research. Boca Raton, FL USA: CRC Press; 1985. p. 283-4.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351-8.
- Ding SY, Shen ZE, Chen YT, Sun SJ, Liu Q, Xie MZ. Pioglitazone can ameliorate insulin resistance in low-dose streptozotocin and high sucrose-fat diet induced obese rats. Acta Pharmacol Sin 2005;26:575-80.
- Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol Res 2005;52:313-20.
- Zhang M, Lv X, Li J, Xu Z, Chen L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res 2008;2008:704045.
- Friedman MI. Hyperphagia in rats with experimental diabetes mellitus: A response to decreased supply of utilizable fuels. J Comp Physiol Psychol 1978;92:109-17.
- Hall JE, Brands MW, Dixon WN, Smith MJ Jr. Obesity-induced hypertension renal function and systemic hemodynamics. Hypertension 1993;22:292-9.
- Hicks KK, Seifen E, Stimers JR, Kennedy RH. Effects of streptozotocin-induced diabetes on heart rate, blood pressure and cardiac autonomic nervous control. J Auton Nerv Syst 1998;69:21-30.
- Sahu A, Sninsky CA, Kalra PS, Kalra SP. Neuropeptide Y concentration in micro dissected hypothalamic regions and in vitro release from the medial basal hypothalamus - preoptic area of streptozotocin-diabetic rats with and without insulin substitution therapy. Endocrinology 1990;126:192-8.
- Chavez M, Seeley RJ, Havel PJ, Friedman MI, Matson CA, Woods SC, et al. Effect of high-fat diet on food intake and hypothalamic neuropeptide gene expression in streptozotocin diabetes. J Clin Invest 1998;102:340-6.
- Zhao S, Chu Y, Zhang C, Lin Y, Xu K, Yang P, et al. Diet-induced central obesity and insulin resistance in rabbits. J Anim Physiol Anim Nutr (Berl) 2008;92:105-11.
- D'Souza T, Mengi SA, Hassarajani S, Chattopadhayay S. Efficacy study of the bioactive fraction (F-3) of Acorus calamus in hyperlipidemia. Indian J Pharmacol 2007;39:196-200.
- Slim RM, Toborek M, Watkins BA, Boissonneault GA, Hennig B. Susceptibility to hepatic oxidative stress in rabbits fed different animal and plant fats. J Am Coll Nutr 1996;15:289-94.
- Nakhaee A, Bokaeian M, Saravani M, Farhangi A, Akbarzadeh A. Attenuation of oxidative stress in streptozotocin-induced diabetic rats by Eucalyptus globulus. Indian J Clin Biochem 2009;24: 419-25.