- *Corresponding Author:
- B. Medhi
Department of Pharmacology, Postgraduate Institute of Medical Education and Research, Chandigarh-160 012, India
E-mail: drbikashus@yahoo.com
| Date of Submission | 02 August 2014 |
| Date of Revision | 17 January 2015 |
| Date of Acceptance | 13 November 2015 |
| Indian J Pharm Sci 2015; 77(6):687-693 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Adverse drug reactions associated with the use of anticancer drugs are a worldwide problem and cannot be ignored. Adverse drug reactions can range from nausea, vomiting or any other mild reaction to severe myelosuppression. The study was planned to observe the suspected adverse drug reactions of cancer chemotherapy in patients aged >18 years having cancer attending Postgraduate Institute of Medical Education and Research, Chandigarh. During the study period, 101 patients of breast cancer and 73 patients of lung cancer were screened for occurrence of adverse drug reactions during their treatment with chemotherapy. About 87.36% patients experienced adverse drug reactions, 90.09% and 83.56% of breast and lung cancer patients experienced at least one adverse drug reaction respectively. In breast cancer patients, 41.58% patients were prescribed fluorouracil+doxorubicin+cyclophosphamide while paclitaxel was prescribed to 22.77% patients. Alopecia (54.94%), nail discolouration (43.96%), dysgeusia (38.46%), anorexia (30.77%), nausea (29.67%), and neuropathy (29.67%) were found to be very common in breast cancer patients treated with single/combined regimen. In lung cancer group of patients, cisplatin with docetaxel, cisplatin with pemetrexed and cisplatin with irinotecan were prescribed to 30.14, 24.65 and 17.81% patients, respectively. Dysgeusia (40.98%), diarrhoea (39.34%), anorexia (32.77%) and constipation (31.15%) and alopecia (31.15%) were commonly observed adverse drug reactions having lung cancer patients. Causality assessments using World Health Organization causality assessment scale showed that observed adverse drug reactions were of probable (64.67%) and possible (35.33%) categories. Alopecia, dysgeusia, anorexia, constipation diarrhoea, nausea, nail discoloration were more prevalent amongst the cancer patients undergoing chemotherapy.
Keywords
Pharmacovigilance, adverse drug reactions, chemotherapy
Adverse drug reactions (ADRs) are a worldwide problem associated with the use of drugs for curbing the ailments. According to World Health Organisation (WHO), ADR can be defined as ‘A response to a drug, which is noxious and unintended, and which occurs at doses normally used in man for the prophylaxis, diagnosis, or therapy of disease, or for the modifications of physiological function [1]. During the last decade it has been demonstrated by a number of studies that drug induced morbidity and mortality is one of the major problem for public health. With the large number of drugs being marketed it is becoming pertinent to monitor ADRs amongst the patients being treated with one or other drug. ADRs often impose a huge financial burden on healthcare system of a country. Some countries spend up to 20% of their hospital budget dealing with drug complications [2,3]. Worldwide, efforts are on-going to identify the ADRs, monitor the drug’s use and improve prescribing habits of practitioners to ultimately make use of medicines more rational [4]. The incidence of ADRs varies with studies, which show incidences ranging from as low as 0.15% to as high as 30% [5-7]. Elderly and hospitalized patients are reported to be more susceptible to ADRs than the adult population (16.6% vs. 4.1%) [6]. Recent epidemiological studies estimated that ADRs are fourth to sixth leading cause of death [8]. Impact of ADRs on patients includes the lowering of quality of life, increase in number of hospitalizations, increased economic burden on health management and increased rate of mortality. The prevalence of ADRs of anticancer drugs in Indian context is 10-12% [9]. Cancer is one of the leading causes of death worldwide with estimated 12% deaths annually [10]. The number of global cancer deaths is projected to increase by 45% from 2010 to 2030. In India the incidence of cancer is about 70-90 per 100 000 persons. Cancer prevalence in India is estimated approximately 2.5 million, with over 800 000 new cases, and claiming around 555 000 deaths in 2010. Currently, 23 per 100 000 women in India are suffering from breast cancer. In India, the incidence of breast cancer has increased over the years and as many as 100 000 new cases are being detected every year. In developing countries older women are likely to develop breast cancer than younger women [11]. Deaths due to lung cancer are projected to raise to 10 million by 2030 with 7 out of 10 deaths in the developing countries. According to a report, it has been found that new cases of lung cancer per lakh population increased by around 160% in Chennai, 40% in Delhi and 100% in Bangalore but decreased by 60% in Mumbai [12].
A million of current 5 million deaths in the world, and 2.41 million in developing countries are contributed by India and in 2020 the figure is projected to be 1.5 million [13,14]. Anticancer drug therapies are more prone to cause ADRs as these agents are cytotoxic and can damage the normally dividing cells along with the cancerous cells. Another reason of more ADRs in patient receiving anticancer drugs is that such patients remain on multi drug treatments making them more vulnerable to ADRs [9,15].
There is a dearth of ADRs data associated with chemotherapy drugs in countries like India. The present study of monitoring the ADRs is to collect data and estimation of incidence of ADRs in north Indian patients receiving treatment in a tertiary care hospital. So, the study was aimed to determine the adverse drug reactions of the anticancer drugs prescribed (either alone or in combination) for the treatment.
Materials and Methods
All the patients of breast cancer and lung cancer undergoing chemotherapy in day care centre during the study time period were recruited. Patient receiving anticancer agents either as single drug regimen or in combination of two or more drugs, aged >18 years were included in the study. Adverse events caused by administration errors, noncompliance or overdose were excluded from the study [2].
Study design and procedure
This observational prospective study was designed to assess the safety of chemotherapy drugs used in lung and breast cancer patients. The study was performed in Post Graduate Institute of Medical Education and Research, Chandigarh. During the study period, the prescription pattern of oncologists was observed accordingly and patients were interviewed for the occurrence of ADRs in the presence of their healthcare team i.e. nurses or doctors.
ADRs were collected by filling the suspected ADR form for each patient who experienced ADR. During the patient interview; patient information, drug information, past medical history, laboratory investigations were accessed. After collecting the information on ADR, causality assessment was done with the help of pharmacovigilance expert (Pharmacovigilance Centre Coordinator).
Results
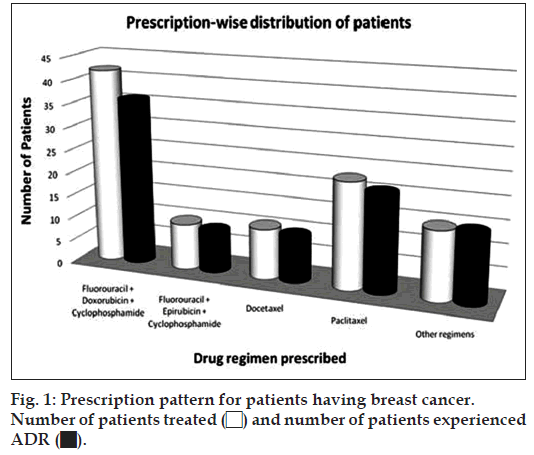
The study sample comprised of 174 patients out of which 101 breast cancer and 73 lung cancer patients receiving chemotherapy were screened for adverse drug reactions. Out of 174 patients 152 (87.36%) patients experienced at least one ADR and 22 (12.64%) patients did not develop any ADR. A summarized table is given below (Table 1): Among the different types of anticancer drug therapy the patients of breast cancer were prescribed different drug regimens of fluorouracil+doxorubicin +cyclophosphamide (41.58%), paclitaxel (22.77%), docetaxel (10.89%), fluorouracil+epirubicin+cyclo phosphamide (9.90%), and some other regimens, which involve docetaxel+carboplatin+trastuzumab (3.96%), trastuzumab (2.97%), epirubicin+docetaxel (1.98%), fluorouracil+docetaxel+cyclophosph amide (1.98%), cisplatin+vinorelbine (0.99%), cisplatin+vinblastin (0.99%), gemcitabine+cisplatin (0.99%), docetaxel+doxorubicin (0.99%); fig. 1 illustrates the prescription pattern in breast cancer group of patients:
| Breast cancer Lung cancer | Total | ||
|---|---|---|---|
| Total number of patients | 101 | 73 | 174 |
| Patients experienced ADR | 91 | 61 | 152 |
| Patients did not experienced ADR | 10 | 12 | 22 |
| Incidence of ADR (%) | 90.09 | 83.56 | 87.36 |
Table 1: Percentage of adr in breast and lung Cancer
Out of 101 patients of breast cancer 91 (90.09%) patients experienced at least one ADR but 10 (9.90%) did not experience any ADR at all. The combined regimen of fluorouracil, doxorubicin and cyclophosphamide was prescribed to a total of 42 (41.58%) patients out of which 36 (85.71%) developed adverse events. The number of patients receiving paclitaxel alone was 23 (22.77%) out of which 21 (91.30%) patients’ experienced adverse events. Docetaxel treatment therapy was received by 11 (10.89%) patients and 10 (90.90%) of them have experienced ADR. A combined drug regimen of fluorouracil+epirubicin+cyclophosphamide was also prescribed to 10 (9.90%) patients out of whom 9 (90%) patients experienced ADR.
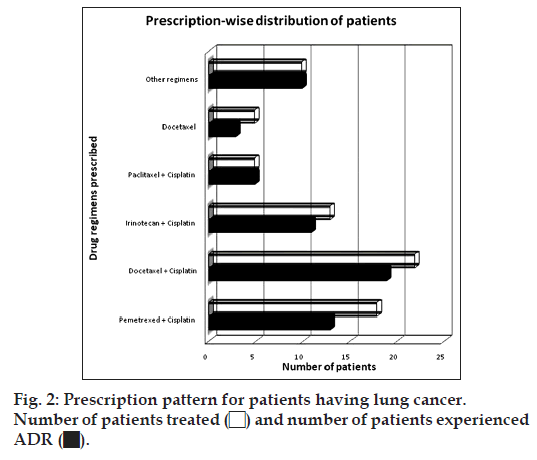
Some other drug regimens in combination as well as single drug regimens were also prescribed like trastuzumab+docetaxel+carboplatin to 4 patients, epirubicin+docetaxel to 2 patients, fluorouracil+doxo rubicin+cyclophosphamide to 2 patients, trastuzumab to 3 patients. Less frequently used drug regimens like docetaxel+doxorubicin, cisplatin+gemcitabine, cisplatin+vinblastine, and cisplatin+vinorelbine were prescribed to 1 patient each of the study population, which developed atleast one single ADR including alopecia, breathlessness, cough, diarrhoea, nausea, neuropathy, pain and restlessness etc. Percentage of patients experienced different ADRs are tabulated below (Table 2): Out of total 73 patients of lung cancer, pemetrexed+cisplatin, docetaxel+cisplatin, irinotecan+cisplatin were administered to 24.65, 30.14 and 17.81% patients, respectively. On the other hand, some other regimens as docetaxel (6.85%) and paclitaxel with cisplatin (6.85%). While docetaxel and paclitaxel in combination with cisplatin and/or carboplatin were also prescribed to some patients. Fig. 2 illustrates the prescription pattern of patients of lung cancer:
| 5-fluorouracil +doxorubicin +cyclophosphamide | 5-fluorouracil+ epirubicin +cyclophosphamide | Docetaxel | Paclitaxel | Otherregimens | Totalpatients | |
|---|---|---|---|---|---|---|
| Percentage of patients treated (n) | 41.58 (42) | 9.90 (10) | 10.89 (10) | 22.77 (23) | 14.85 (15) | 100 (101) |
| Percentage of patients experienced ADR | 85.71 (36) | 90 (9) | 90.90 (10) | 91.30 (21) | 100 (15) | 90.09 (91) |
| Alopecia | 61.11 (22) | 55.55 (05) | 30 (03) | 66.67 (14) | 40 (06) | 54.94 (50) |
| Anorexia | 33.33 (12) | 44.44 (04) | 20 (02) | 38.09 (08) | 13.33 (02) | 30.77 (28) |
| Breathlessness | 5.55 (02) | 0 | 10 (01) | 0 | 6.67 (01) | 4.39 (04) |
| Constipation | 16.67 (06) | 11.11 (01) | 10 (01) | 4.76 (01) | 13.19 (12) | |
| Cough | 2.78 (01) | 22.22 (02) | 10 (01) | 4.76 (01) | 6.67 (01) | 6.59 (06) |
| Diarrhoea | 11.11 (04) | 22.22 (02) | 40 (04) | 28.57 (06) | 20 (03) | 20.88 (19) |
| Dysgeusia | 41.67 (15) | 33.33 (03) | 50 (05) | 33.33 (07) | 40 (06) | 39.56 (36) |
| Fever | 16.67 (06) | 33.33 (03) | 20 (02) | 19.05 (04) | 20 (03) | 19.78 (18) |
| Headache | 19.44 (07) | 22.22 (02) | 20 (02) | 4.76 (01) | 6.67 (01) | 14.28 (13) |
| Hyper-pigmentation | 16.67 (06) | 11.11 (01) | 0 | 4.76 (01) | 6.67 (01) | 9.89 (09) |
| Insomnia | 2.78 (01) | 11.11 (01) | 30 (03) | 9.52 (02) | 0 | 7.69 (07) |
| Itching | 0 | 0 | 0 | 9.52 (02) | 6.67 (01) | 3.29 (03) |
| Menstrual abnormality | 2.78 (01) | 11.11 (01) | 10 (01) | 14.28 (03) | 6.67 (01) | 7.69 (07) |
| Nail discoloration | 50 (18) | 66.66 (06) | 50 (05) | 52.38 (11) | 0 | 43.96 (40) |
| Nausea | 41 (15) | 33.33 (03) | 20 (02) | 19.05 (04) | 20 (03) | 29.67 (27) |
| Neuropathy | 13.89 (05) | 0 | 10 (01) | 66.67 (14) | 46.67 (07) | 29.67 (27) |
| Oral ulceration | 8.33 (03) | 66.66 (06) | 30 (03) | 19.05 (04) | 33.33 (05) | 23.08 (21) |
| Pain | 13.89 (05) | 22.22 (02) | 50 (05) | 23.81 (05) | 13.33 (02) | 20.88 (19) |
| Rashes | 0 | 0 | 0 | 4.76 (01) | 20 (03) | 4.39 (04) |
| Restlessness | 5.55 (02) | 0 | 0 | 14.28 (03) | 6.67 (01) | 6.59 (06) |
| Teary eyes | 2.78 (01) | 0 | 20 (02) | 0 | 6.67 (01) | 4.39 (04) |
| Vertigo | 2.78 (01) | 0 | 20 (02) | 9.52 (02) | 0 | 5.49 (05) |
| Vomiting | 36.11 (13) | 22.22 (02) | 20 (02) | 14.28 (03) | 0 | 21.98 (20) |
| Weakness | 5.55 (02) | 0 | 0 | 9.52 (02) | 6.67 (01) | 5.49 (05) |
Table 2: Adr Profile of Different Prescribed Drug Regimens of Breast Cancer Patients
Out of 73 patients of lung cancer 61 (83.56%) patients experienced adverse events and rest of them did not develop any ADR. During treatment, 24.65% patients were prescribed combined regimen of pemetrexed+cisplatin out of which 72.22% patients experienced adverse events like dysgeusia (69.23%), anorexia (38.46%), constipation (38.46%), neuropathy (30.77%) and fever (30.77%). Docetaxel+cisplatin was another regimen, which was prescribed to 30.14% lung cancer patients out of whom 86.36% experienced adverse drug events such as diarrhoea (63.16%), alopecia (63.16%), anorexia (42.10%), dysgeusia (31.58%), oral ulceration (31.58%). Irinotecan+cisplatin was prescribed to 17.81% patients out of those patients 84.61% patients experienced at least one ADR like nausea, constipation (45.45%) anorexia, diarrhoea, dysgeusia (36.36%). Docetaxel was prescribed to 6.85% out of which 60% patiets experienced ADR and paclitaxel+cispaltin was prescribed to 6.85% patients and all the 100% patients experienced ADR.
Apart from the above mentioned regimens some other regimens were also used. 5.56% of patients were receiving combined regimen of docetaxel+carboplatin. Carboplatin in combination with pemetrexed was given to 4.17% patients and 2.78% patients were on combination of paclitaxel with carboplatin. Patients treated with these regimens had constipation, fever, dysgeusia, neuropathy, diarrhoea, rashes, oedema, pain, insomnia, oral ulceration, nail discoloration and peeling of skin. Table showing the ADR profile is given below (Table 3): In the present study we have identified the prescription pattern of oncologist. In which we found that combined drug regimens were commonly used for the treatment of breast and lung cancer. While single drug regimens were also prescribed to some patients. ADRs associated with chemotherapy were very often even in single drug regimens.
| Events | Pemetrexed +cisplatin | Docetaxel +cisplatin | Irinotecan +cisplatin | Paclitaxel +cisplatin | Docetaxel | Other regimens | Total patients |
|---|---|---|---|---|---|---|---|
| Percentage of patients treated (n) | 24.65 (18) | 30.14 (22) | 17.81 (13) | 6.85 (05) | 6.85 (05) | 13.80 (10) | 100 (73) |
| Percentage of patients experienced ADR | 72.22 (13) | 86.36 (19) | 84.61 (11) | 100 (05) | 60 (03) | 100 (10) | 83.56 (61) |
| Alopecia | 7.69 (01) | 63.16 (12) | 18.18 (02) | 40 (02) | 33.33 (01) | 10 (01) | 31.15 (19) |
| Anorexia | 38.46 (05) | 42.10 (08) | 36.36 (04) | 20 (01) | 66.67 (02) | 0 | 32.77 (20) |
| Bleeding gum | 0 | 0 | 0 | 20 (01) | 33.33 (01) | 20 (02) | 3.28 (02) |
| Breathlessness | 7.69 (01) | 0 | 0 | 20 (01) | 0 | 10 (01) | 4.92 (03) |
| Constipation | 38.46 (05) | 26.31 (05) | 45.45 (05) | 0 | 33.33 (01) | 30 (03) | 31.15 (19) |
| Cough | 0 | 5.26 (01) | 0 | 20 (01) | 0 | 10 (01) | 4.92 (03) |
| Diarrhoea | 23.08 (03) | 63.16 (12) | 36.36 (04) | 20 (01) | 33.33 (01) | 30 (03) | 39.34 (24) |
| Dysgeusia | 69.23 (09) | 31.58 (06) | 36.36 (04) | 40 (02) | 100 (03) | 10 (01) | 40.98 (25) |
| Fever | 30.77 (04) | 15.79 (03) | 0 | 0 | 33.33 (01) | 30 (03) | 18.03 (11) |
| Headache | 30.77 (04) | 10.53 (02) | 0 | 0 | 0 | 0 | 9.84 (06) |
| Hives | 7.69 (01) | 10.53 (02) | 0 | 0 | 0 | 0 | 4.92 (03) |
| Hyper-pigmentation | 7.69 (01) | 5.26 (01) | 0 | 0 | 33.33 (01) | 0 | 4.92 (03) |
| Insomnia | 0 | 0 | 9.09 (01) | 40 (02) | 0 | 30 (03) | 4.92 (03) |
| Itching | 7.69 (01) | 0 | 9.09 (01) | 0 | 33.33 (01) | 0 | 4.92 (03) |
| Nail discoloration | 30.77 (04) | 15.79 (03) | 0 | 20 (01) | 0 | 10 (01) | 14.75 (09) |
| Nausea | 0 | 21.05 (04) | 45.45 (05) | 0 | 33.33 (01) | 0 | 16.39 (10) |
| Neuropathy | 30.77 (04) | 15.79 (03) | 27.27 (03) | 0 | 33.33 (01) | 10 (01) | 19.67 (12) |
| Oedema | 0 | 0 | 0 | 0 | 33.33 (01) | 10 (01) | 3.28 (02) |
| Oral ulceration | 7.69 (01) | 31.58 (06) | 0 | 0 | 33.33 (01) | 0 | 13.11 (08) |
| Pain | 23.07 (03) | 21.05 (04) | 9.09 (01) | 20 (01) | 0 | 10 (01) | 16.39 (10) |
| Peeling of skin | 0 | 5.26 (01) | 0 | 0 | 0 | 10 (01) | 3.28 (02) |
| Rashes | 7.69 (01) | 5.26 (01) | 0 | 0 | 0 | 20 (02) | 6.56 (04) |
| Restlessness | 15.38 (02) | 5.26 (01) | 9.09 (01) | 0 | 33.33 (01) | 20 (02) | 11.47 (07) |
| Vomiting | 23.07 (03) | 26.31 (05) | 18.18 (02) | 20 (01) | 0 | 0 | 18.03 (11) |
| Weakness | 7.69 (01) | 5.26 (01) | 18.18 (02) | 0 | 33.33 (01) | 0 | 8.20 (05) |
Table 3: Adr Profile of Different Prescribed Drug Regimens of Lung Cancer Patients
After performing the causality assessment it has been found that most of the ADRs falls in probable or possible categories of WHO’s scale of causality assessment suggesting the real existence of ADRs in sample studied, which has been shown in the table given below (Table 4). Some premedications like ranitidine and ondansetrone were administered intravenous bolus prophylactically to prevent the ADR.
| Category | Number of cases (%) |
|---|---|
| Certain | 0 |
| Probable | 97 (64.67) |
| Possible | 53 (35.33) |
| Unlikely | 0 |
| Unclassified | 0 |
| Unassessable | 0 |
Table 4: Causality assessment according to who causality categories
Discussion
Reporting of new ADRs is to be encouraged but one should not ignore already reported and well known ADRs because there might be a pattern shift of ADRs over the time. Clinical trials of new drug therapies provide information about the nature and number of serious adverse events (SAEs) [16]. As these trials are conducted in very controlled environment rare and delayed ADRs may fail get to get reported or reported less and sometimes may not accurately simulate the experiences in general population [17]. Studies have demonstrated that SAEs that do not occur during clinical trials can occur after a drug is approved for marketing and consumed by the public [18]. In fact, a study of SAEs identified that after approval more than 20 cancer drugs were found linked with SAEs [19]. ADRs associated with chemotherapeutic drugs decrease the quality of life, and increases the mortality as well as the healthcare budget [20]. It has been found that the ADR profile of cancer chemotherapeutics is very less reported and the situation is even worse in India [21]. The reason might be that the data collected by regulatory authorities and pharmaceutical industries is inaccessible to public. The total contribution of India in adverse drug reaction information is only 1% in global data, which shows the under-reporting [22] and/ or under-detection as physicians are not so aware about pharmacovigilance or do not have adequate knowledge about ADR monitoring and reporting, due to which the exact incidence of ADR is still unknown [23,24]. As a result of which, Indian regulatory decisions are generally based on mimicking the decisions. Withdrawal of pioglitazone by Drugs Controller General of India (DCGI) is a recent example [25] but now the ban has been revoked.
In the present study, prescription pattern of oncologists was studied and it was found that they have used single, double and/or triple regimen in the treatment therapy of breast and lung cancer. Our study is also in conformation with earlier studies showing commonly occurring ADRs in patients undergoing chemotherapy. In our study 152 (87.36%) patients developed ADRs from the total of 174 patients underwent cancer chemotherapy. Our finding is in contrast to a study by Malik et al. [26], which shows 42% incidence and a study by Goyal et al. [27] shows a incidence of 70% of ADRs in the study population. Our results show that dysgeusia, diarrhoea, alopecia, anorexia, nausea and vomiting were found to be very common ADRs usually associated with every chemotherapeutic regimen. Chemotherapy induced nausea and vomiting in cancer patients were found common, which is similar to other study findings previously published [28,29]. In our study female patients of breast cancer were more prone to ADRs 90% against lung cancer male patients 83% [26]. The variation may be due to difference in medications and treatment guidelines followed for treating the cancer in different set up. Dermatological reactions like alopecia 43.39%, nail discolouration 32.24%, were more frequently experienced ADRs by the patients. Other dermatological ADRs like hives 1.97%, hyper pigmentation of skin 7.98%, itching 3.95% and rashes 5.26% were also experienced. Gastrointestinal ADRs like 19.18% of total study population experienced oral ulceration, out of which 23.08% in breast cancer patients, which is in comparison to males 13.11% is more and it is supported by Sanches et al. [30]. In comparison to the previous studies, which showed that ADRs were more common in females as compared to males, which is supported by a study from Nepal [29].
Pheniramine maleate and hydrocortisone were the common drugs used to manage the ADRs like restlessness, breathlessness and rash while granisetron hydrochloride and ondansetron were used to manage nausea with or without vomiting experienced during chemotherapy cycles.
In the present study we have observed the prescription pattern of oncologist. In which we found that combined drug regimens were commonly used for the treatment of breast and lung cancer. While single drug regimens were also prescribed to some patients. ADRs associated with chemotherapy were seen very often even in single drug regimens. After comparing the different regimens i.e. single and combined, it was observed that there was no significant difference in the ADR profiles. Since the study time period was only 2 months, the number of patients screened was less due to which we could not apply the extensive statistics therefore, further long duration studies covering large population are needed to validate the data. Also the haematological data was not assessable due to administrative restrictions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Glossary of Terms Used in Pharmacovigilance; January, 2013. Available from: http://www.who-umc.org/DynPage.aspx?id=97224&mn1=7347&m n2=7252&mn3=7257. [Last cited on 2013 Jul 30].
- De A. Monitoring of suspected adverse drug reactions in oncology unit of an urban multispeciality teaching hospital. Int J Res Pharm Biomed Sci 2010;1:1-32.
- Rottenkolber D, Schmiedl S, Rottenkolber M, Farker K, Saljé K, Mueller S, et al. Adverse drug reactions in Germany: Direct costs of internal medicine hospitalizations. Pharmacoepidemiol Drug Saf 2011;20:626-34.
- World Health Organization. The importance of pharmacovigilance: Safety monitoring of medicinal products. Geneva: World Health Organization and the Uppsala Monitoring Centre; 2002. p. 9-10.
- Beijer HJ, de Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): A meta-analysis of observational studies. Pharm World Sci 2002;24:46-54.
- Jose J, Rao PG. Pattern of adverse drug reactions notified by spontaneous reporting in an Indian tertiary care teaching hospital. Pharmacol Res 2006;54:226-33.
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA 1998;279:1200-5.
- Brown SD Jr, Landry FJ. Recognizing, reporting, and reducing adverse drug reactions. South Med J 2001;94:370-3.
- Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: Prospective analysis of 18 820 patients. BMJ 2004;329:15-9.
- Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst 2011;103:117-28.
- Shetty P. India faces growing breast cancer epidemic. Lancet 2012;379:992-3.
- Rapiti E, Jindal SK, Gupta D, Boffetta P. Passive smoking and lung cancer in Chandigarh, India. Lung Cancer 1999;23:183-9.
- Rao DN, Ganesh B. Estimate of cancer incidence in India in 1991. Indian J Cancer 1998;35:10-8.
- Murthy NS, Chaudhry K, Rath GK. Burden of cancer and projections for 2016, Indian scenario: Gaps in the availability of radiotherapy treatment facilities. Asian Pac J Cancer Prev 2008;9:671-7.
- Harman JG, Limbird LE. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. New York: McGraw Hill; 1996. p. 886.
- Fromme EK, Eilers KM, Mori M, Hsieh YC, Beer TM. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the quality-of-life questionnaire C30. J ClinOncol 2004;22:3485-90.
- Ioannidis JP, Lau J. Completeness of safety reporting in randomized trials: An evaluation of 7 medical areas. JAMA 2001;285:437-43.
- Rothwell PM. External validity of randomised controlled trials: “To whom do the results of this trial apply?”. Lancet 2005;365:82-93.
- Ladewski LA, Belknap SM, Nebeker JR, Sartor O, Lyons EA,Kuzel TC, et al. Dissemination of information on potentially fatal adverse drug reactions for cancer drugs from 2000 to 2002: First results from the research on adverse drug events and reports project. J ClinOncol 2003;21:3859-66.
- Niraula S, Seruga B, Ocana A, Shao T, Goldstein R, Tannock IF, et al. The price we pay for progress: A meta-analysis of harms of newly approved anticancer drugs. J ClinOncol 2012;30:3012-9.
- Kshirsagar NA, Karande SC, Potkar CN. Adverse drug reaction monitoring in India. J Assoc Physicians India 1993;41:374-6.
- Lumpkin MM. International pharmacovigilance: Developing cooperation to meet the challenges of the 21st century. PharmacolToxicol 2000;86Suppl 1:20-2.
- Alvarez-Requejo A, Carvajal A, Bégaud B, Moride Y, Vega T, Arias LH. Under-reporting of adverse drug reactions. Estimate based on a spontaneous reporting scheme and a sentinel system. Eur J ClinPharmacol 1998;54:483-8.
- Hazell L, Shakir SA. Under-reporting of adverse drug reactions: A systematic review. Drug Saf 2006;29:385-96.
- Prasad R. Diabetes Drug: Maybe I Erred in My Judgement. Chennai:The Hindu; 17 July, 2013. Available from: http://www.thehindu.com/ news/diabetes- drug- maybe-i-erred-in-my-judgement/article4921604.ece. [Last cited on 2013 Jul 30].
- Mallik S, Palaian S, Ojha P, Mishra P. Pattern of adverse drug reactions due to cancer chemotherapy in a tertiary care teaching hospital in Nepal. Pak J Pharm Sci 2007;20:214-8.
- Goyal YN, Solanki KC, Mistry RA, Joshi ND, Singh AP, Gajera MV. Pattern of adverse drug reactions due to cancer chemotherapy in tertiary care teaching hospital in Gujarat. Int J Sci Res 2014;3:333-5.
- Navari RM. Management of chemotherapy-induced nausea and vomiting: Focus on newer agents and new uses for older agents. Drugs 2013;73:249-62.
- Thapaliya K, Shrestha A, Prajapati S, Subedi R, Giri S. Study of pattern of adverse drug reaction due to cancer chemotherapy and their management in hospitalized patient in BP Koirala Memorial Cancer Hospital. J Chitwan Med Coll 2014;4:24-8.
- Sanches Junior JA, Brandt HR, Moure ER, Pereira GL, Criado PR. Adverse mucocutaneous reactions to chemotherapeutic agents: Part I. An Bras Dermatol 2010;85:425-37.
 ).
).