- *Corresponding Author:
- T. Sumathi
Department of Medical Biochemistry, Dr. A. L. M. Post Graduate Institute of Basic Medical Sciences, University of Madras, Taramani Campus, Chennai-600 113, India
E-mail sumsthangarajan@gmail.com
| Date of Submission | 19 January 2010 |
| Date of Revision | 11 July 2011 |
| Date of Acceptance | 20 July 2011 |
| Indian J Pharm Sci 2011 73 (4): 409-415 |
Abstract
In the present study, we investigated the protective effect of bacoside-A the active principle isolated from the plant Bacopa monniera against oxidative damage induced by morphine in rat brain. Morphine intoxicated rats received 10- 160 mg/kg b.w. of morphine hydrochloride intraperitoneally for 21 days. Bacoside-A pretreated rats were administered with bacoside-A (10 mg/kg b.w/day) orally, 2 h before the injection of morphine for 21 days. Pretreatment with bacoside-A has shown to possess a significant protective role against morphine induced brain oxidative damage in the antioxidant status (total reduced glutathione, superoxide dismutase, catalase, glutathione peroxidase and lipid peroxidation) and membrane bound ATP-ases(Na+/K+ ATPase. Ca2+ and Mg2+ ATPases) activities in rat. The results of the present study indicate that bacoside-A protects the brain from oxidative stress induced by morphine.
Keywords
Bacoside-A, brain damage, morphine, membrane-bound ATPases, oxidative stress
The opioid analgesics commonly exemplified by morphine, represent the best option for the treatment of severe pain and for the management of chronic pain states. Morphine abuse is a worldwide health problem that provoked many investigations to study its mechanism of action. Focal glomerulosclerosis [1], hepatotoxicity with a reduced glutathione superoxide dismutase, catalase, glutathione peroxidase levels [2], histamine liberation from mast cells [3], neurotoxicity with spongiform leucoencephalopathy [4], neuroteratogenicity [5] and oxidative damage of DNA [6] are some of the pathological features in chronic morphine toxicity generated by local rearrangement of anti-oxidant homeostasis.
Recently, the development of a systemic oxidative stress was noted as a result of opioid–stimulated free radicals production. An increased lipid peroxidation was found in vitro and in vivo in heroin consumption [7,8]. Chronic morphine abuse resulted in the decreased level of antioxidants and antioxidases status [9,10]. Measurement of the levels of free radical or ROS production, metabolites of lipid peroxidation such as malondialdehyde and antioxidant capacity may give information about an antioxidant homeostasis under morphine stimulation and morphine-induced toxicity.
Morphine enhances neuronal Na+/K+ ATPase activity, enzyme responsible for ionic exchange across the cellular membrane [11]. This enzyme of membrane bound may be used as a metabolic probe to study the effects of morphine in rat brain [12], due to mechanism involved in this effect are not well known. Previous studies have shown that significant depletion of reduce glutathione (GSH) in peripheral organs following acute systemic or central administration of opioids [13]. ROS or free radicals may be involved in the morphine and opiate abuse [2,14]. It is well recognized that the use of morphine is associated with degeneration in brain cells [2]. Hence, administration of a compound that has membrane-stabilizing as well as antioxidant properties would therefore be expected to prevent morphine induced oxidative stress in rat brain.
Bacoside-A is the dammarene type triterpenoid saponin isolated from the plant Bacopa monniera [15], which is held in high repute as a potent nerve tonic [16]. Bacopa monniera Linn. is used in the indigenous systems of medicine for the treatment of various nervous system ailments such as insomnia, anxiety, epilepsy, hysteria etc [17]. The plant has been shown as a potent free radical scavenger and antioxidant [18]. Besides it also exhibits vasodilatory [19], calcium antagonistic [20], muscle relaxant [21], mast cell stabilizing [22], and antiulcer [23] properties. Recently, protective effect of bacoside-A against chronic exposure to cigarette smoke has been reported by Anbarasi et al. [24] Thus, the aim of the present study was designed to explore the protective effect of bacoside-A on morphine induced oxidative stress in terms of antioxidant status and membrane bound ATPases in rat brain.
Earlier we reported, several papers with respect to protective effect of Bacopa monniera extract against morphine toxicity, but this is the first report with respect to the bioactive compound level. Hence the present study is undertaken to evaluate the effect of bacoside-A against morphine induced oxidative stress in rat brain.
Materials and Methods
Morphine hydrochloride used in the present study was obtained from MOTI and Company, Lakshmipuram, Royapettah, Chennai, Tamil Nadu, India. All other reagents used were of analytical grade and obtained from Himedia, India.
Isolation of bacoside-A
The plant Bacopa monniera was collected in and around Chennai, India and authenticated by Dr. P. Brindha, Central Research Institute (Siddha), Chennai, India. The dammarene type triterpenoid saponin bacoside-A was isolated from the plant by the procedure carried out by Deepak et al. [25]. The activity-guided isolation of Bacoside A was carried out using the brine shrimp lethality assay to direct the fractionation of a methanolic proportion of methanol. The fractions eluted with chloroform:methanol 85:15 were combined and concentrated under vacuum (7 g). This fraction was subjected to flash chromatography on silica gel (100–200 mesh) and the fractions eluted with chloroform:methanol 85:15 were combined, concentrated under vacuum and crystallised from 70% methanol in water to obtain bacoside-A (2.5 g). Aqueous suspension of bacoside-A was given orally to the experimental animals at a dosage of 10 mg/kg b.w/day for 21 days.
Animals
Adult male albino rats of Wistar strain (150-200 g) were used for the present study. The rats were provided with standard pelleted rat feed and water ad libitum. They were acclimatized to the laboratory conditions and maintained less than 12 h light/dark cycle at 25±2º. The experiments were carried out in accordance with the guidelines provided by the Institutional Animal Ethics Committee.
Experimental design
The animals were divided into four groups of six animals each. Group I–control, Group II - rats received morphine hydrochloride (10-160 mg/kg b.w, i.p) [26], for 21 days, Group III - rats received bacoside-A (10 mg/kg b.w/day, p.o) [24], 2 h before the administration of morphine hydrochloride for 21 days, Group IV - rats received bacoside-A alone orally for 21 days. At the end of the experimental period the animals were sacrificed by cervical decapitation. The brain tissues were collected and homogenized with motor driven Teflon coated homogenizer in ice-cold 0.1M Tris-HCl buffer pH 7.4 to get 10% homogenate and used for biochemical studies.
HPLC-finger print analysis of Bacopa monniera extract
HPLC-Finger Print analysis of Bacopa monniera was performed using the conditions given in Table 1.
| Parameters | Condition used | ||
|---|---|---|---|
| HPLC system | Shimadzu HT2010 chromatographic system with in combination with Class LC 10A software and UV detection | ||
| Column | RP C-18 Luna phenomenex (250x4.6 mm) | ||
| Column oven temperature | 257° | ||
| Mobile phase | A-0.25% Orthophosphoric acid in water B-Acetonitrile |
||
| Flow rate | 1.5 ml | ||
| Injection volume | 25.0 μl | ||
| Gradient | Time | Concentration of A | Concentration of B |
| 0 | 75 | 25 | |
| 25 | 60 | 40 | |
| 35 | 40 | 60 | |
| 38 | 75 | 25 | |
| 45 | 75 | 25 | |
| Detection wavelength | 205 nm | ||
| Run time | 45 min | ||
| Sample preparation | Weigh accurately 500 mg Extract to a 100 ml volumetric flask dissolve in 50 ml methanol, sonicate for 10-15 min. Cool then make up to 100 ml with methanol. Filter through 0.45 microns membrane filter paper | ||
Table 1: Parameters For Hplc Analysis
Biochemical analysis
The activity of SOD was determined by the method of Marklund and Marklund [27]. Activity of catalase was assayed by the method of Sinha [28]. Glutathione peroxidase was assayed by the method of Rotruck et al. [29]. The level of reduced glutathione was measured by the method of Moron et al [30]. Lipid peroxidation was determined by the method of Hogberg et al. [31]. Ferrous sulphate and ascorbate induced lipid peroxidation was carried out by the method of Devasagayam and Tarachand [32]. The activity of Na+/K+-ATPase was assayed by the method of Bonting [33]. Ca2+-ATPase activity was assayed according to the method of Hjerten and Pan [34]. The activity of Mg2+-ATPase was assayed by the method of Ohnishi et al [35]. Inorganic phosphorous was estimated by the method of Fiske and Subbarow [36]. The protein carbonyl content was quantified by the method of Levine et al. [37]. Protein was estimated by Lowry et al. [38] method.
Statistical analysis
Results were expressed as mean±SD (n=6), analysed by oneway ANOVA, followed by Tukey’s post-hoc test by SPSS version 7.0. Student’s t-test was used for the statistical evaluation of the data obtained. Statistical comparisons were made between Group II Vs Group I and Group III Vs Group II. A value of P<0.001 was considered to be statistically significant.
Results
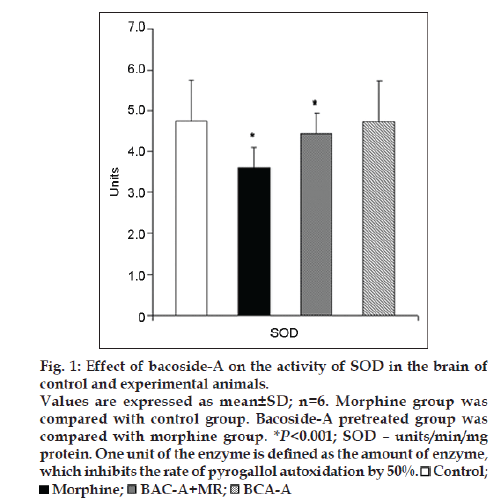
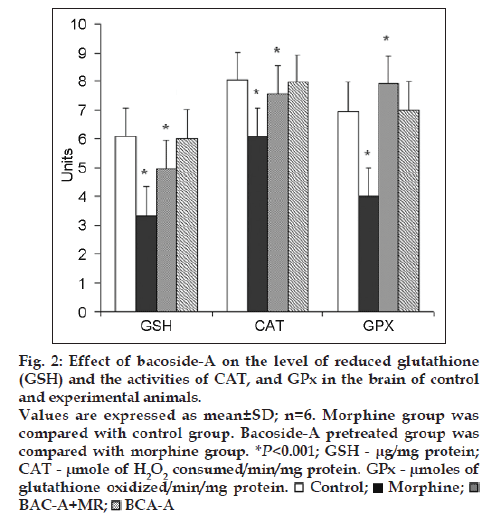
Figs. 1 and 2 represent the effect of bacoside-A on the activities of SOD, CAT, GPx enzymes and the level of glutathione (GSH) in the brain of control and experimental animals. The activities of all these enzymes along with GSH level (P<0.001) were found to be significantly decreased viz; SOD (P<0.001), CAT (P<0.001), GPx (P<0.001) in morphine-induced rats when compared to Group I rats. Whereas pretreatment with bacoside-A significantly alters the changes caused by morphine and found to increase the activities of all these antioxidant enzymes to near normal level when compared to Group II animals. Bacoside-A alone treated rats (Group IV) had no effect on the activities of these enzymes when compared with control rats.
Figure 1: Effect of bacoside-A on the activity of SOD in the brain of
control and experimental animals.
Values are expressed as mean±SD; n=6. Morphine group was
compared with control group. Bacoside-A pretreated group was
compared with morphine group. *P<0.001; SOD – units/min/mg
protein. One unit of the enzyme is defined as the amount of enzyme,
which inhibits the rate of pyrogallol autoxidation by 50%. 
Figure 2: Effect of bacoside-A on the level of reduced glutathione
(GSH) and the activities of CAT, and GPx in the brain of control
and experimental animals.
Values are expressed as mean±SD; n=6. Morphine group was
compared with control group. Bacoside-A pretreated group was
compared with morphine group. *P<0.001; GSH - µg/mg protein;
CAT - µmole of H2O2 consumed/min/mg protein. GPx - µmoles of
glutathione oxidized/min/mg protein. 
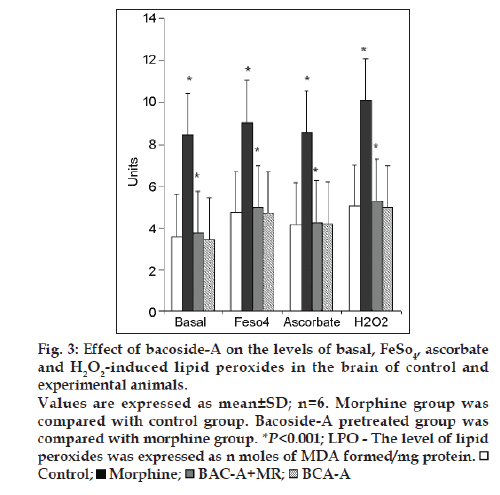
Fig. 3 illustrates the effect of bacoside-A on the levels of Basal, FeSO4, Ascorbate and H2O2 induced lipid peroxides in the brain of control and experimental rats. Group II morphine induced rats registered a significant (P<0.001) increase in the levels of lipid peroxides when compared to group I control rats. A significant decrease (P<0.001) in the levels of lipid peroxide was noted in group-III rats when compared to Group-II rats. Bacoside-A alone treated rats (group IV) did not show any changes in the level of lipid peroxides when compared to control rats.
Figure 3: Effect of bacoside-A on the levels of basal, FeSO4, ascorbate
and H2O2-induced lipid peroxides in the brain of control and
experimental animals.
Values are expressed as mean±SD; n=6. Morphine group was
compared with control group. Bacoside-A pretreated group was
compared with morphine group. *P<0.001; LPO - The level of lipid
peroxides was expressed as n moles of MDA formed/mg protein. 
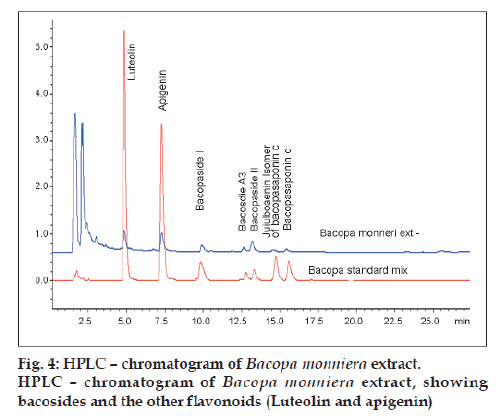
Fig. 4 shows the overlaid chromatogram of Bacopa monniera extract by HPLC along with bacosides and other flavonoids (luteolin and apigenin).
Table 2 shows the effect of bacoside-A on the level of protein carbonyl content in the brain of control and experimental rats. The level of protein carbonyl content was significantly (P<0.001) increased in morphine-induced group II rats when compared to control animals. Whereas the level of protein carbonyl content was maintained at near normal in bacoside-A pretreated rats when compared to group II rats. Group- IV rats showed no significant changes in the level of protein carbonyl when compared to control rats.
| Groups | Protein carbonyl content (n mole/mg protein) |
|---|---|
| Control | 3.00 ± 0.15 |
| Morphine | 4.06 ± 0.18* |
| Bacoside-A + MR | 2.09 ± 0.14* |
| Bacoside-A | 2.94 ± 0.22 |
Table 2: Effect of bacoside-a on the level Of protein carbonyl content in the brain of Control and experimental animals
Table 3 represents the effect of bacoside-A on the activities of Na+/K+-ATPase, Ca2+-ATPase and Mg2+-ATPase in the brain of control and experimental rats. A significant decrease in the activities of all the membrane bound enzymes viz., Na+/k+-ATPase (P<0.001), Ca2+-ATPase (P<0.001) and Mg2+-ATPase (P<0.001), was noted in morphineinduced rats when compared to control rats. Pretreatment with bacoside-A (group-III) prevented the morphine-induced changes in the activities of all these ATP-ases when compared to group II rats. Bacoside-A alone treated rats resembled the control rats.
| Groups | Na+/K+-ATPase (mmol of phosphorous liberated/ min/mg protein) | Ca2+-ATPase (mmol of phosphorous liberated/ min/mg protein) | Mg2+-ATPase (mmol of phosphorous liberated/ min/mg protein) |
|---|---|---|---|
| Control | 0.58 ± 0.05 | 0.56 ± 0.01 | 0.67 ± 0.01 |
| Morphine | 0.25 ± 0.04* | 0.32 ± 0.02* | 0.31 ± 0.02* |
| Bacoside-A + MR | 0.54 ± 0.03* | 0.53 ± 0.01* | 0.64 ± 0.03* |
| Bacoside-A | 0.56 ± 0.02 | 0.55 ± 0.02 | 0.66 ± 0.01 |
Table 3: Effect of bacoside-a on the activities of Na+/k+-atpase, Ca2+-atpase and Mg2+-atpase in the Brain of control and experimental animals
Discussion
Brain is highly susceptible to peroxidative damage, since it contains a relatively high degree of unsaturated fatty acids in relation to its level of antioxidants and consumes more oxygen [39]. Morphine is able to induce oxidative cell injury in neuronal cells and neurodegeneration [40]. It has been reported that single dose of morphine administration increased dopamine and xanthine oxidative metabolism with a consequent increase in ROS production [2]. In addition, morphine could also be metabolized into free radicals [3] and the over production of ROS could lead to oxidative damage.
We have found that the level of GSH in the brain were lower in morphine administered rats than in the control animals. This decrease in the GSH level in the brain suggests that morphine unprotected the brain from oxidative stress. These results could be in accordance with the report of Goudas et al. [41] who observed acute decreases of GSH levels in cerebrospinal fluid samples taken from patients after intracerebroventricular doses of morphine for intractable cancer pain. Such doses of morphine may, by depleting the GSH, render the central nervous system vulnerable to damage from oxidative stress. It has also been reported that the oxidative stress is induced by NO production synthesized in select neuron populations during the opiate syndrome [42], which alters GSH metabolism in the brain.
It has been observed from our study that the protein carbonyl contents and lipid peroxide levels in the brain were significantly higher with the concomitant decrease in antioxidant status in morphine administered animals than in the control.
Furthermore, it has been reported that oxidative damage in the brain is often observed in association with morphine, which has pro-oxidative properties [14]. Morphine mediated oxidative damage in the brain is compounded by the fact that brain ROS distribution is non-uniform, being particularly high unsaturated fatty acids contents in areas sensitive to neurodegeneration [2,40]. It has been reported that the supplementation of exogenous antioxidants were able to abate the oxidative damages of DNA, protein and lipid, caused by morphine [2].
It has been reported that after administration with heroin, the mice were not only showed, decrease in the total antioxidant status in serum and also there were significant decrease in the antioxidant enzymes such as SOD, CAT, GSH-Px in brain and also exhibited oxidative damages to DNA, protein and lipid [40].
In our present study, we observed that there was a significant decrease in Na+/k+-ATPases, Ca2+ATPase and Mg2+ ATPase activities in morphine induced rat brain. Masocha et al. [43] indicated from their findings that the activation of Na+/k+-ATPases play a vital role in the analgesic effect of morphine and the Na+/k+-ATPases activity were greatly decreased in adult morpine induced rats. These results agree with Brase et al. [44], who suggest that morphine induction increases the intracellular sodium in brain and decreases Na+, k+-ATPases activity, indicating that the development of morphine tolerance may be accompanied by changes in the disposition of sodium. These results agree with Pillai et al. [45], stating that, morphine produced a significant decrease in Ca2+, Mg2+ ATPase activity in synaptosomal fractions, as consequence of containing a high density of opiate receptors (morphine's μ-opioid receptor).
Several authors have shown that Bacopa monniera was able to prevent lipid peroxidation in vitro and in vivo [46,47,18], and quench superoxide, hydroxyl and nitric oxide radicals effectively in vitro [48,49]. In our study, a decrease in lipid peroxide level was observed in group-III, bacoside-A pretreated rats when compared to group-II rats, thus establishing its antilipid peroxidative property. Earlier it has been reported by many authors that Bacopa monniera has a potential to prevent lipid peroxidation in vitro and in vivo. This property of the Bacopa monniera extract could have been possible due to the presence of one of its major active component namely bacoside-A.
Earlier we reported that Bacopa monniera prevented morphine induced rat brain mitochondrial damage [50] that might have allowed the availability of ATP synthesis. This energy promoting action may be responsible for the improved mitochondrial function and maintenance of ATPases in our study, upon administration of bacoside-A. The results of the present study clearly imply that antioxidant and antilipid peroxidative properties of bacoside-A could be attributed to the improved activity of ATPases and maintenance of Na+/k+, Ca2+, Mg2+ ionic equilibrium in morphine induced oxidative stress in rat brain. By eliciting a facilitatory effect, bacoside-A offered protection to the brain by stabilizing the membrane and thereby retaining the structural and functional integrity of the membrane. Further studies are needed to study the possible mechanism of action of bacoside-A on morphine-induced oxidative stress in rat brain, in terms of antioxidants and membrane bound enzymes.
References
- Patel J, Manappan N, Bhat R. Role of oxidative stress and hemeoxygenase activity in morphine induced glomerular epithelial cell growth. Am J Physiol Renal Physiol 2003;285:861-9.
- Zhang YT, Zheng QS, Pan J, Zheng RL. Oxidative damage of biomolecules in mouse liver induced by morphine and protected by antioxidants. Basic ClinPharmacolToxicol 2004;95:53-8.
- Di Bello MG, Masini E, Ioannides C, FomusiNdisang J, Raspanti S, Banisacchi T, et al. Histamine release from mast cells induced by the metabolic activation of drugs of abuse into free radicals.Inflam Res 1998;47:122-30.
- Kriegstein AR, Shungu DC, Millar WS, Armitage BA, Brust JC, Chillrud S, et al. Leukoencephalopathy and raised brain lactate from heroin vapor inhalation (?chasing the dragon?). Neurology 1999;53:1765-773.
- Slotkin TA, Seidler FJ, Yania J. Heroin neuroteratogenicity: Targeting adenylyl cyclase as an underlying mechanism. Brain Res Dev 2001;132:69-79.
- Xu B, Wang Z, Li G, Li B, Lin H, Zheng R, et al. Heroin administered mice involved in oxidative stress and exogenous antioxidant-alleviated withdrawal syndrome. Basic ClinPharmacolToxicol 2006;99:153-61.
- Konstantinopolskii MA, Pirozhkov SV, Soloveva AG, Panchenko LF, Barkov NK. The withdrawal syndrome and lipid peroxidation during the chronic administration of narcotic analgesics to rats. EkspKlin Farmakol 1992;55:21-4.
- Panchenko LF, Pirozhkov SV, Nadezhdin VIU, Usmanova NN. Lipid peroxidation, peroxyl-radical-scavering system of plasma and liver and heart pathology in adolescence heroin users. Vopr Med Khim 1999;45:501-6.
- Nazral Islam SK, Hossain KJ, Ahsan M. Serum vitamin E, C and A status of drug addicts undergoing detoxification: Influence of drug habit, sexual practice and Lifestyle factors. E J Clin Nut 2001;55:1022-7.
- Zhou JF, Yan XF, Ruan ZR, Peng FY, Cai D, Yuan H, et al. Heroin abuse and nitric oxide, oxidation, peroxidation, lipid peroxidation. Biomed Environ Sci 2000;13:131-9.
- Hernandez RJ. A Serotonin agonist ? antagonist reversible effect on Na+, K+-ATPase activity in the developing rat brain. DevNeurosci 1982;5:326-31.
- Wan-Kan O, Hosein EA. Synaptosomal Na+, K+-ATPase as a membrane probe in studying the in vivo action of morphine. Can J Biochem 1981;59:687-92.
- Goudas LC, Carr DB, Maszczynska I, Marchand JE, Wurm WH, Greenblatt DJ, et al. Differential effect of central versus parenteral administration of morphine sulfate on regional concentrations of reduced glutathione in rat brain. Pharmacology 1997;54:92-7.
- Guzman DC, Vazquez IE, Brizuela NO, Alvarez RG, Mejia GB, Garcia EH, et al. Assessment of oxidative damage induced by acute doses of morphine sulfate in postnatal and adult rat brain. Neurochem Res 2006;31:549-54.
- Garai S, Mahato SB, Ohtani K, Yamasaki K. Dammarane type triterpenoidsaponins from Bacopamonniera. Phytochemistry 1996;42:815-20.
- Chopra RN, Nayar SL, Chopra IC. Glossary of Indian medicinal plants. New Delhi: CSIR; 1956. p. 32.
- Nadkarni KM. Indian materiamedica. Bombay: Popular PrakashanPvt Ltd.; 1976. p. 624-5.
- Tripathi YB, Chaurasia S, Tripathi E, Upadyay A, Dubey GP. BacopamonnieraLinn. As an antioxidant: Mechanism of action. Indian J ExpBiol 1996;34:523-6.
- Channa S, Dar A, Yaqoob M, Anjum S, Sultani Z, Rahman A. Bronchovasodilatory activity of fractions of pure constituents isolated from Bacopamonniera. J Ethnopharmacol 2003;86:27-35.
- Dar A, Channa S. Calcium antagonistic activity of Bacopamonniera on vascular and intestinal smooth muscles of rabbit and guinea pig. J Ethnopharmacol 1999;66:167-74.
- Dar A, Channa S. Relaxant effect of ethanolic extract of Bacopamonnieraon Trachea, pulmonary artery and aorta from rabbit andguinea pig. Phytother Res 1997;11:323-5.
- Samiulla DS, Prashanth D, Amit A. Mast cell stabilizing activity of Bacopamonnieri. Fitoterapia 2001;72:284-5.
- Sairam K, Rao CV, Babu MD, Goel RK. Prophylactic and curative effects of Bacopamonniera in gastric ulcer models. Phytomedicine 2001;8:423-30.
- Anbarasi K, Vani G, Balakrishna K, Shyamala Devi CS. Creatine kinase isoenzyme patterns upon chronic exposure to cigarette smoke: Protective effect of Bacoside A. Vascular Pharmacol 2005;42:57-61.
- Deepak M, Sangli GK, Arun PC, Amit A. Quantitative determination of the major saponin mixture Bacoside-A on Bacopamonnieri by HPLC. Phytochem Anal 2005;16:24-9.
- Lurie E, Soloviova AG, Alyabieva TN, Kaplun A, Panchenko LF, Shvets V. Effect of novel aromatic derivative of GABA on lipid peroxidation in chronically morphinized rats. BiochemMolBiolInt 1995;36:13-9.
- Marklund S, Marklund G. Involvement of Superoxide anion radical in the auto oxidation of pyragallol and a convenient assay for superoxide dismutase. E J Biochem 1974;47:469-74.
- Sinha AK. Colorimetric assay of catalase. Anal Biochem 1972;47:389-94.
- Rotruck JT, Pope AL, Ganther HE. Selenium: Biochemical role as a component of glutathione peroxidase purification and assay. Science 1973;179:588-90.
- Moron K. Levels of glutathione, glutathione reductase and glutathione-S-transferrase activities in rat lung and liver. Biochem Biophys Acta 1979;582:67-8.
- Hogberg J, Larson RE, Kristoferson A, Orrenius S. NADPH-dependent reductasesolubilised from microsomes by peroxidation and its activity. Biochem Biophys Res Commun 1974;56:836-42.
- Devasagayam TP, Tarachand U. Decreased lipid peroxidation in rat kidney during gestation. Biochem Biophys Res Commun 1987;145:134-8.
- Bonting SL. Sodium-potassium activated adenosine triphosphatase and cation transport. In: Bittar EE, editor. Membrane and ion transport. London: Wiley?Interscience; 1970. p. 257-63.
- Hjerten S, Pan H. Purification and characterization of two forms of a low affinity Ca2+-ATPase from erythrocyte membranes. BiochemBiophysActa 1983;728:281-8.
- Ohnishi T, Suzuki T, Suzuki Y, Ozawa K. A comparative study of plasma membrane Mg2+-ATP ase activities in normal, regenerating and malignant cells. Biochim Biophys Acta 1982;684:67-74.
- Fiske CH, Subbarow Y. The colorimetric determination of phosphorous. J BiolChem 1925;66:375-400.
- Levine RL, Garland D, Oliver CN, Amici A, Climond I, Lenz AG, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 1990;186:464-87.
- Lowry OH, Rose brough NJ, Farr AL, Randall RJ. Protein measurement with the Folin-Phenol reagent. J BiolChem 1951;193:265-75.
- Halliwell B, Gutteridge JM. Oxygen radicals and the nervous system. Trends Neurosci 1985;8:22-6.
- Xu B, Wang Z, Li G, Li B, Lin H, Zheng R, et al. Heroin administered mice involved in oxidative stress and exogenous antioxidant alleviated withdrawal syndrome. Basic ClinPharmacolToxicol 2006;99:153-61.
- Goudas LC, Langlade A, Serrie A, Matson W, Milbury P, Thurel C,et al. Acute decreases in cerebrospinal fluid glutathione levels afterintracerebroventricular morphine for cancer pain.AnesthAnalg 1999;89:1209-15.
- Jhamandas JH, Harris KH, Petrov T, Jhamandas KH. Activation of nitric oxide-synthesizing neurons during precipitated morphine withdrawal. Neuro Rep 1996;7:2843-6.
- Masocha W, Horvath G, Agil A, Ocana M, Del Pozo E, Szikszay M, et al. Role of Na+, K+-ATPase in morphine ?induced antinociception. J PharmacolExpTher 2003;306:1122-8.
- Brase DA. Is intracellular sodium involved in the mechanism of tolerance to opioid drugs? Med Hypotheses 1990;32:164-7.
- Pillai NP, Ross DH. Effects of opiates on high-affinity Ca2+, Mg2+ATPases in brain membrane sub fractions. J Neurochem 1996;47:1642-6.
- Sumathy T, Subramanian S, Govindasamy S, Balakrishna K, Veluchamy G. Protective role of Bacopamonniera on morphine induced hepatotoxicity in rats. Phytother Res 2001;15:643-5.
- Rohini G, Sabitha KE, Devi CS. Bacopamonniera Linn. Extract modulate antioxidant and marker enzyme status in fibrosarcoma bearing rats. Indian J ExpBiol 2004;42:776-80.
- Pawar R, Gopalakrishnan C, Bhutani KK. Dammaranetriterpenesaponin from Bacopamonniera as the superoxide inhibitor in polymorphonuclear cells. Planta Med 2001;67:752-4.
- Russo A, Borrelli F, Izzo AA, Renis M, Vanella A. Free radical scavenging capacity and protective effect of Bacopamonniera on DNA damage.Phytother Res 2003b;17:870-5.
- Sumathy T, Govindasamy S, Balakrishna K, Veluchamy G. Protective role of Bacopamonniera on morphine-induced brain mitochondrial enzyme activity in rats. Fitoterapia 2002;73:381-5.