- Corresponding Author:
- A. Semalty
Department of Pharmaceutical Sciences, H.N.B. Garhwal University, Srinagar - 246 174, India
E-mail: semaltyajay@gmail.com
| Date of Submission | 20 March 2006 |
| Date of Revision | 9 August 2007 |
| Date of Acceptance | 7 November 2007 |
| Indian J Pharm Sci, 2007, 69 (6): 741-747 |
Abstract
Although most protein pharmaceuticals are usually formulated as a solution or suspension and delivered by invasive routes such as subcutaneous injections, major efforts in both academic and industrial laboratories have been directed towards developing effective oral formulations and increasing the oral absorption of intact protein through the use of formulations that protect the macromolecule and/or enhance it's uptake into the intestinal mucosa. However, in spite of these major attempts, relatively little progress has been made. For the efficient delivery of peptides and proteins by non-parenteral route, in particular via the gastrointestinal tract, novel concepts are needed to overcome significant enzymatic and diffusion barriers. The properties of protein and peptides, which are of major interest in oral delivery, are highlighted in the article. This article reviews the various problems associated and novel approaches for formulation and development of oral protein and peptide drug delivery systems.
Keywords
Oral protein delivery, protein and peptide, oral insulin
Peptide and polypeptides are low and/or high molecular weight biopolymers, which yield two or more amino acid on hydrolysis. Peptides and polypeptides are the principle component of the protoplasm of cells and are high molecular weight compounds consisting of alpha amino acid connected together by peptide linkages. These proteins serve as enzymes, structural element, hormones or immunoglobulin and are involved in metabolic process, cell growth, immunogenic defense mechanisms and other biological activities [1-4].
Peptides and polypeptides or proteins are an important class of biological substances which are not only the essential nutrients of human body, but some of the polypeptide hormones like insulin are used in treating various diseases resulting from hormonal deficiency [5]. As this use of peptides and polypeptides for systemic treatment of certain diseases is well accepted in medical practice, research activities are being directed towards the synthesis of large quantities by rDNA technology.
The most common route of administration for protein and peptide drug delivery has been parenteral, although many other routes have been tried with varying degree of success. Routes such as intranasal, transdermal, buccal, intraocular, rectal, vaginal and pulmonary route will deliver the drug to the systemic circulation while avoiding transit through the digestive system [6-12]. A major factor that limits the usefulness of these substances for their intended therapeutic application is that they are easily metabolized by plasma proteases when they reach the peripheral circulation. In addition, adverse effects associated with applying these drugs to the pulmonary or the other mucosal surfaces, may be limiting.
Delivering therapeutically active protein and peptides by the oral route has been a challenge and a goal for many decades. Currently only two biotechnology drugs (Interferon alpha and human growth hormone) that can be given orally are known to be in clinical development in the US [13]. For such drugs to be absorbed through the gastrointestinal tract, they must be protected from enzyme and must traverse through the luminal barriers into the blood stream in an unchanged form. This article reviews the problems associated with the oral delivery of proteins and peptides and presents approaches for the formulation of the delivery system for the same.
Absorption Properties of Peptides
Molecular weight and size
Molecular weight and size influence the diffusion of drugs through the epithelial layer. As a general rule very large molecule have lower diffusivities and only small molecules (<75-100 Dalton) appear to cross the barriers rapidly [14]. However permeability falls of markedly as the molecular size increases. Several authors have investigated the effects of the molecular weight upon oral absorption of various hydrophilic compounds [15-17].
Conformation, stereospecificity and immunogenicity
Unlike conventional drugs, peptide drugs generally have primary, secondary and tertiary structures and in solution may adopt several different conformations depending upon their size. It is the prime requisite to preserve the pharmacologically active conformation during the process of formulation and sterilization. The change in conformation can influence membrane permeability. The stereospecificity of the drug must also be preserved since the permeation systems are thought to be stereoslective [18-23]. Peptides are also recognized as often being immunogenic and the use of inert polymers like PEG, PVP and albumin for peptide delivery has been shown to increase resistance to proteolysis and simultaneously decrease peptide immunogenicity [24,25].
Electrostatic charges
Charge distribution on the peptide change may be even more important than the value of the partition coefficient in predicting permeability of peptides through oral mucosa. Terminal charges or zwitterionic peptide have a negative effect on membrane permeability even though the effective partition coefficient is relatively high [25]. The effect of charge density can be modified to promote peptide absorption by changing the pH of the medium and thus the degree of ionization of the peptides.
Physico-Chemical Properties
Solubility and partition coeffi cient
Peptides, being amphoteric, usually have complex solubility versus pH profile. Aqueous solubility of peptide is strongly dependent upon pH, presence of metallic ion, ionic strength and temperature. At isoelectric point the aqueous solubility of peptide is minimal where the drug is neutral or has no net charge. Unless the N-and C- termini are blocked, peptides are very hydrophilic with a very low octanolwater partition coefficient [26-31] (Table 1). Therefore, to improve the absorption of peptides by passive diffusion, their lipophilicity should be increased.
It is generally recognized from human buccal absorption data that the absorption of drugs from whole oral cavity obeys the pH-partition hypothesis which implies a passive diffusion mechanism. Majority of the proteins are destroyed in the very low pH of the gastric region.
| Peptide | Partition coefficient (n-octanol/buffer, pH 7.4) |
|---|---|
| Insulin | 0.0215 |
| Thyrotropin-releasing hormone | 0.0376 |
| Luteinizing hormone-releasing hormone | 0.0451 |
| Glucagons | 0.0633 |
| Substance P | 0.2750 |
| Met-enkephalin | 0.0305 |
| Leu- enkephalin | 1.1200 |
Peptides show very low octanol-water partition coefficient
Table 1: Lipophilicity of selected peptides [38]
Aggregation, self association and hydrogen bonding
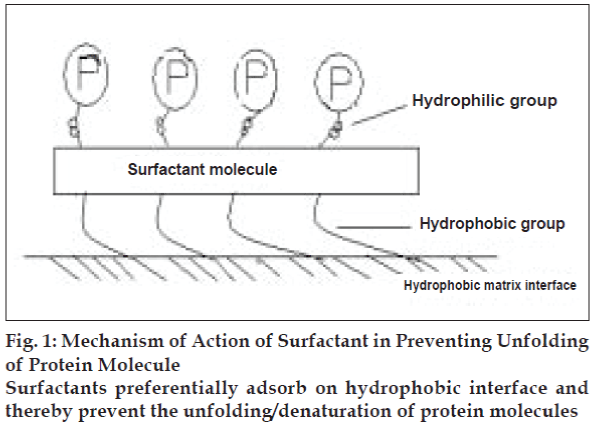
Self-aggregation tendency of peptides modifies their intrinsic properties. Human insulin was found to be more self-aggregating than porcine or bovine insulin32-34. In a study it has been reported that additions of additive like non ionic surfactants (Pluronic F 68) stabilize the peptide formulation against self aggregation (fig. 1).
In aqueous solution, the three dimensional structure of a protein in its native conformation results in more hydrophobic residues being buried within the interior and more hydrophilic amino acid residues exposed to the aqueous solution. However, when the same protein comes into contact with a hydrophobic surface (like delivery matrix interface), there will be an entropic driving force for the hydrophobic residues that are normally buried within the three dimensional structure to interact with the surface and hence causing unfolding or denaturing of protein molecules. Non ionic surfactants and many other additives were found solve the problem by preferential adsorption on hydrophobic interface (Table 2). Intermolecular hydrogen bonding with water decreases the permeability of protein in lipid membrane [35-37].
| Stabilizing additive | Mechanism of action | Protein stabilized | Ref. |
|---|---|---|---|
| Sugars-trehalose, sucrose | Increase Tg thereby enhancing thermal | Collagan, ribonuclease, ovalbomin | 39,40 |
| Maltose, glucose | stability of proteins | ||
| Salts- potassium phosphate, | Increase Tg of proteins and self association | Collagan, ribonuclease, ovalbomin | 39,40 |
| sodium citrate, amm.sulphate | of proteins, reduce the solubility | ||
| Cyclodextrins-hydroxypro- | Not clear; probably by changing the | Porcine growth hormone | 41 |
| pylcyclodextrins | properties of solvent | ||
| Heparin | Increase the unfolding temperature | Acidic fibroblast growth factor | 42 |
| by 15-30° | |||
| Metals - zinc | Complexation | hGH against urea induced denaturation Insulin | 43-45 |
| Chelating agent- EDTA | Complexation and decrease catalytic | Acidic fibroblast growth factor ribonuclease A | 42-46 |
| degradation by metal | |||
| Surfactant - Non ionic- | Preferential adsorption on hydrophobic | NutropinR (r-hGH) with polysorbates; | 47-51 |
| polysorbates | interface of delivery matrix; | hGH loaded PLG polymer matrix | |
| Cationic-cetrimide | |||
| Anionic - SLS | Membrane perturbation |
Various additives serve to stabilize the protein delivery matrix by a variety of mechanisms which are shown in the table with the protein stabilized by them
Table 2: Stabilizing additives in protein delivery
Basis of oral delivery of Proteins
It was observed that the small amount of intact protein and peptide can enter the circulation under normal circumstances [52-54]. After these studies, some finding suggests that at higher peptide dosage the fraction absorbed may be expected to increase due to saturability of the degradation. These finding led the possibility of developing oral peptide delivery system.
Potential problem associated with oral protein delivery
The oral administration of peptide and protein drugs faces two formidable problems. The first is protection against the metabolic barrier in GIT. The whole GIT and liver tend to metabolize proteins and peptides into smaller fragments of 2-10 amino acids with the help of a variety of proteolytic enzyme (proteases), which are of four major types; aspartic protease (pepsin, rennin), cystinyl proteases (papain, endopeptidase), metallo proteases (carboxypeptidase-A, ACE) and serinyl proteases (thrombin, trypsin). The second problem is the absence of a carrier system for absorption of peptides with more than three amino acids.
Approaches to circumvent metabolic barriers
The approaches to circumvent proteolytic action should be based entirely upon the principle sight of degradation of the peptide drug; intracellular, luminal or the brush border. The approaches may include [55]; prodrug approach, co-administration of protease inhibitors, use of penetration enhancers and surfactants, use of carrier system and/or formulation approaches.
Prodrug approach
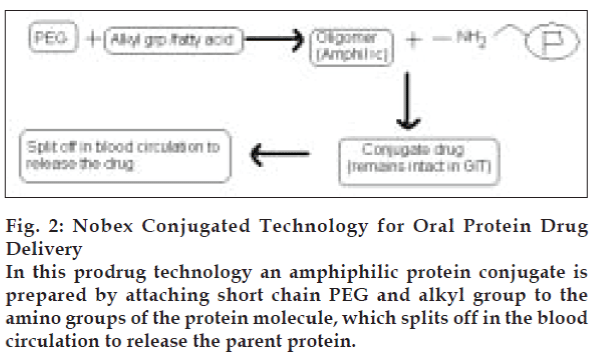
Proteins are labile due to susceptibility of the peptide backbone to proteolytic cleavage, as well as their molecular size and complex secondary, tertiary and sometimes even quaternary structures. Therefore proteins can be modified chemically to give more stable prodrugs with increased plasma half-lives (Table 3). Some strategies for prodrug formation include olefenic substitution, d-amino acid substitution, dehydro amino acid substitution [56], carboxyl reduction, retro inversion modification [57], polyethylene glycol (PEG) attachment to amino group [58] and thio-methylene modification. In a recent technology known as Nobex Technology, an amphiphilic protein conjugate is prepared (fig. 2). This technology reduces self-association, increases penetration and increases compatibility with formulation ingredients than parent drug [59]. By this technology Nobex’s conjugated insulin has also been prepared. In this technology short chain PEG and alkyl group are attached to Lys-29 of beta chain. Prepared conjugated insulin was found to be more absorbed and effective. Calcitonin oligomer prepared by this technology showed increased stability and absorption.
Figure 2: Nobex Conjugated Technology for Oral Protein Drug Delivery
In this prodrug technology an amphiphilic protein conjugate is prepared by attaching short chain PEG and alkyl group to the amino groups of the protein molecule, which splits off in the blood circulation to release the parent protein.
| Parent protein/peptide | Prodrug |
|---|---|
| S-Gonadotropin Releasing Hormone | S-Gn-RH-A |
| Nonopeptide with D-Arg-6 | |
| Growth Hormone | GHRP-6 |
| Luteinizing hormone-releasing hormone | Buserelin, |
| luproreline, gosereline | |
| Vasopressin | Desmopressin |
| Somatostatin | Sandostatin |
Some important Proteins and their respective prodrugs (Chemically modified proteins to give more stable form with increased plasma half-lives) are shown in the table
Table 3: List of prodrugs of proteins/peptides
Protease inhibitors
To alter the environment for maximum enzyme stability, protease inhibitors are co-administered with protein and peptides. Various protease inhibitors have been examined with respect to their ability to suppress proteolytic activity (Table 4). Positive results were observed in the oral absorption of tetragastrin, insulin, arginin, vasopressin, rennin inhibitors [60-63].
| Drug | Protease inhibitor | Result(s) | Ref. |
|---|---|---|---|
| Insulin | Aprotinin, bactracin, bestatin, | Significant reduction in insulin digestion | 64 |
| Camostatmesilate, chymotrypsin inhibitor | and improvement in its intestinal | 65 | |
| FK – 448, sodium glycocholate, soyabean | absorptionprofile. | ||
| trypsin inhibitor | |||
| Insulin | Camostatmesilate | 66 | |
| Plasma glucose levels decreased in a dose | |||
| dependent manner. | |||
| Vasopressin | Aprotinin | Improvement in the activity profile of the drug | 65 |
| And its analogues | |||
| Calcitonin | Camostatmesilate | Significant improvement in calcitonin delivery | 66 |
Co-administration of protease inhibitors with protein and peptides suppress the proteolytic activity of the enzymes and thereby improve the stability and oral bioavailability of proteins
Table 4: Studies of protein drug delivery with protease inhibitors
Use of penetration enhancers
Penetration enhancers are compounds which, when added to a solute, increase its absorption across biological membranes. Peptides and proteins, due to their molecular size, often require penetration enhancers to achieve therapeutically significant levels of luminal absorption [67]. Surfactants are one of the classes of penetration enhancers. Addition of a surfactant can stabilize a protein against denaturation during several stages from incorporation to the release at the site of delivery. Use of surfactants decreases the self-association and absorption of protein on hydrophobic interface of delivery matrix. They increase penetration and stability of protein and peptide formulations. Besides penetration enhancement, sodium glycocholate inhibits leucine amino peptidase and protect insulin from proteolysis [68].
Use of a carrier systems
Special types of carriers are used for the poorly absorbed proteins and peptides, which are unstable in the gastro intestinal lumen for their targeting to a specific tissue or organ. A well designed carrier system protects the drug from the intestinal proteases and localizes the drug at or near the cellular membrane to maximize their driving force for passive permeation [69]. Various novel carrier systems for protein and peptide drug delivery have been studied like lipid vesicles, particulate systems, emulsions, bioadhesive systems etc.
Formulation approaches
A variety of approaches are adopted in formulating oral peptide delivery systems as per the nature of peptide drugs and the delivery matrix. Table 5 shows the general formulation strategies for protein and peptide formulation. An azo polymer, which is stable in GIT but decomposes at the ileocaecal junction have been used for insulin delivery and found to be very promising for oral insulin delivery [70]. Chitosan-EDTAprotease inhibitor conjugates have been used for many peptide delivery [71]. A new class of molecules-N acylated non-α, aromatic amino acid compound was found to increase the absorption of human growth hormone (hGH) by altering the conformation of molecule reversibly and facilitate transport across intestinal mucosa [72]. Several formulations tested for the oral protein delivery include emulsions, liposomes, nanoparticles, soft gelatin coated capsules [73,74].
| Protein’s nature | Formulation approach | |
|---|---|---|
| Unstable in solution | Lyophilization using cryoprotectants and incorporating drug into delivery matrix as a | |
| solid powder. | ||
| Adsorb to delivery matrix (PLG ) | 1. | Incorporation of hydrophilic surfactants (Polysorbate 20/80, Pluronic F.68) |
| 2. | Addition of another protein as a competitor for adsorption surface | |
| High protein concentration required in | 1. | Addition of surfactant to reduce self-association |
| delivery system-prone to aggregation | 2. | Use of less soluble prodrug e.g.- complexation with metal (zinc-insulin) |
| Poor stability at low pH | 1. | Lyophilization |
| 2. | Formulation in high pH buffer | |
| 3. | Addition of soluble basic salt in delivery matrix to neutralize acid degradation | |
| products of delivery matrix | ||
| 4. | Formulation of microporous delivery matrix rather than monolithic device | |
| Heat sensitivity | Using low temperature homogenization encapsulation process. | |
On the basis of the nature of protein, various approaches can be explored to formulate the oral protein drug delivery system.
Table 5: Approaches of formulation of oral protein drug delivery system [81].
General method for production of protein formulations are emulsification, coacervation, extrusion, spray drying and polymerization. In all these processes it is highly emphasized that high stress, high temperature, heat and crosslinking agent must be avoided (or minimized), to ensure the stability during the formulation [75,76]. Special procedures like double emulsion method and Prolease microsphere technology may also be adopted [77]. The Prolease process is a spray method of producing microparticles containing proteins using a cryogenic process. In this method, the protein drug is incorporated as a lyophilized powder, and all manipulations involving the matrix polymer (PEG) and the proteins are performed at low temperature (≤- 80o).
As proteins are more stable in solid state than in liquid, its incorporation in solid form in delivery matrix is advantageous. Spray drying and lyophillization are widely used for formulation of protein and peptide delivery system [78]. In a study, hGH was lyophilized to get stable form with reduction in aqueous solubility. It decreased the potential for degradation during release due to decrease in protein molecule mobility and thereby ensured the stability and improved the bioavailability of orally administered peptide hormone.
Recent Advances In Oral Protein Drug Delivery
Biosante Pharmaceuticals has developed a delivery system based on calcium phosphate to administer an oral form of insulin called CAPIC. Calcium phosphate particles containing insulin was synthesized in the presence of PEG-3350 and modified by aggregating the particles with caseins (the principle protein of milk) to obtain the calcium phosphate-PEG-insulincasein (CAPIC) oral insulin delivery system. The formulation CAPIC was created through a nanoparticulate technology, using microscopic particles of calcium phosphate. Studies in diabetic mice showed that oral insulin administration through the new system was effective in reduction and maintenance of normal blood glucose levels [79,80].
A group of research scientists developed mucoadhesive oral Insulin delivery systems using lectin functionalized complexation hydrogels [82,83]. They developed a class of environmentally responsive complexation hydrogels composed of methacrylic acid grafted with ethylene glycol chains (P(MAA-g-EG)) functionalized with wheat germ agglutinin (WGA) to overcome the challenges of oral administration. The drug carriers were designed to firstly minimize the effects of the harsh environment of the gastrointestinal tract and secondly to target delivery of insulin to the upper small intestine by exploiting the pH shift between the stomach and the upper small intestine. Insulin entrapment in the polymer network was unaffected by the WGA functionalization and loading efficiency was determined to be 75% in both functionalized and unfunctionalized microparticles. Recently, on 27 January 2006, Pfizer Inc. obtained the US FDA approval for launching human insulin (rDNA origin) inhalation powder (Exubera) spray. The product had been introduced in the US market in December 2006 [84].
Conclusions
The scientific community has reached a new stage in the understanding of the properties of peptides and proteins and in the manufacturing of these therapeutic agents. In the past, administration of peptides and proteins was believed to be impossible, while nowadays it is expected that the obstacles for effective delivery of therapeutic peptides and proteins will be overcome and delivery systems with better compliance would be made available to the patients.
References
- Dence JE. Steroids and Peptide: Selected Chemical Aspects for Biology, Biochemistry and Medicine. New York: John Willey and Sons; 1980, p. 89.
- Hey DH, John DI. Amino acids Peptides and Related Compounds, In; Organic Chemistry. Baltimore: University Park Press; 1978, p. 5.
- Matthews DM. Intestinal absorption of peptides. Physiol Res 1975;55:537-607.
- Walker WA, Isselbacher KJ. Uptake and transport of macromolecules by the intestine: Possible role in clinical disorders. Gastroenterology 1974;67:59-70.
- Klostermeyer H, Humble RE. Chemistry and biochemistry of insulin. Agnew Chem Intern 1966;5:807-11.
- Nair M, Chein YW. Development of anticandidal delivery systems: II. Int J Pharm 1993;89:41.
- Lesch CA, Squier CA, Crutchley A, Willams DM, Speight P. the permeability of human oral mucosa and skin to water. J Dent Res 1989;68:1345.
- Rathbone MJ, Drummond BK, Tucker IG. Oral cavity as a site for systemic drug delivery. Adv Drug Del Rev 1994;13:1-22.
- Chein YW, Su KS, Chang SF. editors, Nasal Systemic Drug Delivery. New York: Marcel Dekker Inc; 1989.
- Su KS. Intranasal delivery of peptides and proteins. Pharm Int 1986;7:8-11.
- Van de Donk HJ, Van der Heuvel AG, Zuidema J, Merkus FW. The effects of nasal drops and their additives on human mucociliary clearance. Rhinology 1982;20:127-37.
- De Boer AG, Breimer DD, Mattie H, Pronk J, Gubbens-Stibbe JM. Rectal absorption of drugs. ClinPharmacolTher 1979;1:441.
- Clark AR, Shire SJ. Protein formulation and delivery. In: McNally EJ, editors. Drugs and the Pharmaceutical Science. Vol. 99. New York: Marcel Dekker; 2000. p. 201-12.
- Siegel IA. Permeability of the rat oral mucosa to organic solutes measured in vivo. Arch Oral Biol 1984;29:13-6.
- McMartin C, Hutchinson LE, Hyde R, Peters GE. Analysis of structural requirements for the absorption of drugs and macromolecules from the nasal cavity. J Pharm Sci 1987;76:535-40.
- Maitani Y, Machida Y, Nagai T. Influence of molecular weight and charge on nasal absorption of dextran and DEAE-dextran in rabbits. Int J Pharm 1989;49:23-7.
- Donovan MD, Flynn GL, Amidon GL. Absorption of polyethylene glycols 600 through 2000: The molecular weight dependence of gastrointestinal and nasal absorption. Pharm Res 1990;7:863-8.
- Green PG, Hinz RS, Kim A, Szoka FC, Guy RH. Iontophoretic delivery of a series of tripeptides across the skin in vitro. Pharm Res 1991;8:1121-7.
- Delie F, Letourneux Y, Nisato D, Puisieux F, Couvreur P. Oral administration of peptides: Study of a glycerolipidicprodrug. Int J Pharm 1995;115:45-52.
- Burnham NL. Polymers for delivering peptides and proteins. Am J Hosp Pharm 1999;51:210-8.
- Palm K, Luthman K, Ungell AL, Strandlund G, Artursson P. Correlation of drug absorption with molecular surface properties. J Pharm Sci 1996;85:32-9.
- Ho NFH, Day JS, Barsuhn CL, Burton PS, Raub TJ. Biophysical model approaches to mechanistic transepithelial studies of peptides. J Control Release 1990;11:3-24.
- Merkle HP, Wolany GJ. Intraoral peptide absorption. In: Audus KL, Raub TJ, editors. Biological barriers to protein delivery. New York/ London: Plenum; 1993. p. 131-60.
- Burnham N. Polymers for delivering peptides and proteins. Am J Hosp Pharm 1994;51:210-8.
- Reddy IK, Madan PL. Controlled/sustained release: Proteins and peptides. Pharm Times 1992;58:132.
- Siegel IA, Izutsu KT, Watson E. Mechanisms of nonelectrolyte penetration across dog and rabbit oral mucosa in vitro. Arch Oral Biol 1981;26:357-61.
- Corbo DC, Huang YC, Chein, YW. Nasal delivery of progestational steroid in ovariectomized rabbits, II: Effect of penetrant hydrophilicity. Int J Pharm 1989;50:253-60.
- Huang CH, Kimura R, Bwarshi-Nassar R. Mechanism of nasal absorption of drugs, II: Absorption of l-tyrosine and the effect of structural modiÆcation on its absorption. J Pharm Sci 1985;74:1298-301.
- Hirai S, Yashiki T, Matsuzawa T, Mima H. Absorption of drugs from the nasal mucosa of rat. Int J Pharm 1981;7:317-25.
- Hansun LB, Christrup LL, Bundgaard H. Enhanced delivery of ketobemidone through porcine buccal mucosa in vitro via more lipophilic ester prodrugs. Int J Pharm 1992;88:237-42.
- Hansun LB, Jorgenson A, Rasmussen SN, Christrup LL, Bundgaard H. Buccal absorption of ketobemidone and various ester prodrugs in the rat. Int J Pharm 1992;88:243-50.
- Toniolo C, Bonora GM, Stavropoulos G, Cordopatis P, Theodoropoulos D. Self-association and solubility of peptides: Solvent-titration study of N-protected C-terminal sequences of substance P. Biopolymers 1986;25:281.
- Touitou E. Enhancement of intestinal peptide absorption. J Control Release 1992;21:139-44.
- Banga AK, Chein YW. Systemic delivery of therapeutic peptides and proteins. Int J Pharm 1988;48:15-50.
- Conradi RA, Hilgers AR, Ho NF, Burton PS. The influence of peptide structure on transport across caco-2 cells. Pharm Res 1992;8:1453-60.
- Conradi RA, Hilgers AR, Ho NF, Burton PS. The influence of peptide structure on transport across caco-2 cells, II: Peptide bond modification which results in improved permeability. Pharm Res 1992;9:435-9.
- Burton PS, Conradi RA, Hilgers AR, Ho NFH, Maghiora LL. The relationship between peptide structure and transport across epithelial cell monolayers. J Control Release 1992;19:87-98.
- Lee VH. Enzymatic barriers to peptide and protein absorption. Crit Rev Ther Drug Carrier Syst 1988;5:69-97.
- Arakawa T. The stabilization of P-lactoglobulin by glycine and NaC1. Biopolymers 1989;28:1397.
- Arakawa T, Kita Y, Carpenter JF. Protein-solvent interactions in pharmaceutical formulations. Pharm Res 1991;8 :285-91.
- Charman SA, Mason KL, Charman WN. Techniques for assessing the effects of pharmaceutical excipients on aggregation of porcine growth hormone. Pharm Res 1993;10:954-62.
- Tsai PK, Volkin DB, Dabora JM, Thompson KC, Bruner MW, Gress JO, Matuszewska B. Formulation design of acidic fibroblast growth factor. Pharm Res 1993;10:649-59.
- Gonda I. Inhalation therapy with recombinant human deoxyribonuclease I. Adv Drug Deliv Rev 1996;19:37-46.
- Johnson OL, Cleland JL, Lee HJ. A month-long effect from a single injection of microencapsulated human growth hormone. Nat Med 1996;2:795-9.
- Izutsu KI, Yoshioko S, Kojima S. Physical stability and protein stability of freeze-dried cakes during storage at elevated temperatures. Pharm Res 1994;11:995-9.
- Townsend MW, Byron PR, Deluca PP. Effects of. formulation additives on the degradation of freeze-dried ribonuclease A. Pharm Res 1990;7:1086-91.
- Horbett TA. Stability of Protein Pharmaceuticals. Part A. In: Ahem TJ, Manning MC, editors. Chemical and Physical Pathways of Protein Degradation. New York: Plenum Press; 1992. p. 195.
- Costantino HR, Langer R, Klibanov AM. Moisture-induced aggregation of lyophilized insulin. Pharm Res 1994;11:21-9.
- Thurow H, Geisen K. Stabilisation of dissolved proteins against denaturation at hydrophobic interfaces. Diabetolgia 1984;27:212-8.
- Mumenthaler M, Hsu CC, Pearlman R. Feasibility study on spray drying protein pharmaceuticals by improved optical methods. Pharm Res 1994;11:12-20.
- Cleland JL, Jones AJ. Development of stable protein formulations for microencapsulation in biodegradable polymers: Proceedings of an International Symposium on Controlled Release of Bioactive Materials. Seattle: Controlled Release Society; 1995.
- Gardener MLG. Intestinal assimilation of intact peptides and proteins from the diet: A neglected field? BiolRev 1984;59:289-331.
- Gardener MIG, Illingworth KM, Kelleher J, Wood D. Intestinal absorption of the intact peptide carnosine in man and comparison with intestinal permeability to lactulose. J Physiol 1991;439:411.
- Gebert G. For enteral absorption intact β protein molecules. AlgeminMedizin 1991;19:125.
- Jain GK. Oral Protein Drug Delivery. In: Jain NK, editor. Advances in Controlled and Novel Drug Delivery. New Delhi: CBS Publishers; 2001. p. 232.
- Wyvratt, MJ, Patchett AA. Recent developments in the design of angiotensin-converting enzyme inhibitors. Med Res Rev 1985;5:483-531.
- Brewster D, Waltham K. TRH degradation rates vary widely between different animal species. BiochemPharmacol 1981;30:619-22.
- Davis S, Abuchowski A, Park YK, Davis FF. Alteration of the circulating life and antigenic properties of bovine adenosine deaminase in mice by attachment of polyethylene glycol. ClinExpImmunol 1981;46:649-52.
- Christopher HP. Nobex Corporation: Crossing Barrier for Better. Drug Delivery 2003;3:12.
- Jennewein HM, Waldeck F, Konz W. The absorption of tetragastrin from different sites in rats and dogs. Arzneimittelforschung 1974;24:1225-8.
- Kidron M, Bar OJ, Berry EM, Ziv E. Alteration of the paracellular space of enterocytes with glucose to modulate the passive intestinal absorption of insulin. Life Sci 1982;31:2837.
- Saffran M, Bedra C, Kumar GS, Neckers DC. Vasopressin: A model for the study of effects of additives on the oral and rectal administration of peptide drugs. J Pharm Sci 1988;77:33-8.
- Takaori K, Burton J, Donowitz M. The transport of an intact oligopeptide across adult mammalian jejenum. Biochem Biophys Res Commun 1986;137:682-7.
- Morishita M, Morishita I, Takayama K, Machida Y, Nagai T. Sitedependent effect of aprotinin, sodium caprate, Na2EDTA and sodiumglycocholate on intestinal absorption of insulin. Biol Pharm Bull 1993;16:68-72.
- Lueben HL, Lehr CM, Rentel CO, Noach AB, Boer AG, Verhoel JC, et al. Bioadhesive polymers for the peroral delivery of peptide drugs.J Control Rel 1994;29:329-38.
- Tozaki H, Nishioka J, Komoike J, Okada N, Fujita T, Muranishi S, etal. Enhanced absorption of insulin and (Asu(1,7)) eel-calcitonin usingnovel azopolymer-coated pellets for colon-specific drug delivery. J Pharm Sci 2001;90:89-97.
- Vyas SP, Khar RK. Controlled Drug Delivery. New Delhi: VallabhPrakashan; 2002. p. 524.
- Hirai S, Yashiki T, Mima H. Absorption of drugs from the nasal mucosa of rat. Int J Pharm 1981;9:317-25.
- Chen H, Langer R. Oral particulate delivery: Status and future trends. Adv Drug Deliv Rev 1998;34:339-50.
- Saffran M, Kumar GS, Savariar C, Burnham JC, Williams F, Neckers DC. A new approach to the oral administration of insulin. Science 1986;233:1081.
- Bernkop-Schnurch A, Scerbe-Saiko A. Synthesis and in vitro evaluation of chitosan-EDTA-protease-inhibitor conjugates which might be useful in oral delivery of peptides and proteins. Pharm Res 1998;15:263-9.
- Leon-Bay A. Ho KK, Agarwal R, Baughman RA, Chaudhary K, DeMorin F, et al. 4-{4-[(2-Hydroxyben-zoyl)amino]phenyl}butyric acid as a novel oral delivery agent for recombinant human growth. hormone. J Med Chem 1996;39:2571-8.
- Oppenheim RC, Stewart NF, Gordon L, Patel HM. The Production and Evaluation of Orally Administered Insulin Nanoparticles. Drug Develop Ind Pharm 1982;8:531-46.
- Touitou E, Rubinstein AA. Targeted enteral delivery of insulin to rats. Int J Pharm 1986;30:95-99.
- Lewis DH. Biodegradable Polymers as Drug Delivery System. In: Chasin M, Langer R, editors. Biopolymers. New York: Marcel Dekker; 1990. p. 8-24.
- Tabata Y, Langer R. Polyanhydride. microspheres that display near-constant release of water-soluble model drug. compounds. Pharm Res 1993;10:391-9.
- Gombotz WR, Healy MS, Brown LR. US Patent No. US5019400, 1991.
- Hanson MA, Roun SK. Stability of Protein Pharmaceuticals. Part B. In: Ahern, TJ, Manning MC. editors. In Vivo Pathways of Degradation and Strategies for Protein Stabilization. New York: Plenum Press; 1992. p. 209.
- News release. New delivery system to administer insulin orally. Pharm Tech 2003;27:17.
- Morçöl T, Nagappan P, Nerenbaum L, Mitchell A, Bell SJ. Calcium phosphate-PEG-insulin-casein (CAPIC) particles as oral delivery systems for insulin. Int J Pharm 2004;277:91-7.
- Johnson OL. Oral protein drug delivery In: McNally EJ, editors. Protein Formulation and Delivery. New York: Marcel Dekker; 2000. p. 251.
- Kristy MW, Peppas NA. [Last accessed on 2005 Oct 28]. Available from: http://aiche.confex.com/aiche/2005/preliminaryprogram.
- Peppas NA. Devices based on intelligent biopolymers for oral protein delivery. Int J Pharm 2004;277:11-7.
- News Section, First human insulin inhalation powder approved by USFDA. Nat Med J India 2006;19:53.