- *Corresponding Author:
- Kondareddypally Nanjundappa Anitha
Department of Pharmacology,
College of Pharmaceutical Sciences, Dayananda Sagar University,
Bangalore, Karnataka 560078,
India
E-mail: AnithaKN.res-shs-pharmacy@dsu.edu.in
| Date of Received | 11 August 2021 |
| Date of Revision | 06 October 2021 |
| Date of Acceptance | 07 January 2022 |
| Indian J Pharm Sci 2022;84(1):41-48 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Nigella sativa seeds methanolic extract was prepared by a simple maceration process. The extract was subjected to preliminary phytochemical screening and high-performance thin-layer chromatography for linoleic and linolenic acid and tested for in vitro soluble epoxide hydrolase inhibition activity. Then the extract (100, 150 and 200 mg/kg) was evaluated against ethanol-induced stomach ulcer in rats using 1-(1-propanoylpiperidin-4-yl)-3-(4-trifluoromethoxy)phenyl) urea (1 mg/kg) and omeprazole (20 mg/ kg) as reference standards. The plant extract contains flavonoids, alkaloids, proteins, free amino acids, tannins, flavonoids and linoleic acid. The in vitro soluble epoxide hydrolase inhibition test results reveal that Nigella sativa seeds methanolic extract inhibit human soluble epoxide hydrolase enzyme (half maximal inhibitory concentration=2.619 μg/ml). The Nigella sativa seeds methanolic extract reversed macroscopic and microscopic alteration and attenuated the levels of myeloperoxidase, tumour necrosis factor-alpha, interleukin-6, 5-hydroxyeicosatetraenoic acid in ulcer rats. These results were comparable with standard control. In conclusion, Nigella sativa seeds methanolic extract has a protective action against ethanolinduced ulcers in rats.
Keywords
Nigella sativa, stomach ulcer, soluble epoxide hydrolase inhibition, 5-hydroxyeicosatetraenoic acid, 1-(1-propanoylpiperidin-4-yl)-3-(4-trifluoromethoxy)phenyl) urea
Peptic ulcer is the characteristic mucosal damage due to many factors such as infections, alcohol intake, smoking or some drugs[1]. Ethanol penetrate rapidly into the mucosa, damages cell membrane and causes inflammation that leads to gastric ulcer[2]. Ethanol- induced gastric ulcer mediated through the free radical generation that leads to depletion of endogenous antioxidants and increases the lipid peroxide levels in mucosa[3]. Because of differences inalcoholmetabolizing enzyme, serum cytokines and inflammatory responses also varies[4]. Hence, pre-treatment with drugs having anti-inflammatory, antioxidant and restorative properties along with cytochrome 2E1 modulating ability is the first right step in preventing Ethyl Alcohol (EtOH) induced gastric ulcer.
So far, histamine-2 receptor antagonists, Proton Pump Inhibitors (PPIs) and antacids are the commonly used antiulcer agents which work with the principle of reducing or neutralization the stomach acid[5].
Nonetheless, due to high relapse of stomach acids, the use of the same is limited. Practicing antibiotic treatment to treat Helicobacter pylori is restricted with a fear of developing antibiotic resistance[6]. However, to treat ethanol-induced gastric ulcers, antioxidants are used, as the gastric damage is caused due to the direct action of epithelium which causes lipid peroxidation. But, excess of antioxidants poses side effect like tumour cells protection[7]. Hence, an exploration of new anti-ulcer agents, with different treating pathways is in progress.
As the hypothesis is already set, the defensive use of soluble Epoxide (sEH) inhibitors could decrease the Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) induced ulcers with increase in anti-inflammatory Epoxyeicosatrienoic Acids (EETs) which are produced from arachidonic acids. sEH inhibition reduces the metabolism of EETs to form 5-Hydroxyeicosatetraenoic Acids (5-HETE) and ease inflammatory bowel disease along with fibrotic diseases[8,9]. In metabolism of arachidonic acid, EETs are artefacts of cytochrome P450 epoxygenase pathway, which affects several biological utilities associated with anti-inflammation, anti-hypertension as well as anti-apoptosis[10,11]. Hence, administration of sEH inhibitors will decrease the gastric ulcer levels with an increase in EETs and epoxy fatty acids. 1-(1-Propanoylpiperidin-4-yl)-3-(4- Trifluoromethoxy)Phenyl) Urea (TPPU) is one such sEH inhibitor and its efficacy is proven to inhibit the gastric ulcer with the help of biomarkers[12]. Research has been done to explore the presence of TPPU or TPPU like sEH inhibitors in herbal plants and succeeded.
Indian herbs are known to bless with many medicinal values. With that, researchers have investigated many plants consisting of sEH inhibitors. An isolate of Glycosmis stenocarpa areal plant is found to show sEH inhibition action[13]. A Vietnamese medicinal plant Docynia indica[14], root of the plant Pentadiplandra brazzeana[15] and Cimicifuga dahurica (Turcz.) Maxim[16] were also investigated for the presence of sHE inhibitors.
Nigella sativa belonging to family of Ranunculaceae is a miracle herb widely used as medicinal plant throughout the world[17]. Seeds and oil of Nigella sativa have a long history of folklore usage in various systems of medicines and food. Extensive research has been done on the biological and therapeutic potentials of Nigella sativa such as antimicrobial, spasmolytic, gastro and renal protective, anti-inflammatory and antioxidant properties[17]. Seeds of the plant are used as a liver tonic which could fight several complications like bronchitis, asthma and skin-related issues. The seeds are known to elevate milk production in nursing women and keepup immunity by fighting parasitic infections[18-20].
Nigella sativa is considered a miracle drug and still exhibits unveiled biological applications. In the present study, the insight on presence of sEH inhibition in the methanolic extracts of seeds of Nigella sativa was investigated. The extract was further tested for its efficacy to treat an ethanol-induced gastric ulcer. The antiulcer properties were evaluated by observing pH of the gastric contents, macroscopic and microscopic observations of the gastric tissue, ulcer scoring, estimation of Myeloperoxidase (MPO) in gastrointestinal tissue and estimation of inflammatory biomarkers in blood serum (Tumour Necrosis Factor alpha (TNF-α), Interleukin-6 (IL-6) and 5-HETE).
Materials and Methods
Materials:
TPPU a sEH inhibitor was received as a gift from the Department of Entomology, University of California. Human 5-HETE Enzyme-Linked Immunosorbent Assay (ELISA) kit was procured from Fine Test (Fine Biotech, Wuhan, China), inflammatory markers TNF-α and IL-6 ELISA kits were procured from Krishgen Biosystems, Mumbai, India. All the other chemicals used in the study were of analytical grade.
Animals:
Adult female Wistar rats (7-8 w) were used in the present study. The animals were housed under standard laboratory conditions, maintained on a 12 h light:12 h dark cycle and free access to food and water. Animals were acclimatized to laboratory conditions before the test. The experimental protocols were approved by the Institutional Animal Ethics Committee (DSU/PhD/ IAEC/12/2017-18). All the procedures performed were in accordance with the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines, under Ministry of Animal Welfare Division, Government of India, New Delhi.
Plant material:
The seeds of Nigella sativa were collected from the local market of Tirupati and authenticated from Department of Botany, Sri Venkateshwara University, Tirupati (India). The voucher specimen was kept in the department herbarium for future reference.
Preparation of Nigella sativa Seeds Methanolic Extract (NSME):
The dried seeds of Celastrus paniculatus (300 g) were coarsely powdered and extracted with absolute methanol (1000 ml) by cold maceration process for 7 d and methanol was evaporated to get a dark brown coloured NSME (yield: 15 %).
Preliminary phytochemical screening of NSME:
Phytochemical constituents in NSME were performed for qualitative analysis of alkaloids (Mayer’s test, Dragendorff’s test, Wagner’s test, Hager’s test), carbohydrates (Molisch’s test, Fehling’s test, Benedict’s test), glycosides (Legal’s test, Libermann-Burchard’s test), phytosterols (Liebermann-Burchard’s test), fixed oils and fats (spot test), saponins (foam test), phenolic compounds and tannins (ferric chloride test, lead acetate test), proteins and free amino acids (Millon’s test, Ninhydrin test), gums and mucilage, flavonoids (Shinoda’s test, alkaline reagent test)[21,22].
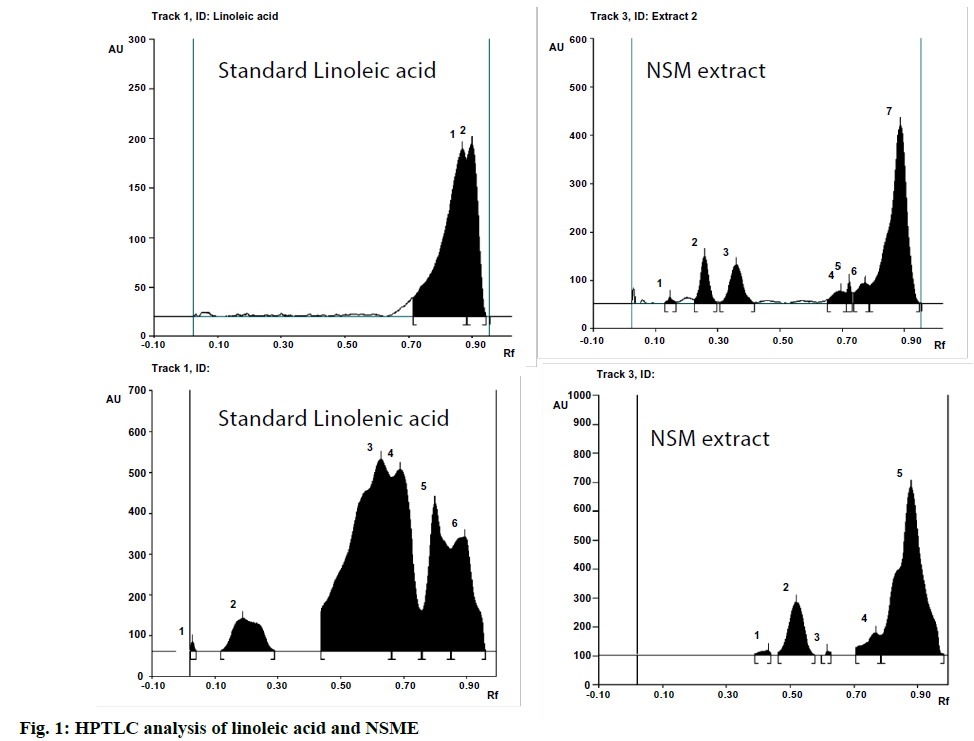
High-Performance Thin-Layer Chromatography (HPTLC) was employed for the qualitative estimation of linoleic and linolenic acid. Camag HPTLC system with a Linomat V sample applicator, Camag Thin-Layer Chromatography (TLC) Scanner 3 and WINCATS 4 software was used for the interpretation of the data. An aluminium plate (20×10 cm) precoated with silica gel 60F254 (E Merck) was used as the adsorbent. The plates were developed using n-hexane:ethyl acetate (5:4) as the mobile phase for NSME in a Camag twin trough chamber and scanned at 254, 366 and 425 nm. The Retention Factor (Rf) values were determined using WINCATS 4 software. The developed plates were photo-documented at 254 nm, 366 nm and 425 nm using a Camag 3 Reprostar. The Rf values of NSME were compared with the Rf value of standard linoleic acid and confirmed by an overlay of spectra.
In vitro sEH inhibition activity of NSME:
Plant extracts were solubilized at 10 mg/ml in Dimethylsulfoxide (DMSO) and inhibitory potencies were measured against the human recombinant sEH using a fluorescent reporting system as per the method prescribed by Jones et al.[23]. Shortly, half maximal Inhibitory Concentration (IC50) values were determined using Cyano (2-Methoxynaphthalen-6-yl) Methyl Trans-(3-Phenyl-Oxyran-2-yl) Methyl Carbonate (CMNPC) as a fluorescent substrate. Recombinant sEH (1 nM) was incubated with inhibitors for 5 min in 100 mM sodium phosphate buffer (pH 7.4) containing 0.1 mg/ml of Bovine Serum Albumin (BSA) at 30° prior to substrate introduction ([S]=5 μM). The activity was measured by determining the appearance of the 6-methoxy-2-naphthaldehyde with an excitation wavelength of 330 nm and an emission wavelength of 465 nm for 10 min.
In vivo antiulcer activity of NSME:
Weighed quantity of the NSME was suspended in distilled water using Tween 80 (1 % v/v) and administered orally to experimental animals at a constant volume of 3 ml/kg. 3 doses of NSME (100, 150 and 200 mg/kg, orally (p.o.)) were selected based on our earlier studies.
Total 42 rats were randomly divided into 7 groups consisting of six each, Group I served as normal control, Group II served as ulcer control, Groups III and IV served as a standard control and Groups V to VII were test groups.
Animals were deprived of food for 18 to 24 h but had free access to water. Then animals from Group I and II received 1 ml/kg of Tween 80 (1 % v/v) orally; animals from group III and IV received TPPU (1 mg/kg, Intraperitoneal (IP)) and omeprazole (20 mg/kg, p.o.), respectively. Group V, VI and VII, orally received NSME at the dose of 100, 150 and 200 mg/kg respectively.
To induce gastric ulcer in the animals, 1 h after the offered mentioned treatments, animals from Group II to Group VII are orally administered with a single dose of ethanol (90 %) 5 ml/kg to induce gastric ulcers. Group I animals served as normal control and received 5 ml/ kg of normal saline[24].
1 h after the ethanol treatment blood samples were collected; serum was separated and used for biochemical estimations. Then animals were sacrificed with cervical dislocation and abdomen was opened through a midline incision and the stomach was removed and opened along the greater curvature and carefully collected the gastric content pH determined with a digital pH meter. The stomach tissue was washed with phosphate- buffered saline and used for ulcer scoring, biochemical estimations and histopathological studies[25].
Macroscopic and microscopic observations:
Ulcer scoring: The severity of ulcer was evaluated by an independent observer who was blinded to the treatment. Macroscopic ulcer scores were assigned based on clinical features of the stomach using the following scoring pattern. Red colouration was counted as 0.5 points, spot ulcers as 1 point, hemorrhagic streaks counted as 1.5 points, if up to four mucosal erosion or ulcers then 2 points were added to the score and if more than five ulcerations or erosions then 3 points were added to the score. Thus, each stomach tissue could get multiple scores and the ulceration index was the sum of its scores, with a maximal score of 6. After scoring the stomach tissue was washed with saline and part of tissue was preserved for histopathological studies and the remaining was used for estimation of MPO.
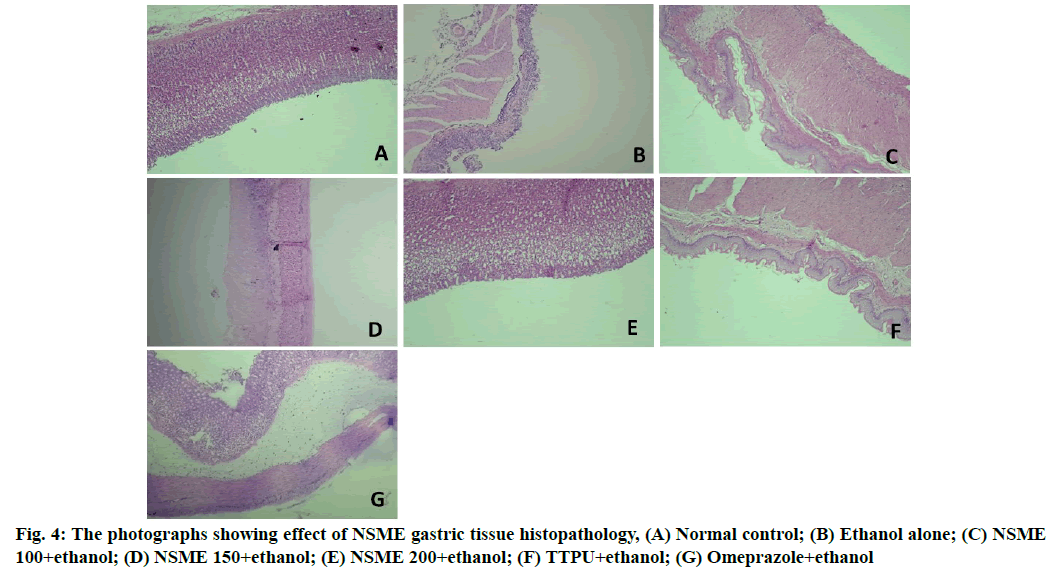
Histopathological observations: A portion of the tissue specimen from each rat (n=3) was fixed with 10 % formalin, embedded in paraffin wax and cut into sections of 5 mm thickness. The sections were stained with Hematoxylin and Eosin (H and E) dye for histopathological observations. The stained sections were examined for any inflammatory changes like infiltration of the cells, necrotic foci, damage to tissue structures like payers patches, damage to nucleus etc.
Estimation of MPO levels:
MPO was estimated in gastrointestinal tissues as per ELISA kit manufacturer protocol. Shortly, 40 µl of samples, 10 µl of biotin conjugate, 50 µl of Horseradish Peroxidase (HRP) conjugate were used for the study. The plate with above-mentioned mixture was incubated for 1 h at 37. Incubation was further continued with substrate solutions given in the kit and finally, 50 µl of stop solution was added and the colour change was studied by measuring absorbance at 450 nm.
Estimation of TNF-α, IL-6 and 5-HETE in serum:
The blood samples collected were subjected to centrifugation for 10 min, 4000 rpm at 4°. The serum was separated and used for estimation of TNF-α, IL-6 and 5-HETE using a microplate reader as per ELISA kit manufacturer protocol.
Statistical analysis:
Statistical analysis was done with Graphpad prism 5.01 software. The data is presented in the form of mean±Standard Error of the Mean (SEM). For statistical significance, one-way Analysis of Variance (ANOVA) followed by Tukey’s multiple comparison test was employed. With the significance value p<0.05, between the groups was considered significant.
Results and Discussion
Preliminary phytochemical screening indicates that NSME contains components such as alkaloids, carbohydrates, glycosides, proteins, free amino acids and flavonoids, whereas phytosterols, fixed oils, fats, saponins, gums and mucilage were absent. The qualitative analysis results are shown in Table 1. These results are in accordance with the literature published previously[26-28].
| S. No | Test components | Test methods | Result |
|---|---|---|---|
| 1 | Alkaloids | Mayer’s Test | + |
| Dragendorff’s test | + | ||
| Wagner’s test | + | ||
| Hager’s test | + | ||
| 2 | Carbohydrates | Molisch’s test | + |
| Fehling’s test | + | ||
| Benedict’s test | + | ||
| 3 | Glycosides | Legal’s test | + |
| Libermann-Burchard’s test | + | ||
| 4 | Phytosterols | Liebermann-Burchard‘s test | - |
| 5 | Fixed oils and fats | Spot test | - |
| 6 | Saponins | Foam test | - |
| 7 | Phenolic compounds and tannins | Ferric chloride test | + |
| Lead acetate test | - | ||
| 8 | Proteins and free amino acids | Millon’s test | - |
| Ninhydrin test | + | ||
| 9 | Gums and mucilage | Swelling property | - |
| 10 | Flavonoids | Shinoda’s test | + |
| Alkaline reagent test | + |
Note: (+) sign indicates presence and (–) sign indicates absence of that particular component
Table 1: Qualitative Analysis of Presence of Various Chemical Components in NSME
The HPTLC fingerprint studies were carried out for establishing the presence of the biomarker compound linoleic acid in NSME. The Rf value of standard linoleic acid was found to be 0.87 at 366 nm. The NSME revealed a spot having Rf 0.87 corresponding to that of standard linoleic acid at 366 nm. The presence of linoleic acid in NSME was confirmed by overlay spectrum study of standard linoleic acid at 366 nm.
The standard linoleic acid, NSME exhibited blue fluorescence under 254 nm, bright blue fluorescence under 366 nm and no fluorescence under 425 nm. The NSME was also evaluated for the presence of linolenic acid but could not be confirmed by overlay spectrum study of standard linolenic acid at 254 nm. The HPTLC chromatograms of standard linoleic acid and linolenic acid and the respective ones for NSME are presented in fig. 1.
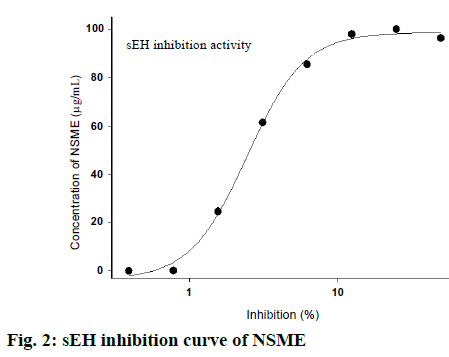
The in vitro sEH inhibition test results reveal that NSME inhibits human sEH enzyme activity. The IC50 values of the in vitro sEH inhibition test were found to be 2.619 µg/ml. The sEH inhibition activity curve is shown in fig. 2. This data indicates that NSME has strong inhibitory actions against sEH activity. Inhibition of sEH is an emerging novel mean for treating various disorders associated with inflammation, cardiac disorders and hypertension depending on their ability to maintain the EETs levels in plasma and tissues[29]. Recently, one of the research groups has demonstrated the efficacy of TPPU against NSAIDs-induced gastric ulcer via sEH inhibition pathway. TPPU is proven to increase the levels of anti-inflammatory EETs[12]. Herein, for in vivo antiulcer study, we considered TPPU as a reference plant standard following sEH inhibition pathway to compare with NSME.
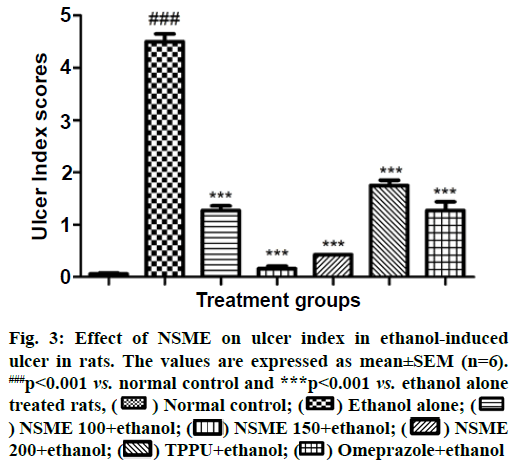
The in vivo evaluation of effect of NSME on ethanol- induced ulcer in rat suggests that it has a protective action against ethanol-induced ulcer in rats. From macroscopic and microscopic observation of gastric tissues it is observed that ethanol alone treated animals received highest ulcer scores and mucosal damage in the gastric mucosa which confirms the ethanol-induced lesions. Excess intake of ethanol is reported to cause severe gastric mucosal lesions via neutrophil infiltration and serve as major incentive of stomach ulcer in human beings. Ethanol-induced gastric ulcers are associated with oxygen-free radical release[30]. On the other hand administration of NSME to the ethanol-treated animals well protected the animals from ethanol-induced gastric lessons. The ulcer index score and microscopic photographs of gastric tissue histopathology are shown in fig. 3 and fig. 4, respectively.
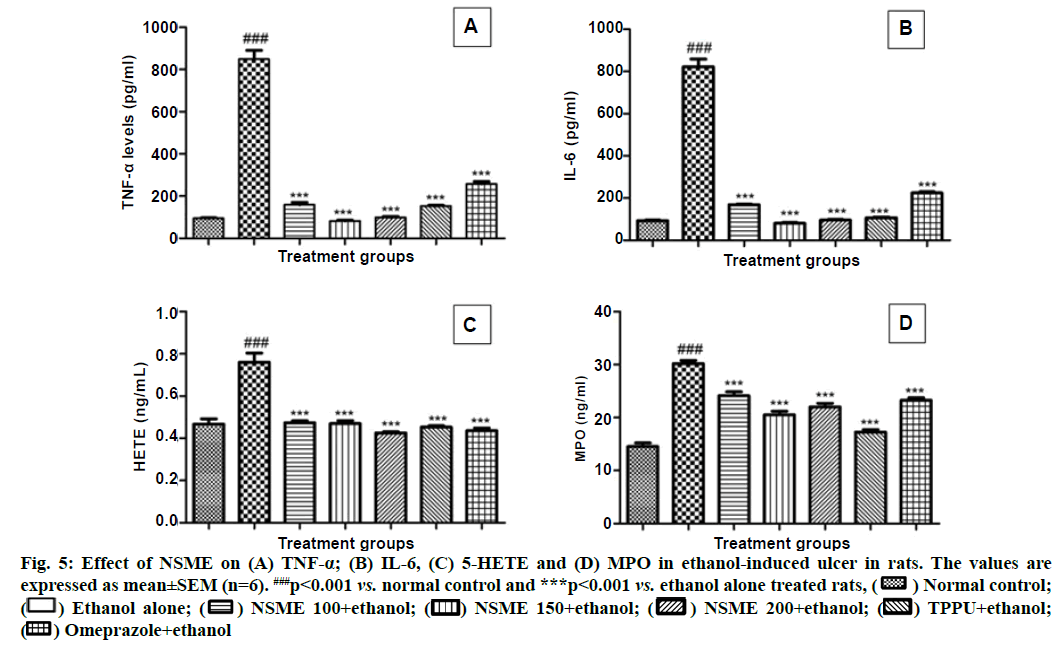
The production of inflammatory mediators is also a key aspect of the lesions mechanisms. TNF-α and IL-6 are the pro-inflammatory cytokines, which are released due to the activation of innate immune system, triggered by ethanol ingestion[31]. Some of the previous researchers have confirmed the increased levels of these markers in gastric tissue induced by ethanol[32]. The present study reports well and support the above findings. The drastically increased levels of TNF-α and IL-6 were observed in ethanol alone treated animals when compared with normal control animals. The study demonstrated the reduced levels of these two cytokines in NSME treated animals compared to the ethanol alone treated group. The medium and higher dosage of NSME showed nearly equal levels to TPPU and normal control proving their potency equal to TPPU. The detailed results of TNF-α and IL-6 estimations are represented in fig. 5A and fig. 5B.
After cytokines, release of leukocytes was estimated by release of 5-HETE. Herein, the increase in levels of 5-HETE was observed in ethanol alone administered group and rest all the groups showed nearly equal amounts of 5-HETE. This report indicates that treatment with NSME restricted ethanol-induced elevation of 5-HETE levels in the ethanol-treated animals (fig. 5C).
Neutrophil infiltration is another important factor on which the inflammation is dependent. The process aids accumulation as well as proclamation of tissue- disrupting components in several tissues along with gastric mucosal lesions[33], which further aids serious gastric mucosal lesions. Aggregation of neutrophil infiltration in those lesions is evaluated by levels of MPO. The reduced levels of MPO indicate the exhibition of anti-inflammatory property. In the present study, the increased levels of MPO in ethanol alone treated animals showed the inflammation caused by ethanol administration. Whereas, the decrease in MPO levels (p<0.01) in NSME treated groups indicated the reduced inflammation (fig. 5D).
The plant extract which has potency in treating stomach ulcers were screened to have chemical constituents such as flavonoids, alkaloids, proteins, free amino acids, tannins, flavonoids and in accordance our NSME is found to show all those potential chemical constituents. HPTLC chromatograms presented the linolenic acid existence in NSME.
Acknowledgements:
Authors would like to thank Professor Bruce D. Hammock and Christophe Morisseau, Department of Entomology, University of California for gifting TPPU and helping out with in vitro screening of extracts for sEH inhibition.
Conflict of interests:
The authors declared no conflict of interest.
References
- Al Batran R, Al-Bayaty F, Jamil Al-Obaidi MM, Abdualkader AM, Hadi HA, Ali HM, et al. In vivo antioxidant and antiulcer activity of Parkia speciosa ethanolic leaf extract against ethanol-induced gastric ulcer in rats. PLoS One 2013;8(5):e64751.
[Crossref] [Google Scholar] [PubMed]
- Franke A, Teyssen S, Singer MV. Alcohol-related diseases of the esophagus and stomach. Dig Dis 2005;23(3):204-13.
[Crossref] [Google Scholar] [PubMed]
- Hernández-Muñoz R, Montiel-Ruíz C, Vázquez-Martínez O. Gastric mucosal cell proliferation in ethanol-induced chronic mucosal injury is related to oxidative stress and lipid peroxidation in rats. Lab Invest 2000;80(8):1161-9.
[Crossref] [Google Scholar] [PubMed]
- Szabo G, Mandrekar P, Dolganiuc A, Catalano D, Kodys K. Reduced alloreactive T?cell activation after alcohol intake is due to impaired monocyte accessory cell function and correlates with elevated IL?10, IL?13, and decreased IFN-γ levels. Alcohol Clin Exp Res 2001;25(12):1766-72.
[Crossref] [Google Scholar] [PubMed]
- MacAllister CG. A review of medical treatment for peptic ulcer disease. Equine Vet J 1999;31(S29):45-9.
[Crossref] [Google Scholar] [PubMed]
- Graham DY, Fischbach LA. Empiric therapies for Helicobacter pylori infections. Can Med Assoc J 2011;183(9):E506-8.
[Crossref] [Google Scholar] [PubMed]
- Conklin KA. Dietary antioxidants during cancer chemotherapy: Impact on chemotherapeutic effectiveness and development of side effects. Nutr Cancer 2000;37(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci USA 2005;102(28):9772-7.
[Crossref] [Google Scholar] [PubMed]
- Harris TR, Bettaieb A, Kodani S, Dong H, Myers R, Chiamvimonvat N, et al. Inhibition of soluble epoxide hydrolase attenuates hepatic fibrosis and endoplasmic reticulum stress induced by carbon tetrachloride in mice. Toxicol Appl Pharmacol 2015;286(2):102-11.
[Crossref] [Google Scholar] [PubMed]
- Bianco RA, Agassandian K, Cassell MD, Spector AA, Sigmund CD. Characterization of transgenic mice with neuron-specific expression of soluble epoxide hydrolase. Brain Res 2009;1291:60-72.
[Crossref] [Google Scholar] [PubMed]
- Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res 2009;50:S52-6.
[Crossref] [Google Scholar] [PubMed]
- Goswami SK, Wan D, Yang J, Da Silva CA, Morisseau C, Kodani SD, et al. Anti-ulcer efficacy of soluble epoxide hydrolase inhibitor TPPU on diclofenac-induced intestinal ulcers. J Pharmacol Exp Ther 2016;357(3):529-36.
[Crossref] [Google Scholar] [PubMed]
- Kim JH, Morgan AM, Tai BH, Van DT, Cuong NM, Kim YH. Inhibition of soluble epoxide hydrolase activity by compounds isolated from the aerial parts of Glycosmis stenocarpa. J Enzyme Inhib Med Chem 2016;31(4):640-4.
[Crossref] [Google Scholar] [PubMed]
- Xuan Duy L, Le Ba V, Gao D, Hoang VD, Quoc Toan T, Yang SY, et al. Soluble epoxide hydrolase inhibitors from Docynia indica (Wall.) Decne. Nat Prod Res 2021;35(23):5403-8.
[Crossref] [Google Scholar] [PubMed]
- Kitamura S, Morisseau C, Inceoglu B, Kamita SG, De Nicola GR, Nyegue M, et al. Potent natural soluble epoxide hydrolase inhibitors from Pentadiplandra brazzeana Baillon: Synthesis, quantification and measurement of biological activities in vitro and in vivo. PLoS One 2015;10(2):e0117438.
[Crossref] [Google Scholar] [PubMed]
- Thao NP, Kim JH, Luyen BT, Dat NT, Kim YH. In silico investigation of cycloartane triterpene derivatives from Cimicifuga dahurica (Turcz.) Maxim. roots for the development of potent soluble epoxide hydrolase inhibitors. Int J Biol Macromol 2017;98:526-34.
- Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddique NA, et al. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac J Trop Biomed 2013;3(5):337-52.
[Crossref] [Google Scholar] [PubMed]
- Assayed ME. Radioprotective effects of black seed (Nigella sativa) oil against hemopoietic damage and immunosuppression in gamma-irradiated rats. Immunopharmacol Immunotoxicol 2010;32(2):284-96.
[Crossref] [Google Scholar] [PubMed]
- Boskabady MH, Mohsenpoor N, Takaloo L. Antiasthmatic effect of Nigella sativa in airways of asthmatic patients. Phytomedicine 2010;17(10):707-13.
[Crossref] [Google Scholar] [PubMed]
- Abel-Salam BK. Immunomodulatory effects of black seeds and garlic on alloxan-induced diabetes in albino rat. Allergol Immunopathol 2012;40(6):336-40.
[Crossref] [Google Scholar] [PubMed]
- Trease GE, Evans WC. Textbook of Pharmacognosy. 13th ed. London, UK: Balliese, Tindall and Co. Publishers; 1989.
- Doss A. Preliminary phytochemical screening of some Indian medicinal plants. Anc Sci Life 2009;29(2):12-6.
- Jones PD, Wolf NM, Morisseau C, Whetstone P, Hock B, Hammock BD. Fluorescent substrates for soluble epoxide hydrolase and application to inhibition studies. Anal Biochem 2005;343(1):66-75.
[Crossref] [Google Scholar] [PubMed]
- Bhattacharya S, Chaudhuri SR, Chattopadhyay S, Bandyopadhyay SK. Healing properties of some Indian medicinal plants against indomethacin-induced gastric ulceration of rats. J Clin Biochem Nutr 2007;41(2):106-14.
[Crossref] [Google Scholar] [PubMed]
- Jain NK, Kulkarni SK, Singh A. Modulation of NSAID-induced antinociceptive and anti-inflammatory effects by α2-adrenoceptor agonists with gastroprotective effects. Life Sci 2002;70(24):2857-69.
[Crossref] [Google Scholar] [PubMed]
- Islam MH, Ahmad IZ, Salman MT. Antibacterial activity of Nigella sativa seed in various germination phases on clinical bacterial strains isolated from human patients. E3 J Biotechnol Pharm Res 2013;4(1):8-13.
- Zribi I, Omezzine F, Haouala R. Variation in phytochemical constituents and allelopathic potential of Nigella sativa with developmental stages. S Afr J Bot 2014;94:255-62.
- Yessuf AM. Phytochemical extraction and screening of bio active compounds from black cumin (Nigella sativa) seeds extract. Am J Life Sci 2015;3(5):358-64.
- Ghosh S, Chiang PC, Wahlstrom JL, Fujiwara H, Selbo JG, Roberds SL. Oral delivery of 1, 3-dicyclohexylurea nanosuspension enhances exposure and lowers blood pressure in hypertensive rats. Basic Clin Pharmacol Toxicol 2008;102(5):453-8.
[Crossref] [Google Scholar] [PubMed]
- Szabo S, Vattay P, Scarbrough E, Folkman J. Role of vascular factors, including angiogenesis, in the mechanisms of action of sucralfate. Am J Med 1991;91(2):S158-60.
[Crossref] [Google Scholar] [PubMed]
- Salga MS, Ali HM, Abdulla MA, Abdelwahab SI. Gastroprotective activity and mechanism of novel dichlorido-zinc (II)-4-(2-(5-methoxybenzylideneamino) ethyl) piperazin-1-iumphenolate complex on ethanol-induced gastric ulceration. Chem Biol Interact 2012;195(2):144-53.
[Crossref] [Google Scholar] [PubMed]
- Mei X, Xu D, Xu S, Zheng Y, Xu S. Novel role of Zn (II)–curcumin in enhancing cell proliferation and adjusting proinflammatory cytokine-mediated oxidative damage of ethanol-induced acute gastric ulcers. Chem Biol Interact 2012;197(1):31-9.
[Crossref] [Google Scholar] [PubMed]
- Abdel-Salam OM, Czimmer J, Debreceni A, Szolcsányi J, Mózsik G. Gastric mucosal integrity: Gastric mucosal blood flow and microcirculation. An overview. J Physiol Paris 2001;95(1-6):105-27.
[Crossref] [Google Scholar] [PubMed]