- *Corresponding Author:
- Jiannan Zhang
Department of Anesthesiology, Traditional Chinese Medicine Hospital of Wuxi, Wuxi, Jiangsu 214071, China
E-mail: zjn_wxzy@126.com
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “163-170” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The repair effect of baicalin on rat hippocampal neuronal cells after injury was explored in this study. According to the concentration gradient, glutamate which induced hippocampal neuronal cells damage was categorized into four groups; control, 62.5 μM, 125 μM and 250 μM. Baicalin was pretreated with 0, 1, 10, 20 and 40 μM concentrations for 1 h. The morphological changes of neuronal cells were detected by reactive oxygen species, and cell proliferation was detected by cell counting kit-8. The apoptosis of hippocampal neuronal cells was detected by flow cytometry. The degree of cell damage was determined by the amount of lactate dehydrogenase released in the extracellular system. The expression of related proteins was detected by Western blot. Real-time fluorescence-based quantitative polymerase chain reaction was used to detect messenger ribonucleic acid levels of related genes. With an increase of glutamate concentration, the survival rate of neuronal cells decreased gradually, and the apoptosis rate and cytotoxicity increased with the increase of glutamate concentration. Compared with the control group, the reactive oxygen species level of neuronal cells in baicalin-treated group was reduced, which was inversely proportional to the concentration of baicalin. As the concentration of baicalin increases, the survival rate of neuronal cells increases. Compared with the control group, the apoptosis rate of neuronal cells was reduced, and the levels of inflammation-related factors like interleukin-6, interleukin-1 beta and tumor necrosis factor alpha significantly decreased. The protein expressions of N-methyl D-aspartate receptor subtype 2B, phosphorylated-extracellular signal-regulated kinase and phosphorylated-cyclic adenosine 3’,5’-monophosphate response element-binding protein significantly increased after treatment with high concentration of baicalin. Baicalin can increase glutamate-induced neuronal cell injury, reduce the cytotoxicity, reduce the expression of inflammatory factors, and promote the reduction of apoptosis.
Keywords
Baicalin, hippocampal neuronal cells, glutamate, inflammatory factors, interleukin, cytotoxicity
Glutamate which is an acidic amino acid, is found in large quantities in cereal proteins and is abundant in the brains of animals[1]. Glutamate plays an important role in protein metabolism and is involved in many important chemical reactions in animals, plants and microorganisms. Glutamate is a major excitatory neurotransmitter that acts as a signaling molecule between nerve cells and is involved in most of the aspects of normal brain function, including cognition, learning, and memory[2,3]. Glutamate-induced cytotoxicity has been linked to neurodegenerative diseases such as Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, and other conditions, including spinal cord injury and multiple sclerosis[4-6].
As an important component of the limbic system, the hippocampus plays an important role in learning, recent memory, emotional response and regulation of visceral function[7]. Hippocampus is the main concentration of neural stem cells in the central nervous system. In the recent years, inflammation and occurrence of hippocampal neuronal damage have become the focus of research, and a large amount of inflammation can lead to the loss of a large number of hippocampal neurons[8-10]. Hippocampal neurons are the main cellular components of the hippocampus, and their main function is to participate in the regulation of recent memory, emotion and visceral function, which is one of the main concerns of Alzheimer’s disease, epilepsy and other diseases[11,12]. In this study, glutamate was used to treat hippocampal neuronal cells to induce cell damage and similarly baicalin was used to repair the damage. The morphology, proliferation, apoptosis, the extracellular Lactate Dehydrogenase (LDH) level, expression of a series of inflammatory factor genes were observed, the relationship between the damage and repair of neuronal cells and inflammatory factors by baicalin were explored.
Baicalin is the main component of Astragalus membranaceus has antiviral, anti-inflammatory, anti-apoptotic and antioxidant functions[13], and has a protective effect on nerves. It exerts neuroprotective effects through anti-inflammatory and anti-apoptotic pathways, thereby providing an effective protection against cerebral ischemia and other injuries. The effect of baicalin in in vitro models has also shown protective mechanism against Amyloid Beta (Aβ) peptide-induced neuronal damage[14,15]. It is known that baicalin can protect, repair the learning and memory impairment in mice, but its role in hippocampal neuronal after injury and its molecular mechanism are unclear.
Therefore, this study investigated the molecular mechanism of different doses of baicalin on glutamate-induced repair of hippocampal neuronal cell damage in rats from the aspects of hippocampal neuronal cell proliferation, apoptosis, LDH release, inflammatory cytokines and N-methyl D-aspartate Receptor subtype 2B (NR2B)/Extracellular Signal-Regulated Kinase (ERK)/Cyclic adenosine 3’,5’-monophosphate Response Element-Binding Protein (CREB) signaling pathway, to provide a theoretical basis for clinical treatment and drug development of neuroinflammation.
Materials and Methods
Cell culture:
The rat hippocampal neuronal cell line, Immortalized Rat Hippocampal Cells (H19-7) was stored at -80° in the laboratory. The culture based formula of H19-7 cells was cultured in 89 % Dulbecco’s Modified Eagle Medium (DMEM), 10 % fetal bovine serum and 1 % penicillin/streptomycin double anti-suspension. It was cultured in an incubator maintained at 37° containing 5 % Carbon dioxide (CO2). Under the inverted microscope, when the cells grow to 70 %~90 %, the medium in the bottle was completely absorbed the suspension was discarded. The original medium was rinsed gently with Phosphate-Buffered Saline (PBS) for 1-2 times, and an appropriate amount of trypsin was added for 1-2 min. When the cells began to settle down, the complete medium was added to stop the digestion, and the cells were transferred and mixed. After counting the slide of cells were removed. According to the experimental arrangement, appropriate amount of cell suspension was inoculated into a new culture flask and placed in an incubator for further culture.
Cell Counting Kit-8 (CCK-8) analysis:
H19-7 cells were collected in logarithmic growth phase, and the cell suspension concentration was adjusted to 5×104 cells/ml. Single-cell suspensions was seeded into the 96-well plate, and 100 μl of cell suspension was added into each well with 6 replicates per set. 96-well plate was cultured in a 5 % CO2, at 37° in an incubator to make the cells adhere to the wall. The old medium was discarded and 100 μl of medium containing different concentrations of baicalin was added. The cells were incubated in 5 % CO2 at 37° for 24 h and 48 h. 10 μl CCK-8 solution was added to each well of the 96-well plate and the culture was terminated after 1-4 h, depending on changes in cell status. The absorbance of each hole was measured at Optical Density (OD) 450 nm with a full-wavelength enzyme labeler.
Cell survival rate %=(OD of treated cells/OD of control cells)×100 %
Here control does not contain any drug intervention, except cells, culture medium and CCK-8.
Flow cytometry analysis:
In normal cells, Phosphatidylserine (PS) is only distributed in the interior of the lipid bilayer of the cell membrane. In the early stage of cell apoptosis, membrane PS shifts from the inside of the lipid membrane to the outside. Annexin V is a phospholipidbinding protein with high affinity for PS, which binds to the membrane of early apoptotic cells by exposure to extracellular PS. Therefore, annexin V is used as one of the sensitive indicators to detect early apoptosis. During apoptosis, membrane PS eversion occurs before nucleus change, and Propidium Iodide (PI) cannot cross the cell membrane. In the process of cell necrosis, although PS exosection occurs, the permeability of the membrane is significantly increased at this time, and nucleic acid dyes such as PI enter the cells and bind to nucleic acids in the nucleus, emitting red fluorescence. For this reason, annexin V-Fluorescein Isothiocyanate (FITC) (Green fluorescence) is often used in combination with nucleic acid dyes (e.g., PI) that identify cell death to distinguish apoptotic cells from dead cells.
Pancreatic digestion cells without Ethylenediamine Tetraacetic Acid (EDTA) were centrifuged (2000 rpm for 5 min) and 1~5×105 cells were collected by washing cells with PBS twice (2000 rpm for 5 min). 500 μl binding buffer suspension cells were added. After mixing with 5 μl annexin V-FITC, 5 μl PI was added and mixed well. The reaction was observed at room temperature, away from light, for 5~15min; flow cytometry experiment was performed within 1 h.
Determination of LDH:
LDH is a very stable cytoplasmic enzyme that is found in the cytoplasm of normal cells. Once the cell membrane is damaged, LDH is released outside the cell. LDH catalyzes the formation of pyruvate from lactic acid, which reacts with Iodonitrotetrazolium (INT) (tetrazole salts) to form red methyl (Lenzan) compound, which can be detected by enzyme labeling device. The amount of color formed is directly proportional to the number of lysed cells. Obtain absorption data at visible wavelengths using a 96-well plate reader. This assay can be used to measure the integrity of the cell membrane in cellmediated cytotoxicity analysis, in which target cells are lysed by effector cells to determine the extent of cell damage.
LDH cytotoxicity assay kit (Nanjing Jiancheng) detects LDH enzyme activity. H19-7 cells were cultured at 37° for 30 min. The reaction was stopped with a stop solution (1 M Hydrochloric acid (Hcl), 50 μl) and OD was measured at 490 nm.
Cytotoxicity (%)=(experimental group OD value/ control group OD value)×100 %.
Fluorescent determination of Reactive Oxygen Species (ROS):
The principle of cellular ROS fluorescence assay is to detect ROS levels in cells using the fluorescent probe 2’,7’-Dichlorofluorescein Diacetate (DCFHDA). Fluorescent probes are specialized molecules that react with ROS to produce a fluorescent signal. These fluorescent probes are converted to fluorescein intracellularly, and when ROS levels are elevated, the fluorescent signal of fluorescein is also enhanced. The fluorescence intensity of intracellular fluorescein was observed by fluorescence microscope, so as to reflect the level of intracellular ROS.
The detection was performed with the ROS detection kit (KGA7308-100). Primarily, a fluorescent probe was added into the medium to make it enter the cells. The cells were treated into single-cell suspension and the fluorescence intensity was observed by fluorescence microscope. In order to ensure the accuracy of the experimental results, it is necessary to conduct a control group experiment, that is, the cells are treated into a suspension without fluorescent probes, and their fluorescence intensity is measured as the background fluorescence signal. Finally, the level of ROS in the cells is calculated according to the difference in fluorescence intensity.
Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) analysis:
Hippocampal neuronal cells were obtained, and total Ribonucleic Acid (RNA) was extracted by Total RNA Isolation (TRIzol) reagent. Qiagen miRNeasy mini kit is used to extract exosomal microRNA (miRNA). SuperScriptTM 1st-strand synthesis system (Invitrogen) was used to prepare complementary Deoxyribonucleic Acid (cDNA) for qRT-PCR analysis by SYBRTM Green Master Mix (Qiagen). The 2-ΔΔCt method was used to calculate the normalized relative expression of β-actin. Gene primer sequences were shown in Table 1.
| Gene | Primer sequence (5’-3’) | Primer size |
|---|---|---|
| TNF-α | F: GATCGGTCCCAACAAGGAGG | 20 |
| R: TCCCTCAGGGGTGTCCTTAG | 20 | |
| IL-1β | F: TTGCTTCCAAGCCCTTGACT | 20 |
| R: GGTCGTCATCATCCCACGAG | 21 | |
| IL-6 | F: AGAGACTTCCAGCCAGTTGC | 20 |
| R: AGTCTCCTCTCCGGACTTGT | 20 | |
| NR2B | F: GGGTCACGCAAAACCCTTTC | 19 |
| R: CCTTGTTTTTGACGCCCCTG | 20 | |
| ERK | F: CAACCAGAACAACTGGCTGC | 20 |
| R: GCCCAAAGCTCCTGACTTCT | 20 | |
| CREB | F: ACTCAGCCGGGTACTACCAT | 20 |
| R: GCACTGCCACTCTGTTCTCT | 20 | |
| GAPDH | F: GCGAGATCCCGCTAACATCA | 20 |
| R: CTCGTGGTTCACACCCATCA | 20 |
Table 1: Nucleotide Sequences of Primers used for Quantitative Rt-Pcr Detection for mRNA
Western blot:
The protein content of hippocampal neuronal cells was quantified by Bicinchoninic Acid (BCA) method. The total cell lysate was separated by Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and blotted on polyvinylidene fluoride membrane (Millipore). Then, the membrane was blocked with 5 % skimmed milk at room temperature for 1 h and with specific antibodies against NR2B (Abcam, ab183942), ERK (Abcam, ab184699), phosphorylated-ERK (p-ERK) (Abcam, ab76299), CREB (CST, #9197), p-CREB (Abcam, ab32096) and β-actin (Abcam, ab6267). The blot was visualized using SuperSignalTM West Pico PLUS chemiluminescent substrate (SD251210, Thermo Fisher Scientific, Inc).
Statistical analysis:
All data are expressed as mean±Standard Error of the Mean (SEM). We used a two-tailed student’s t-test to compare the two groups. Use analysis of variance to compare multiple groups and then perform Bonferroni post testing. All experiments were repeated at least 3 times, p<0.05 was considered statistically significant.
Results and Discussion
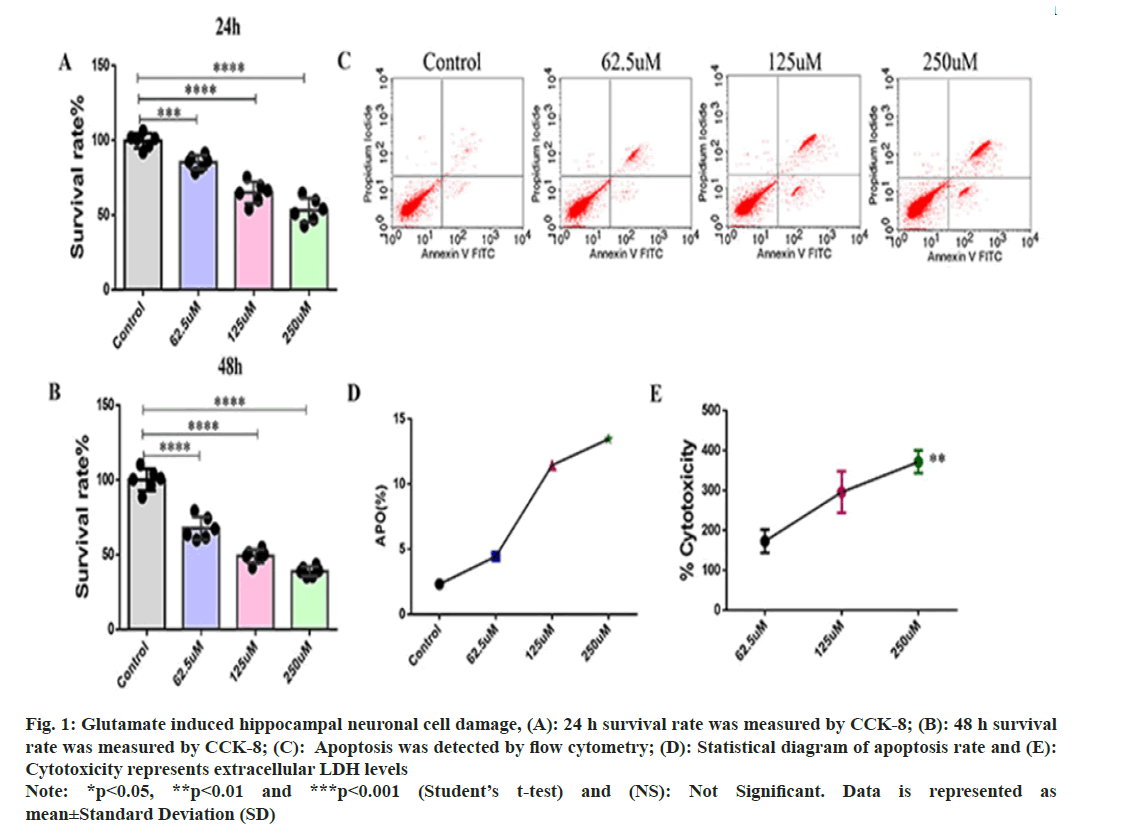
Glutamate induced neuronal cell damage hippocampal neuronal cells in rat was explained in detail. In order to investigate the glutamate damage to rat hippocampal neuronal cells, we used different concentrations of glutamate (62.5, 125 and 250 μM) to induce cell damage. CCK-8 assay was used to detect the proliferation of hippocampal neuronal cells. After 24 h of culture, compared with the control group, the survival rate of hippocampal neuronal cells gradually decreased with the increase of glutamate concentration (fig. 1A), and the proliferation ability of hippocampal neuronal cells decreased with the increase of glutamate concentration after 48 hours of glutamate induction (fig. 1B). Flow cytometry to detect apoptosis. The rate of apoptosis was proportional to the glutamate concentration (fig. 1C and fig. 1D), indicating that glutamate induces apoptosis in hippocampal neuronal cells. At the same time, according to cytotoxicity assays, the amount of extracellular LDH released by glutamate induction increased significantly (fig. 1E).
Fig. 1: Glutamate induced hippocampal neuronal cell damage, (A): 24 h survival rate was measured by CCK-8; (B): 48 h survival rate was measured by CCK-8; (C): Apoptosis was detected by flow cytometry; (D): Statistical diagram of apoptosis rate and (E): Cytotoxicity represents extracellular LDH levels Note: *p<0.05, **p<0.01 and ***p<0.001 (Student’s t-test) and (NS): Not Significant. Data is represented as mean±Standard Deviation (SD)
These results showed that glutamate could inhibit the proliferation of hippocampal neurons, promote apoptosis, and have a toxic effect on cells.
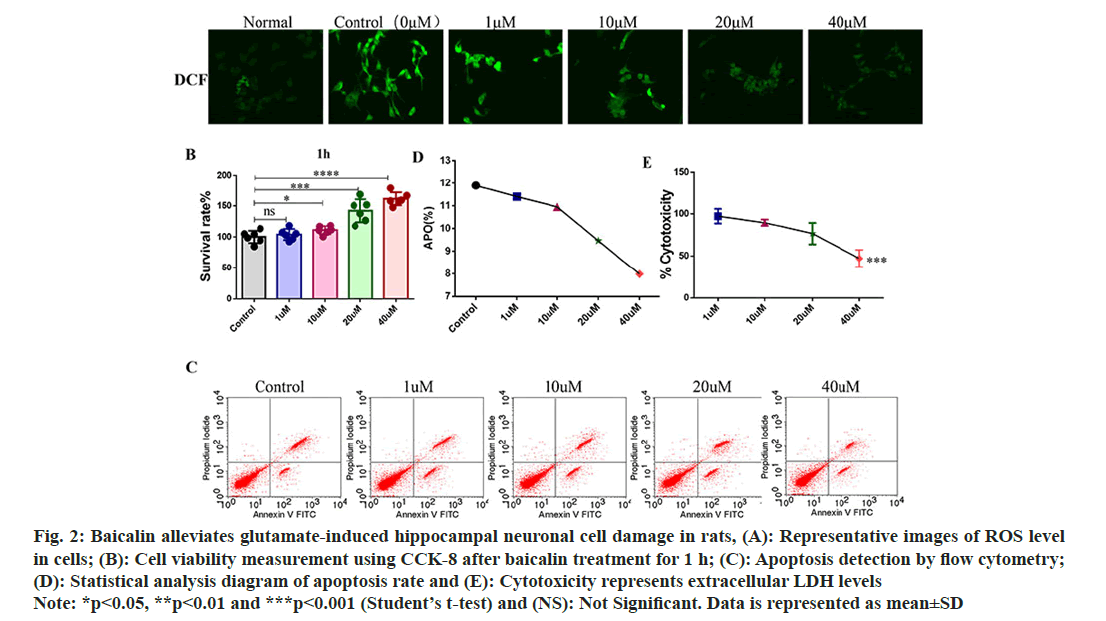
Baicalin improves hippocampal neuronal cell injury in rats. In order to study the repair effect of baicalin on rat hippocampal neuron cell injury, the optimal glutamate concentration of 125 mmol/l was used to induce damage in rat hippocampal neuronal cell culture. 0, 1, 10, 20 and 40 μM baicalin were pretreated for 1 h, and the ROS fluorescence probe was detected after baicalin treatment. The results showed that the ROS fluorescence intensity decreased with the increase of baicalin concentration after baicalin treatment (fig. 2A). Compared with the control group, 1 μM baicalin had no significant effect on neuronal cell proliferation, while 10 μM, 20 μM and 40 μM baicalin significantly promoted hippocampal neuronal cell proliferation rate (fig. 2B); apoptosis was detected using flow cytometry. As the concentration of baicalin increased, the rate of apoptosis in hippocampal neuronal cells decreased (fig. 2C and fig. 2D). Levels of release from extracellular LDH showed that baicalin reduces glutamate-induced cell damage (fig. 2E).
Fig. 2: Baicalin alleviates glutamate-induced hippocampal neuronal cell damage in rats, (A): Representative images of ROS level in cells; (B): Cell viability measurement using CCK-8 after baicalin treatment for 1 h; (C): Apoptosis detection by flow cytometry; (D): Statistical analysis diagram of apoptosis rate and (E): Cytotoxicity represents extracellular LDH levels Note: *p<0.05, **p<0.01 and ***p<0.001 (Student’s t-test) and (NS): Not Significant. Data is represented as mean±SD
These results indicated that baicalin could improve the morphology of neuronal cells, promote cell proliferation, inhibit cell apoptosis and reduce the damage of neuronal cells.
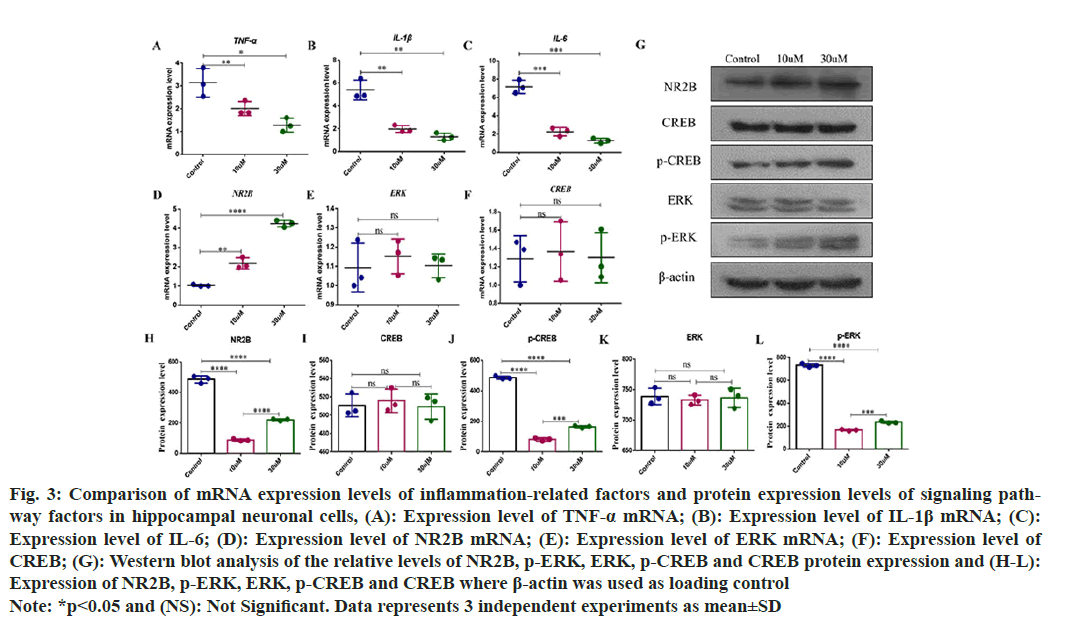
Changes of messenger (m) RNA relative expression of inflammatory factors and NR2B/ERK/CREB signaling pathway in hippocampal neurons was observed. Hippocampal neuronal cells were cultured in vitro and subjected to damage induction by glutamate. Baicalin was used for pretreatment for 1 h, and the mRNA levels of inflammatory factors (Interleukin-1 (IL-1) β, IL-6, and Tumor Necrosis Factor-Alpha (TNF-α), and the mRNA levels of NR2B, ERK and CREB were detected by qRTPCR. Compared to the control group, the mRNA levels of IL-1β, IL-6 and TNF-α decreased with the increasing baicalin concentrations and the difference was statistically significant (fig. 3A-fig. 3C). The expression level of NR2B mRNA in hippocampal neuronal cells increases approximately 2-fold when the baicalin concentration is 10 μM, and increases 4-fold when baicalin concentration is 30 μM (fig. 3D). The protein expression levels of NR2B, ERK, p-ERK, CREB and p-CREB in hippocampal neurons were detected by Westen blot. Compared with the control group, there was no significant change in the mRNA levels of ERK and CREB (fig. 3E and fig. 3F), and compared with the control group, but also the protein expression level in the baicalin group. After hippocampal neuronal cell damaged, the protein expression levels of p-ERK and p-CREB were still lower than those in the control group after treatment with low concentration (10 μM) baicalin. Compared with the low concentration, the protein expression levels of p-ERK and p-CREB were increased after high concentration (30 μM) baicalin treatment, even if they were lower than those in the control group (fig. 3G-fig. 3L).
Fig. 3: Comparison of mRNA expression levels of inflammation-related factors and protein expression levels of signaling pathway factors in hippocampal neuronal cells, (A): Expression level of TNF-α mRNA; (B): Expression level of IL-1β mRNA; (C): Expression level of IL-6; (D): Expression level of NR2B mRNA; (E): Expression level of ERK mRNA; (F): Expression level of CREB; (G): Western blot analysis of the relative levels of NR2B, p-ERK, ERK, p-CREB and CREB protein expression and (H-L): Expression of NR2B, p-ERK, ERK, p-CREB and CREB where β-actin was used as loading control Note: *p<0.05 and (NS): Not Significant. Data represents 3 independent experiments as mean±SD
LDH is widely found in various tissues of the human body, and there is also a large amount of lactate dehydrogenase in central neurons. Under normal physiological conditions, its biochemical properties are stable and rarely leak. When neuronal cells are damaged, LDH is released, and the greater the amount of LDH in the cell supernatant, the more severe the cell damage. Therefore, the LDH content in cell supernatant can be used as an indicator to reflect the degree of hippocampal neuronal damage[16]. The results showed that the extracellular LDH activity and apoptosis expression of hippocampal neurons were significantly increased after glutamate induction. In this study, glutamate was applied to induce hippocampal neuronal cell damage. The results showed that the percentage of PI and annexin V double-positive cells and LDH activity increased significantly after glutamate injury, and the expression of cell activity decreased significantly, which was consistent with the injury response in the hippocampal tissue injury in vivo, and confirmed that glutamate can successfully construct the hippocampal neuronal cell injury model. The degree of glutamateinduced cell damage can be measuring the rate of apoptosis in neuronal cells.
The results of this study suggest that baicalin can reverse glutamate-induced decline in cellular activity. After baicalin treatment, hippocampal neuronal apoptosis and LDH activity were significantly reduced, and ROS levels were also decreased, which confirmed that baicalin could significantly inhibit glutamate-induced damage to hippocampal neuronal cells.
In brain, IL-1β is mainly produced in astrocytes, microglia, neurons and vascular endothelial cells, and IL-1β mRNA expression is positively correlated with the degree of inflammation[9,17]. Both high expression and loss of IL-1β can lead to impaired learning and memory[18,19]. In our previous studies, the mRNA expression levels of TNF-α, IL-6 and IL-1β were increased after splenectomy in aged rats, and the expression levels of inflammatory factors were decreased after baicalin treatment[20]. This study showed that baicalin treatment could reduce the expression level of IL-1β mRNA after glutamate induced cell damage. IL-6 is significantly elevated in injured brain tissue, promotes the adhesion of leukocytes and endothelial cells, causes endothelial cell damage, increases the permeability of the bloodbrain barrier, produces oxygen free radicals, and causes nerve cell death[21]. After baicalin treatment, the expression of IL-6 decreased significantly, indicating that baicalin has neuroprotective effects. Baicalin can downregulate the expression of TNF-α, an inflammatory factor that increases after hippocampal neuron injury in rats. These results indicate that high concentration of baicalin can significantly inhibit the expression of glutamateinduced inflammatory cytokines.
CREB is an intracellular transcription factor[22] and activation of CREB through autophosphorylation has a dramatic impact on intracellular signaling pathways and regulation, as well as on neurons growth, injury, regeneration, and synaptic plasticity[23,24]. ERK is an important upstream signaling molecule of CREB, which can convert various extracellular stimuli into different intracellular responses, and is involved in cell proliferation, differentiation, and synaptic plasticity[25,26]. Related studies have found that inflammatory factors can activate the ERK-CREB pathway and participate in related intracellular reactions[27]. In our previous study, the expression of NR2B mRNA and protein was reduced in hippocampal nerve damage caused by splenectomy in elderly rats, and baicalin treatment could improve their expression[20]. This research confirmed that the mRNA and protein expression levels of NR2B in baicalin treatment group were higher than those in control group after glutamate-induced cell injury, while the mRNA and protein expression levels of ERK and CREB had no significant changes, and the protein expression levels of NR2B, p-ERK and p-CREB were lower than those in the control group. However, compared with the low-concentration baicalin group, the expression levels of NR2B, p-ERK and p-CREB proteins in the high-concentration baicalin group were significantly increased, indicating that highconcentration baicalin coould effectively alleviate glutamate-induced cell damage, and the up-regulation or activation of NR2B/ERK/CREB helped alleviate neuronal damage and exert neuroprotective effects.
There are still shortcomings in this study, so it is necessary to select genes related to ERK, CREB phosphorylation inhibitors, ERK expression knockout or CREB blockers for further research, so as to clarify the in-depth mechanism of baicalin regulating of neuronal cell damage repair.
In conclusion, baicalin may alleviate glutamateinduced hippocampal neuronal cell damage through the NR2B/ERK/CREB pathway.
Ethical approval:
The study was approved by the Ethics Committee of the Wuxi Traditional Chinese Medicine Hospital (approval number: SZGJCX2023021502).
Acknowledgement:
We acknowledge Liang Cai and Jiangnan Zhang, for performing the experiment, designing and manuscript writing. Hongmei Zhou, R. H. Li, Chenhao Jiang, Shajin Liu and Jiannan Zhang are thankful for their participation in this work’s literature search, experimental validation, data analysis, and manuscript writing. Jiannan Zhang participated in experimental design, data collection and statistical analysis. Liang Cai provided administrative support to the experiment. Jiannan Zhang performed critical review for the intellectual content, data analysis/ interpretation of this article. All the authors approved the final version for publication.
Funding:
This research was supported by Wuxi Municipal Health Commission (M202258), Jiangsu Traditional Chinese Medicine Administration (MS2022057), and Nanjing University of Traditional Chinese Medicine (XZR2023025).
Conflict of interests:
The authors declared no conflict of interests.
References
- Onaolapo AY, Onaolapo OJ. Dietary glutamate and the brain: In the footprints of a Jekyll and Hyde molecule. Neurotoxicology 2020;80:93-104.
[Crossref] [Google Scholar] [PubMed]
- Reiner A, Levitz J. Glutamatergic signaling in the central nervous system: Ionotropic and metabotropic receptors in concert. Neuron 2018;98(6):1080-98.
[Crossref] [Google Scholar] [PubMed]
- Belousov AB, O'Hara BF, Denisova JV. Acetylcholine becomes the major excitatory neurotransmitter in the hypothalamus in vitro in the absence of glutamate excitation. J Neurosci 2001;21(6):2015-27.
[Crossref] [Google Scholar] [PubMed]
- Sun C, Cao XC, Liu ZY, Ma CL, Li BM. Polygalasaponin F protects hippocampal neurons against glutamate-induced cytotoxicity. Neural Regen Res 2022;17(1):178-84.
[Crossref] [Google Scholar] [PubMed]
- Baek JY, Jung K, Kim YM, Kim HY, Kang KS, Chin YW. Protective effect of γ-mangostin isolated from the peel of Garcinia mangostana against glutamate-induced cytotoxicity in HT22 hippocampal neuronal cells. Biomolecules 2021;11(2):1-10.
[Crossref] [Google Scholar] [PubMed]
- Iovino L, Tremblay ME, Civiero L. Glutamate-induced excitotoxicity in Parkinson's disease: The role of glial cells. J Pharmacol Sci 2020;144(3):151-64.
[Crossref] [Google Scholar] [PubMed]
- Mark KA, Quinton MS, Russek SJ, Yamamoto BK. Dynamic changes in vesicular glutamate transporter 1 function and expression related to methamphetamine-induced glutamate release. J Neurosci 2007;27(25):6823-31.
[Crossref] [Google Scholar] [PubMed]
- Alvarez‐Arellano L, Pedraza‐Escalona M, Blanco‐Ayala T, Camacho‐Concha N, Cortés‐Mendoza J, Perez‐Martínez L, et al. Autophagy impairment by caspase-1-dependent inflammation mediates memory loss in response to β-amyloid peptide accumulation. J Neurosci Res 2018;96(2):234-46.
[Crossref] [Google Scholar] [PubMed]
- Lopez‐Rodriguez AB, Hennessy E, Murray CL, Nazmi A, Delaney HJ, Healy D, et al. Acute systemic inflammation exacerbates neuroinflammation in Alzheimer's disease: IL-1 beta drives amplified responses in primed astrocytes and neuronal network dysfunction. Alzheimers Dement 2021;17(10):1735-55.
[Crossref] [Google Scholar] [PubMed]
- Chesnokova V, Pechnick RN, Wawrowsky K. Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain Behav Immun 2016;58:1-8.
[Crossref] [Google Scholar] [PubMed]
- Penn Y, Segal M, Moses E. Network synchronization in hippocampal neurons. Proc Natl Acad Sci USA 2016;113(12):3341-6.
[Crossref] [Google Scholar] [PubMed]
- Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron 2000;27(3):623-33.
[Crossref] [Google Scholar] [PubMed]
- Han S, Wang W, Wang S, Yang T, Zhang G, Wang D, et al. Tumor microenvironment remodeling and tumor therapy based on M2-like tumor associated macrophage-targeting nano-complexes. Theranostics 2021;11(6):2892-916.
[Crossref] [Google Scholar] [PubMed]
- Yu HY, Zhu Y, Zhang XL, Wang L, Zhou YM, Zhang FF, et al. Baicalin attenuates amyloid beta oligomers induced memory deficits and mitochondria fragmentation through regulation of PDE-PKA-Drp1 signalling. Psychopharmacology 2022;239(3):851-65.
[Crossref] [Google Scholar] [PubMed]
- Heo HJ, Kim DO, Choi SJ, Shin DH, Lee CY. Potent Inhibitory effect of flavonoids in Scutellaria baicalensis on amyloid beta protein-induced neurotoxicity. J Agric Food Chem 2004;52(13):4128-32.
[Crossref] [Google Scholar] [PubMed]
- Livesey A, Garty F, Shipman AR, Shipman KE. Lactate dehydrogenase in dermatology practice. Clin Exp Dermatol 2020;45(5):539-43.
[Crossref] [Google Scholar] [PubMed]
- Marks JA, Li S, Gong W, Sanati P, Eisenstadt R, Sims C, et al. Similar effects of hypertonic saline and mannitol on the inflammation of the blood-brain barrier microcirculation after brain injury in a mouse model. J Trauma Acute Care Surg 2012;73(2):351-7.
[Crossref] [Google Scholar] [PubMed]
- Yang X, Zhang Q, Gao Z, Yu C, Zhang L. Baicalin alleviates IL-1β-induced inflammatory injury via down-regulating miR-126 in chondrocytes. Biomed Pharmacother 2018;99:184-90.
[Crossref] [Google Scholar] [PubMed]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, et al. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology 2007;32(8-10):1106-15.
[Crossref] [Google Scholar] [PubMed]
- Zhang JN, Zhou HM, Jiang CH, Liu J, Cai LY. Protective effect of baicalin against cognitive memory dysfunction after splenectomy in aged rats and its underlying mechanism. J Integr Neurosci 2020;19(4):679-85.
[Crossref] [Google Scholar] [PubMed]
- Wang GH, Jiang ZL, Li YC, Li X, Shi H, Gao YQ, et al. Free-radical scavenger edaravone treatment confers neuroprotection against traumatic brain injury in rats. J Neurotrauma 2011;28(10):2123-34.
[Crossref] [Google Scholar] [PubMed]
- Han J, Li E, Chen L, Zhang Y, Wei F, Liu J, et al. The CREB coactivator CRTC2 controls hepatic lipid metabolism by regulating SREBP1. Nature 2015;524(7564):243-6.
[Crossref] [Google Scholar] [PubMed]
- Zeng L, Jiang H, Ashraf GM, Liu J, Wang L, Zhao K, et al. Implications of miR-148a-3p/p35/PTEN signaling in tau hyperphosphorylation and autoregulatory feedforward of Akt/CREB in Alzheimer's disease. Mol Ther Nucleic Acids 2022;27:256-75.
[Crossref] [Google Scholar] [PubMed]
- Yan L, Jin Y, Pan J, He X, Zhong S, Zhang R, et al. 7,8-dihydroxycoumarin alleviates synaptic loss by activated PI3K-Akt-CREB-BDNF signaling in Alzheimer's disease model mice. J Agric Food Chem 2022;70(23):7130-8.
[Crossref] [Google Scholar] [PubMed]
- Lavoie H, Gagnon J, Therrien M. ERK signalling: A master regulator of cell behaviour, life and fate. Nat Rev Mol Cell Biol 2020;21(10):607-32.
[Crossref] [Google Scholar] [PubMed]
- Sun N, Wang J, Dou X, Wang Y, Yang Y, Xiao D, et al. A chiral microenvironment promotes retinal progenitor cell proliferation by activating the Akt and ERK pathways. Biomater Sci 2022;10(20):5938-46.
[Crossref] [Google Scholar] [PubMed]
- Cao H, Ren WH, Zhu MY, Zhao ZQ, Zhang YQ. Activation of glycine site and GluN2B subunit of NMDA receptors is necessary for ERK/CREB signaling cascade in rostral anterior cingulate cortex in rats: Implications for affective pain. Neurosci Bull 2012;28(1):77-87.
[Crossref] [Google Scholar] [PubMed]