- *Corresponding Author:
- Zhichun Du
Department of Forensic Medicine, School of Criminal Justice, East China University of Political Science and Law, Shanghai 200042, China
E-mail: 18955299399@163.com
| This article was originally published in a special issue, “Recent Progression in Pharmacological and Health Sciences” |
| Indian J Pharm Sci 2024:86(2) Spl Issue “1-13” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Gamma-hydroxybutyric acid is a kind of endogenous short-chain organic fatty acid, which has pharmacological effects such as euphoric and sedative, but its clinical use is strictly limited due to its high toxicity and easy addiction. In recent years, gamma-hydroxybutyric acid has been abused significantly and even used in drug-assisted crimes. It is difficult to identify gamma-hydroxybutyric acid because of its physiological presence in vivo and it has fast entry and exit characteristics. In vivo, gamma-hydroxybutyric acid is synthesized by the biotransformation of gamma-aminobutyric acid, gamma butyrolactone and 1,4-butanediol, and is metabolized through the gamma-hydroxybutyric acid-gamma-aminobutyric acid, gamma-hydroxybutyric acid-succinic acid-tricarboxylic acid cycle, alpha- and beta-oxidation of gamma‐hydroxybutyrate, and xenobiotic metabolism of gamma-hydroxybutyric acid. A variety of gamma-hydroxybutyric acid metabolism-related compounds are produced in the metabolic process, among which, 2,3-dihydroxybutyric acid, 3,4-dihydroxybutyric acid and glycolic acid are stable in urine, easy to identify and have a long detection window, which is expected to solve the difficult problem of gamma‐hydroxybutyrate identification. The biosynthesis, catabolism and metabolism-related markers of gamma-hydroxybutyric acid were systematically and comprehensively sorted out and summarized in order to provide theoretical reference for related studies on gamma-hydroxybutyric acid identification.
Keywords
Gamma-hydroxybutyric acid, biosynthesis, biological metabolic pathway, biomarkers
Gamma (γ)-Hydroxybutyric acid (GHB) is a central nervous system inhibitory short-chain organic fatty acid with neurotransmitter and neuromodulatory functions[1], which is found in the brain of mammals, in tissues and organs such as the heart, kidneys, liver, skeletal muscle and brown fat[2]. In 1960, GHB was first synthesised by French scientists and used as an anaesthetic[3]. In 1970s and 1980s, GHB was reported to increase the release of growth hormone and was used by body-builders as a dietary supplement to steroids[4]. After 1990, GHB was used as a sleep aid and recreational drug. Subsequently it was found that users of GHB were prone to toxic reactions and even dependence and withdrawal symptoms with prolonged use, so GHB gradually lost its medical value. However, because of its euphoric and sedative pharmacological effects, GHB has tended to be abused in recent decades[5]. The use of GHB is particularly evident in drug-assisted sexual offences. As a result, GHB was banned in the United States in 1990 and was classified as a Schedule I controlled substance by Food and Drug Administration (FDA) in 2000, followed by European countries. In 2007, GHB was placed under control as a class I psychotropic substance in China, and in 2021, its precursor, γ-Butyrolactone (GBL), was also placed under control as a chemical subject to drug control.
Although GHB abuse is less reported than other substances of abuse, the number of people using it as a recreational pastime is increasing[6]. A comparison of the relative propensity to abuse triazolam, pentobarbital and GHB showed that it had the highest rate of overdose-induced intoxication, although the level of abuse was some-where between the first and second[7]. Additionally, because GHB is naturally present in the body and is rapidly metabolized, its detection in blood and urine takes only 6 h and 12 h, respectively[8]. As a result, GHB identification is often difficult to distinguish from its endogenous origin and rationalise its findings[9].
Currently, the study of biomarkers related to GHB metabolism is a hot topic in forensic toxicology research, and a large body of literature has focused on this aspect of research, and some progress has been made. The study of biomarkers requires the identification of the biometabolic pathways of GHB in vivo in order to establish the theoretical relationship between biomarkers and GHB blood concentrations. However, the current studies on GHB biometabolism are scattered, however due to the lack of systematic and comprehensive theoretical studies, this paper attempts to comprehensively sort out and summarize the major items of GHB in order to outline the complete biometabolic pathways and markers of GHB in vivo, and to lay a solid theoretical foundation for GHB identification-related studies.
Formation Mechanism of MDR
The formation mechanism of MDR is complex, which mainly includes transmembrane transporters such as Permeability-glycoprotein (P-glycoprotein), MDR Protein (MRP) and Lung Resistance Protein (LRP) mediated drug effective mechanism, abnormal enzyme activity mediated MDR, enhancement of Deoxyribonucleic Acid (DNA) repair function, increased apoptotic resistance, increased protein kinase C activity, MRP overexpression, etc.[7]. The main mechanism of cancer MDR is shown in fig. 1, and the key signaling pathways to overcome MDR through the regulation of natural drug derived components are shown in fig. 2.
Molecular Structure and Pharmacological Toxicity Profile
The molecular structure of GHB is similar to that of γ-Aminobutyric Acid (GABA), with only one hydroxyl group replacing one of the amino groups of GABA, with the structural formula HO-CH2-CH2-CH2-CH2-COOH. GHB exists in pure form as a colourless, odourless liquid or as a white sodium salt crystal powder, often dissolved in water, beverages or alcohol[10]. The exact pharmacological effects and physiological metabolic mechanisms of GHB in humans have not yet been investigated[11].
It is generally accepted that GHB acts on GABAB receptors, some GABAA subtype receptors and GHB-specific receptors to produce its pharmacological effects[12]. GHB is a biphasic dose-dependent drug with toxic symptoms very similar to those of intoxication. Due to the steep dose-response curve and the narrow range of safe drug use, small dose deviations can cause toxic reactions[13]. Doses between 0.5 g and 1.5 g can produce particularly stimulating, anxiety-relieving and euphoric effects; doses up to 2.5 g can produce significant aphrodisiac and libido-increasing effects; high doses can produce severe sedation and narcotic sleep, and >4 g may produce serious consequences such as coma and respiratory depression[14,15]. The mechanism of GHB intoxication mainly inhibits central nervous and respiratory system functions and less frequently affects cardiovascular and gastrointestinal system functions[16].
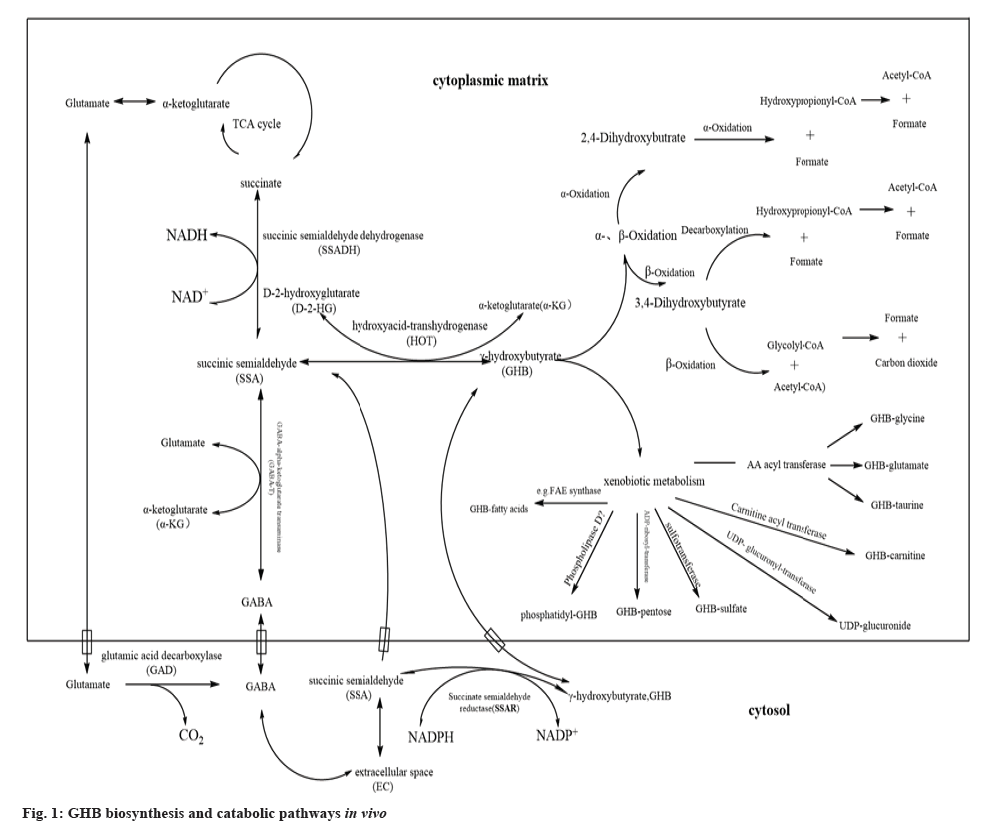
In Vivo Biosynthetic Pathways
GABA biosynthesis of GHB in vivo:
In the physiological state, endogenous GHB in humans is mainly produced by the metabolism of GABA[17] (fig. 1). In vitro experiments have confirmed that experimental mouse brain homogenates can convert glutamate to GHB. 0.125 % of glutamate was converted to GHB within 60 min when the concentration of glutamate in the brain of experimental mice was 10-3 M, at which point the GHB concentration was approximately 10-6 M[18]. In this process, Glutamic Acid Decarboxylase (GAD) is a key enzyme in the metabolism of glutamate to GABA, which has a very similar distribution to GABA in vivo and is found not only in brain but also in the non-neural peripheral tissues such as the liver, kidney and pancreas[19]. In the GABA metabolic bypass, glutamate in the brain cytoplasm is converted to GABA in the presence of GAD and Carbondioxide (CO2) is produced[20]. In the mitochondria of brain cells, GABA is produced in response to GABA-Transaminase (GABA-T), a pyridoxal-5'-phosphate-dependent cofactor, and Succinic Semialdehyde (SSA)[21]. The enzymatic degradation reaction has also been shown to be reversible[22].
The Nicotinamide Adenine Dinucleotide Phosphate Hydrogen (NADPH) dependent enzyme that converts SSA to GHB is located primarily in the cytoplasm of the brain. When SSA enters the brain cytoplasm it is reversibly converted to GHB by the enzymatic action of NADPH-dependent SSA Reductase (SSAR)[23]. In vitro experiments revealed that GHB could not be converted to SSA in the brain cytoplasm at pH 7.4, whereas trace amounts of GHB were converted to SSA at pH 9.5 and with the involvement of NADPH[23], thus suggesting that the enzymatic conversion of SSA to GHB reversible reaction is dominated by the forward reaction.
Although the biosynthetic precursor compound for GHB has been identified as GABA, indications are that GABA may not be the only precursor compound for GHB biosynthesis[24,25]. Because if GABA was the sole source of substrate for GHB biosynthesis, the regions of distribution of GABA and GHB in humans would be identical, but GHB concentrations in rat kidney and brown fat are 10 times higher than GHB concentrations in brain[24] and the regions of distribution of GHB and GABA in the brain are also inconsistent, suggesting that GABA is not the only source of substrate for GHB biosynthesis[25].
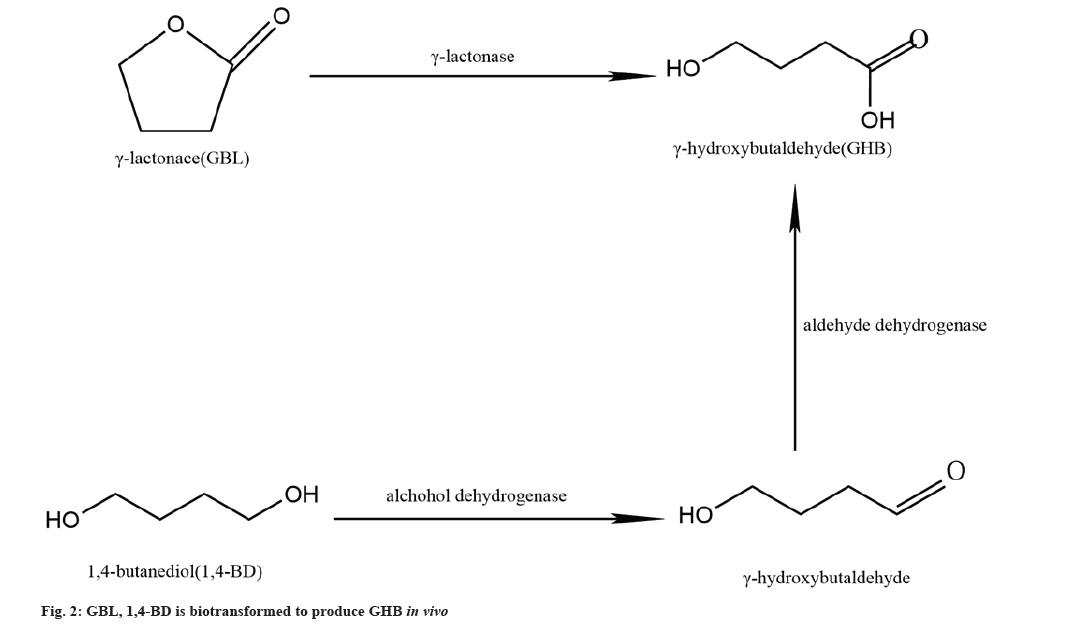
Biosynthesis of GHB from GBL and 1,4-Butanediol (1,4-BD) in vivo:
In vitro, both GBL and 1,4-BD are relatively common industrial reagents that can be converted to GHB. Although the endogenous presence of these compounds has not been detected in humans, endogenous GBL and 1,4-BD has been detected in the brain and liver of experimental rats, respectively[26]. Bergelson et al.[27] suggest that this endogenous 1,4-BD in rat liver is actually a neutral diol ester produced by the metabolism of triglycerides in the liver. In addition, this neutral diol can also be produced in maize seeds and soil yeasts. However, the source of endogenous GBL remains unclear for the time being. Following intraperitoneal injection of [13C] GABA and [13C] 1,4-BD in experimental rats, GBL was detectable in the brain although [13C] GBL was not detected, suggesting that GBL does not originate from GHB and not due to the metabolic production of GABA and 1,4-BD[26]. Thus, the substrate for in vivo endogenous GHB biosynthesis may be derived from GBL and 1,4-BD.
In the liver, Alcohol Dehydrogenase (ADH), the rate-limiting enzyme for the metabolism of 1,4-BD to GHB, plays a key role in the enzymatic reaction, but fomepizole has a significant inhibitory effect on ADH[28]. Disulfiram (DSF) reduces the reaction of 1,4-BD metabolism to GHB by 60 % and it does not completely inhibit the metabolism of 1,4-BD suggesting that 1,4-BD production GHB has another enzyme involved which metabolises GHB aldehyde, which has since been identified as Acetaldehyde Dehydrogenase (ALDH)[29]. 1,4-BD is rapidly converted to GHB by the action of both ADH and ALDH (fig. 2). In vitro, 1,4-BD in rat brain homogenate cultures could also be converted to GHB, but pyrazole and DSF, which are the inhibitors of ADH and ALDH, could not block the conversion of 1,4-BD to GHB, suggesting that its conversion reaction in the brain may not be the same as that in the liver[30]. However, ethanol can competitively block the conversion of 1,4-BD to GHB in brain and liver, and some studies have found that the sedative and hypnotic effects of 1,4-BD are mediated pharmacologically through its conversion to GHB in vivo[31]. GHB was not found in the striatal microdialysis fluid of experimental rats after intra-peritoneal injection of 1,4-BD preceded by fomepizole[32]. Thus, in the brain 1,4-BD may not be converted to produce GHB.
A specific γ-lactonase, which is present in human and experimental rat blood and in rat liver microsomes in the form of a soluble protein, specifically hydrolyses aliphatic short-chain γ-lactones[33]. GBL is rapidly and completely absorbed after oral administration[34]. GHB is rapidly hydrolysed in vivo by γ-lactonase (fig. 2), and this enzymatic reaction occurs only in the blood and liver, no similar enzymatic hydrolysis reaction is found in the brain[35].
In addition, Carter et al.[36] found that after direct intraventricular injection of GBL and 1,4-BD in adult male Sprague-Dawley (SD) rats where the rats’ behavior was not active. However, baclofen and GHB showed a dose-dependent reduction in behavioural reactivity regardless of intracerebral or peritoneal administration, further suggesting that GBL and 1,4-BD may not be converted into GHB in the brain. Thus, the enzymatic conversion of GBL and 1,4-BD to GHB occurs only in tissues other than the central nervous system.
GHB In Vivo Biodegradable Metabolic Pathways and their associated Biomarkers
GHB biodegradable metabolic pathways:
Exogenous GHB enters the body by a variety of routes, mainly through oral absorption into the bloodstream by the gastrointestinal system, but also partially through injection or inhalation into the lungs[37]. GHB enters the body and can be transported throughout the body via the erythrocyte membrane into the red blood cells[38], it can also cross the blood-brain barrier and enter the brain[39]. After entering the body it is rapidly broken down and metabolised, with <2 % of GHB being eliminated in the urine in its prototype state[40]. The catabolism of GHB in vivo is extremely complex and it is thought that there are 3 to 4 metabolic pathways due to which the exact metabolic pathway remains somewhat controversial[41,42].
SSA as a key intermediate in the metabolism of GHB: It is generally accepted that GHB catabolism is first converted to SSA by the action of GHB Dehydrogenase (GHBDH), which is the main metabolite of endogenous GHB degradation and metabolism in vivo[43]. The source of SSA in the mitochondrial matrix of liver and brain is threefold: From the extracellular gap into the inner mitochondrial membrane; conversion of GHB and ketoglutarate to hydroxyglutarate and SSA via Hydroxyacid-Oxoacid Transhydrogenase (HOT) and conversion of GHB to SSA by SSAR in the cytoplasm[20] (fig. 1). In hepatic mitochondria, GHB generates SSA via NAD(P)+-dependent oxidation of GHBDH[44]. GHBDH also has NADP+-dependent D-glucuronide reductase (aldo-keto reductase 1A1) activity, which has been reported to be activated upon ingestion of high concentrations of exogenous GHB and accounts for 82 % of the total activity of GHBDH[45]. Thus, upon entry of exogenous GHB into the body, the dehydrolysis of GHB also converts D-glucuronide to L-gulonic acid, and the D-glucuronide catabolic pathway in turn directly regulates the catabolism of GHB[46]. When D-glucuronide and L-gulonic acid were injected into the body, respectively, the half-life of GHB in plasma and tissue cells was increased or decreased by 33 %, respectively, implying the existence of an enzymatic reaction other than the GHBDH reaction in the body to convert GHB to SSA[47]. In addition, when NADP+-dependent GHBDH activity was inhibited in the cytoplasm of experimental rat kidneys by specific antibodies or valproic acid, it was found that 50 % of [1-14C] GHB could still be metabolized to 14 CO2, whereas the same specific antibody or valproic acid added to filtered mitochondrial debris from brain and kidney cells did not affect [1-14C] GHB catabolism to produce [14C] containing SSA and CO2[48].Since, this enzyme has been shown to be an oxidoreductase with HOT, which is mainly found in the kidney, liver and brain. It is found mainly in the mitochondria of cells such as the kidney, liver and brain[49].
HOT is a NAD+ or NADP+ cofactor-independent oxidoreductase which is active only in the presence of both hydroxy and oxygenated acids. D-2-Hydroxyglutarate (D-2HG), or the 1st two compounds in the presence of both Kaufman et al.[49] and Struys et al.[50] confirmed the presence of HOT in human liver and fibroblasts was confirmed, and it was also found that the enzymatic activity was 5 times higher in the opposite direction when GHB and Alpha-Ketoglutaric acid (α-KG) were used as substrates to produce D-2HG and SSA. But only GHB could be produced in the presence of D-2HG and SSA as substrates, suggesting that this may be due to the inhibition of HOT activity by SSAR. In summary, endo- and exo-derived GHB can be metabolised in vivo to produce SSA by 2 pathways, one through the action of GHBDH in the cytoplasm and the other through the action of HOT in the mitochondria.
GHB-GABA metabolic pathway: In the brain, GHB can be converted to GABA, but not via the GABA bypass pathway following the Tricarboxylic Acid (TCA) cycle, but rather by converting SSA to GABA in the presence of GABA-T (fig. 1). Vayer et al.[51] studied that the addition of (3H)-labelled GHB to in vitro cultures of rat cerebellar sections revealed that [3H]-GABA could be detected in the cultures, but the addition of SSA Dehydrogenase (SSADH) inhibitor did not result in the accumulation of [3H]-GABA. GABA production was effectively blocked by the addition of the GABA-T inhibitor, demonstrating that GHB is metabolised in the cerebellum by transamines rather than by the TCA cycle.
During this transamine metabolism, SSA is converted to GABA via the reverse enzymatic reaction of GABA-T degradation of GABA, which in turn forms the GHB-GABA metabolic pathway. Therefore, it has been suggested that the reduced glutamate biosynthesis of GABA in the brain following GHB administration in experimental rats can be reasonably explained by the metabolic pathway of GHB synthesis of GABA. This is because in vivo GABA concentrations correlate with GAD activity and not so much with GABA-T activity[52]. GHB increases the concentration of GABA in the body through the GHB-GABA metabolic pathway, which in turn can affect GAD activity. However, it has also been suggested that while GABA-T has the ability to reverse the reaction in vitro. This reverse enzymatic reaction is often difficult to occur in vivo because both SSADH and GABA-T are located in the mitochondrial matrix[53]. SSADH is highly susceptible to converting SSA to Succinic Acid (SA) and reducing SSA concentration, in turn prevents the enzymatic reaction of SSA reverse to GABA. Because of the widespread presence of SSADH in the brain[54], it can be inferred that the GHB-GABA metabolic pathway in the brain may not be the main pathway of GHB biometabolism.
GHB-SA-TCA circulating metabolic pathway: In vivo, the catabolic pathway of GHB was investigated by using C-14-GHB isotope labelling, which revealed that half of the exogenous C-14-GHB was catabolically cleared in <5 min, but also revealed an increase in radioactive C-14 isotopes in the TCA cycle and SA in brain neuronal cells[55]. SA eventually enters the TCA cycle to metabolize CO2 and water[56] which in turn forms the GHB-SA-TCA metabolic pathway (fig. 1).
The metabolism of GHB to SA requires a 2-step enzymatic reaction, where the 1st step involves producing SSA in the presence of GHBDH while the 2nd step involves producing SA in the presence of SSADH. Both dehydrogenases are sensitive to inhibitors such as valproate, salicylate, α-ketoisocaproate and phenyl acetate, but GHBDH is more sensitive to these inhibitors and the 1st oxidative step in the metabolism of SA from GHB has significantly inhibited their following use[47]. However, SSADH is the key enzyme in the GHB-SA-TCA cycle metabolic pathway. When SSADH is inhibited, it can effectively block the metabolism of 2 central neuroinhibitory transmitters, GHB and GABA, in vivo, resulting in significant accumulation of GHB and GABA in vivo.
Patients with a rare genetic disorder, SSADH deficiency, also known as GHB aciduria, have significantly elevated levels of GHB in their blood and urine. The disease has been found to be caused by a defect in SSADH due to a mutation in the Aldehyde Dehydrogenase 5 family member A1 (ALDH5A1) gene, which encodes SSADH, resulting in a significant accumulation of GHB and GABA in the body due to impaired metabolism[57]. The impaired metabolism of GHB caused by SSADH deficiency further suggests the exact mechanism by which GHB is metabolized in vivo via the GHB-SA-TCA metabolic pathway.
α and Beta (β) oxidative metabolic pathways of GHB: In vivo, α or β oxidation of fatty acids is an important mode of their degradation in vivo, and a similar mode of degradation may exist for GHB as a short-chain fatty acid. If this hypothesis turns to be true, then GHB will eventually lose carbon atoms on C1 and C1-C2 on the carbon chain after α or β oxidation in vivo, respectively, and it is highly likely that 2 intermediate compounds, 2,4-Dihydroxy Butyric Acid (OH-BA) and 3-Oxo-4-Hydroxybutyric Acid (3,4-OH-BA), will be formed during the oxidation process. Lee et al.[58] found S-3,4-OH-BA and Glycolic Acid (GA) concentrations increasing in urine after a single dose of 1 g GHB was taken orally by 4 adults (M:F/1:1), and also found the presence of 4-OH-BA which was a kind of hydroxy-epoxy isomers in excreted urine. This data strongly suggests that GHB is metabolised by β-oxidation in vivo. Although 3,4-OH-BA is available in humans through food[59], but the corresponding increase in concentration of 3,4-OH-BA in urine and the presence of 3,4-OH-BA after GHB administration are sufficient evidence that the urinary 3,4-OH-BA is derived from the in vivo metabolism of GHB. Zhang et al.[41] further investigated that GHB is metabolised in vivo via the TCA cycle and the GABA pathway, in addition to β-oxidation to produce glycolyl Coenzyme A (CoA) and acetyl-CoA and two other parallel metabolic pathways. One is the removal of the C1 carbon atom from the GHB carbon chain by α-oxidation, and the other is the removal of the carbon atom from C4, but its exact mechanism is not known.
Sadhukhan et al.[42] added 13C-labelled [1,2,3,4-13C]-GHB to rat liver homogenates for incubation and found that only 8 %±2 % of the [1,2,3,4-13C]-labelled SA entered the TCA cycle and another 59 %±6 % of [1,2,3,4-13C] was converted to GABA. The remaining GHB undergoes two pathways, α-oxidation and β-oxidation to produce 2,4-dihydroxybutanoyl CoA and 3,4-dihydroxybutanoyl CoA, respectively, with the β-oxidation metabolic pathway dominating, being 16.8 times more catabolic than the α-oxidation pathway. Further studies revealed that 2,4-dihydroxybutanoyl-CoA was again α-oxidized to produce 3-hydroxypropionyl-CoA and formate, and 3-hydroxypropionyl-CoA was again metabolized to acetyl-CoA and formate. 3,4-dihydroxybutanoyl-CoA was again β-oxidized to produce glycolyl-CoA and acetyl-CoA. Then, glycolyl-CoA was metabolised to formate and CO2.
GHB, a short-chain fatty acid and can be metabolised in human mitochondria through α- and β-oxidation, respectively, in three main ways as α-α-oxidation pathway; β-β-oxidation pathway and β-oxidation-decarboxylation pathway (fig. 1).
Xenobiotic metabolism pathway of GHB: Throughout life, humans continuously ingest exogenous compounds such as drugs, pesticides and food additives, commonly referred to as "xenobiotic substances". Xenobiotic substances follow a unique enzymatic detoxification metabolic pathway in the body called xenobiotic metabolism, which occurs mainly in the liver and is divided into phase I and phase II metabolism[60]. The phase I metabolism is mainly a molecular terminal "oxygenation" reaction, while the phase II metabolism is a "binding reaction" between xenobiotics and endogenous compounds. The 2 phase enzymatic reaction produces less toxic, water-soluble, highly polar form of the xenobiotic conjugate, which is then eliminated from the body via bile, sweat and urine.
Ethanol is a common xenobiotic that is ingested by humans. Some of the ethanol is excreted through the metabolism of xenobiotic substances by the enzymes such as ethanol dehydrogenase and Uridine Diphosphate (UDP)-glucuronidase, which produces Ethyl Glucuronide (EtG) and Ethyl Sulfate (EtS). EtG and EtS are often used as biomarkers for the detection of ethanol concentrations because they are stably detected in urine[61]. Following the ethanol xenobiotic metabolic pathway, endo- and exo-derived GHB may also undergo a similar xenobiotic metabolic pattern in vivo. By testing the theoretical hypothesis, Petersen et al.[62] found that GHB-glucuronide (Gluc) was detected in urine, confirming that GHB has a similar xenobiotic metabolic pathway to ethanol in vivo.
In addition, Harrington et al.[63] found that the drugs ritonavir and saquinavir, which are used to treat Human Immunodeficiency Virus (HIV)-1, inhibit hepatic and intestinal cytochrome P450, and that small doses of GHB can cause lethal toxicity while treating HIV-1. This may block the metabolic pathway of GHB via cytochrome P450, which in turn increases the biotoxicity of GHB. The fact that cytochrome P450 is a key enzyme in the metabolism of xenobiotics phase I is thoroughly supported by the fact that GHB is metabolized in vivo via the xenobiotic metabolic pathway.
As research progressed, the phase II metabolites of exo- and endogenous GHB, GHB-S, GHB-carnitine, GHB-glutamate and GHB-glycine, were discovered in vivo[64] and this GHB conjugate is produced on the basis of the phase I metabolism of GHB by various transferases such as sulfotransferase, carnitine acyl transferase and amino acid acyl transferase. The name of the product is assigned to a variety of transferases, including sulfotransferase, carnitine acyl transferase and amino acid acyl transferase[65] (fig. 1). GHB is metabolised in vivo by this xenobiotic pathway to produce various GHB conjugates, which are then transferred out of the cell membrane by specific transfer proteins on the cell membrane and finally enters the blood system and are excreted in the urine.
GHB metabolism related biomarkers:
According to the different metabolic pathways of GHB, the biomarkers related to GHB metabolism can be divided into 3 categories as GHB conjugates, organic acids and amino acids, and >10 compounds. At present, some of these compounds have been studied in the relevant literature and some progress has been made. It has been found that the compounds related to GHB metabolism have their own characteristics, which can improve the identification of GHB to different degrees.
GHB metabolism related conjugates: GHB conjugates are metabolic intermediates produced in the GHB xenobiotic metabolic pathway and their concentrations are directly correlated with in vivo GHB concentration levels and therefore theoretically are the most potential biomarkers for GHB identification. In 2013, Petersen et al.[62] used liquid chromatography with tandem mass spectrometry to detect the presence of GHB-Gluc in urine for the 1st time and determined its endogenous concentration range of 0.11-5 µg/l. Subsequently, Mehling et al.[66] used liquid chromatography quadrupole time-of-flight mass spectrometry to simultaneously detect the presence of GHB-Gluc, GHB-S in urine, but found that only GHB-Gluc could be detected in blood and that its concentration did not change according to time-dependent manner. Piper et al.[67] recommended exo- and endogenous reference values in urine of 24 µg/ml and 56 µg/ml, respectively, and found that when the characteristic ratio of GHB-Gluc to β-citric acid-glutamate in urine was higher than its endogenous ratio of 40.8 (within the reference population, GHB-Gluc/β-citryl-glutamic acid was found in accordance with Gaussian-distributed after log transformation, at a ratio value of 40.8, which is calculated using a 99.7 % reference limit). It suggests that the current study of GHB-Gluc is on the hydroxy-glycosylated fraction of GHB and does not take into account the carboxy-glycosylated fraction of GHB. Thus the study of GHB-Gluc is limited and needs further comprehensive study[68,69].
Steuer et al.[70,71] used an untargeted metabolomics approach to study GHB conjugates and found that urinary concentrations of GHB-glutamate, GHB-glycine, GHB-taurine and GHB-carnitine increased significantly after 4.5 h of GHB exposure, but decreased rapidly after 8 h. In 2022, Kraemer et al.[72] used liquid chromatography with tandem mass spectrometry in negative ion mode for blood or blood spot samples, and found that 4-Palmitoyloxy butyrate, which is the palmitic ester of GHB (GHB-Pal) could be detected in plasma or serum at high GHB concentrations (>4 µg/ml), but GHB-Pal was found to be highly variable individually and susceptible to sodium fluoride and degradation. Recently, Thimm et al.[73] found that Phoshatidyl-GHB (P-GHB) was highly GHB concentration-dependent in blood, and that when 50 mM GHB was added to the blood, P-GHB concentrations continued to increase until peak concentrations occurred after 72 h (17.4 µg/ml). However, as this is only an exploratory study, further research is needed. Therefore, based on the results of the current study, GHB conjugates are not yet effective in solving the problems associated with the direct identification of GHB.
Organic acids associated with GHB metabolism: 2,4-OH-BA and 3,4-OH-BA are isomers of each other and are intermediates produced in the α and β oxidation pathways in GHB, respectively, while GA is a product of the β oxidation of 3,4-OH-BA[42]. The first 2 isotopes are relatively stable in both urine and blood, and their urinary concentrations are GHB exposure time dependent[71,74]. Jarsiah et al.[75] recommended endogenous cut-off values of 4 mg/l and 5 mg/l for 2,4-OH-BA and 3,4-OH-BA, respectively, in whole femoral venous blood after studying 103 femoral venous blood and 80 urine samples in which none had a history of prenatal exposure to GHB and its precursors. Values >5 mg/l suggested that it might cause ingested exogenous GHB during life. Subsequently, Jarsiah et al. [14] recommended endogenous cut-off values of 2 mg/l, 3 mg/l, 25 mg/l and 50 mg/l for these 2 isomers in serum and urine, respectively. Jarsiah et al. and Küting et al.[76] studied blood and urine samples from 5 patients taking GHB (1.86-3.72 g/dose) sodium salts for narcolepsy using Gas Chromatography Mass Spectrometry (GC-MS) and found that the detection window for 2,4-OH-BA in urine was only 4.5-9.5 h, while the detection window for 3,4-OH-BA in both biological matrices was also only 8 h and 12 h. However, when normalized to creatinine in urine the detection windows in urine for both isomers were extended to 11.5-22 h and 8.5-70 h, respectively, when treated with creatinine in urine.
After studying of 12 (6 males, 6 females) healthy volunteers using Nuclear Magnetic Resonance (NMR) techniques, Palomino-Schätzlein et al.[77] found that using a single low dose (25 mg/kg) of GHB for 1 h resulted in GA concentrations significantly increasing in urinary and that, despite the window of detection of GHB in urine for only 6 h, the GA decreased slowly compared to its pre-dose autologous concentration, and this high concentration state could be maintained for up to 20 h, and even beyond 24 h could still be distinguished from its endogenous origin. Küting et al.[76] also demonstrated a detection window of up to 6.5-22 h for GA in urine, but its concentration in blood did not change significantly. To explain the identification of GA in GHB poisoning cases, Jarsiah et al.[14] recommended cut-off values for GA in blood and urine as 5 mg/l and 400 mg/l respectively. In addition, Seo et al.[78] found that the concentration of 2-Hydroxyglutarate (2-HG) in rat urine increased mostly significantly in GHB exposure experiments, with urine samples from single or multiple doses being >92 % and 81 % experimental controls, respectively, and significant alterations in citric acid, isocitric acid and cis-aconitic acid concentrations were also observed, leading to the conclusion that these compounds are useful in identifying GHB exposure also has an important supporting role.
Amino acids associated with GHB metabolism: In vivo, amino acids are involved in GHB metabolism and the TCA cycle as precursors to organic acids. Seo et al.[79] established 3 experimental models by intraperitoneal injection of GHB in single, multiple doses and experimental control groups. 28 amino acids were found in the urine of the three groups of experimental rats, of which 26 organic acids changed significantly, and five amino acids, including phenylalanine, glutamic acid, aspartic acid, asparagine and methionine, were recommended for study as alternative biomarkers for GHB identification. However, Steuer et al.[71] found little variability in the changes in concentrations of amino acids, acetylcarnitine, TCA intermediates, bile and fatty acids in blood before and after dosing, and it was difficult to distinguish GHB from other drug abuse, and in urine it was not effective in extending the window of detection of GHB, therefore Steuer et al. concluded that these compounds in plasma and urine are not suitable as biomarkers for GHB identification.
In addition, Lee et al.[80] found that urinary concentrations of N1-acetyl spermidine and spermine remained high after single and multiple GHB exposures after 1 dose (600 mg/kg) of GHB was administered intraperitoneally to rats consecutively for 10 d, and therefore recommended the use of these 2 compounds as qualitative biomarkers in cases of GHB exposure and addiction identification.
Biomarker studies to decipher the difficulties of GHB identification:
Difficulties in the direct identification of GHB in biological matrices: In the practice of GHB identification, direct GHB identification is mainly based on the establishment of a cut-off value for endogenous GHB to differentiate between exo- and endogenous GHB and the interpretation of GHB identification results. The cut-off values for GHB in two biological matrices, blood and urine, are relatively consistent in the literature, being 4 or 5 mg/ml and 6 or 10 mg/ml, respectively[81] and recommended their corresponding detection windows of 6 h and 12 h, respectively[8]. However, if 5 mg/ml and 10 mg/ml are used as cut-off values for GHB in the 2 biological matrices, respectively, the exogenous GHB may only have an actual detection window of 3 h and 8 h[82] and thus cannot avoid false negative identification results. Therefore, 4 mg/ml and 6 mg/ml have been recommended in the literature as GHB cut-off values in two biological matrices respectively[83] but Kankaanpaa et al.[84] concluded that setting a cut-off value of 10 mg/ml for GHB in urine was safer and could avoid some of the false positive identification results. Elliott[85] also suggests that 4 mg/ml and 10 mg/ml are the minimum cut-off values for GHB in the 2 biological matrices, and that lower values would not distinguish between exo- and endogenous GHB. However, even if the cut-off values for GHB in blood and urine are adjusted to 4 mg/ml and 10 mg/ml, respectively, the detection window for exogenous GHB in blood is only 2.5 h[66] and it may not be possible to identify it in vivo beyond 4 h[86].
In addition, Guthery et al.[87] concluded that the cut-off values currently recommended in the literature are only reliable within the GHB detection window, but it is difficult to sample within 4 h of GHB exposure in real cases, especially for drug-assisted sexual offences. Attempts have been made to extend the detection window by detecting GHB in hair, chest hair and pubic hair[88,89]. However, although the detection of GHB in chest and pubic hair can assist in the confirmation of GHB in hair[89]. However, due to the wide range of endogenous GHB concentrations in hair (0.5-12 ng/mg), as a result, no suitable cut-off values have been determined in the hair so far[90]. Therefore, the likelihood of exogenous GHB being detected in GHB exposure cases is extremely low, and the GHB identification results lack sufficient scientific theoretical support.
Enhancement of GHB by GHB metabolism-related biomarker studies: Biomarkers are an effective tool with predictive, diagnostic and monitoring functions and are essential in diagnostic and research work in clinical, systemic pharmacology and forensic toxicology[80]. Following a study of metabolism-related markers of GHB in vivo, found that the detectable concentrations of GHB-Gluc and GHB-S in urine were not significantly different from their endogenous concentrations, and that both concentrations in urine remained within the endogenous concentration range after GHB exposure[66]. GHB-Pal is GHB-specific, but it is unstable and easily degraded in biological matrices[72]. SA is extremely unstable in biological matrices and cannot be stored stably even at -20°[74]. In urine, the identification window for GHB conjugates with glutamate, glycine, taurine and carnitine was short and declined rapidly after 8 h of GHB exposure, which did not effectively enhance the detection window for GHB[71].
However, in related studies, there are also subjects with high value studies. 2,4-OH-BA, 3,4-OH-BA, and GA are relatively stable in blood and urine sample matrices[71,74] and also have long detection windows in urine and can be distinguished from their endogenous and exogenous sources[14,76]. The cut-off values for 2,4-OH-BA, 3,4-OH-BA, and GA in blood and urine were 2 mg/l, 3 mg/l, 5 mg/l, 25 mg/l, 50 mg/l and 400 mg/l, respectively[14] and the creatinine-normalized 2,4-OH-BA and 3,4-OH-BA could respectively prolong GHB identification window to 11.5-22 h and 8.5-70 h[76] and high GA concentrations in urine can be maintained for 20 h, even after 24 h of dosing, and can still be identified with their autologous physiological concentrations[77]. In addition, phosphatidyl-GHB (16:0/18:1) has a concentration-dependent and time-dependent GHB exposure and it remains at high levels after 72 h of GHB exposure, but it has been studied relatively low. The results of GHB biomarker studies have demonstrated the correct direction of indirect identification of GHB[73], which can effectively solve the problems of short GHB identification window and differentiation between endogenous and exogenous GHB. Due to the limitations of the study, the statistical relationship between GHB metabolism-related biomarkers and GHB exposure has not been fully established, and the quantitative identification of GHB exposure by biomarkers is still not possible, and further research is needed.
Conclusion
GHB exists in the body as a physiological compound and is metabolised by a variety of pathways, creating a stable endostasis in the body. The entry of exogenous GHB into the body may alter the equilibrium of this endostasis, which is directly manifested by changes in the concentrations of various physiological compounds in biological samples. Therefore, it is theoretically possible to indirectly identify exogenous GHB by detecting compounds related to GHB metabolism. Currently, research on GHB identification has focused on the discovery of biomarkers that can be substituted for the detection of exogenous GHB[91], such as the search for biomarkers related to GHB metabolism in biological matrices such as blood and urine through targeted and untargeted metabolomics approaches, and the theoretical basis supporting such studies is a comprehensive compendium of GHB biometabolic pathways and their related biomarkers in vivo, therefore, this paper provides a review of the various possible pathways of GHB biometabolism and their biomarkers in vivo, with a view to provide theoretical references for the pharmacokinetics and pharmacology of GHB and indirect identification methods for the direct identification of GHB and the detection of its metabolism-related biomarkers.
Conflict of interests:
The authors declared no conflict of interests.
References
- Nicholson KL, Balster RL. GHB: A new and novel drug of abuse. Drug Alcohol Depend 2001;63(1):1-22.
[Crossref] [Google Scholar] [PubMed]
- Mamelak M. Gammahydroxybutyrate: An endogenous regulator of energy metabolism. Neurosci Biobehav Rev 1989;13(4):187-98.
[Crossref] [Google Scholar] [PubMed]
- Gahlinger PM. Club drugs: MDMA, Gamma-Hydroxybutyrate (GHB), rohypnol, and ketamine. Am Fam Physician 2004;69(11):2619-27.
[Google Scholar] [PubMed]
- Giorgetti A, Busardò FP, Giorgetti R. Toxicological characterization of GHB as a performance-enhancing drug. Front Psychiatry 2022;13:1-16.
[Crossref] [Google Scholar] [PubMed]
- Dijkstra BA, Beurmanjer H, Goudriaan AE, Schellekens AF, Joosten EA. Unity in diversity: A systematic review on the GHB using population. Int J Drug Policy 2021;94:1-8.
[Crossref] [Google Scholar] [PubMed]
- Sumnall HR, Woolfall K, Edwards S, Cole JC, Beynon CM. Use, function, and subjective experiences of Gamma-Hydroxybutyrate (GHB). Drug Alcohol Depend 2008;92(1-3):286-90.
[Crossref] [Google Scholar] [PubMed]
- Carter LP, Richards BD, Mintzer MZ, Griffiths RR. Relative abuse liability of GHB in humans: A comparison of psychomotor, subjective, and cognitive effects of supratherapeutic doses of triazolam, pentobarbital, and GHB. Neuropsychopharmacology 2006;31(11):2537-51.
[Crossref] [Google Scholar] [PubMed]
- Busardò FP, Jones AW. Interpreting γ-hydroxybutyrate concentrations for clinical and forensic purposes. Clin Toxicol 2019;57(3):149-63.
[Crossref] [Google Scholar] [PubMed]
- Marclay F, Saudan C, Vienne J, Tafti M, Saugy M. Source inference of exogenous Gamma-Hydroxybutyric Acid (GHB) administered to humans by means of carbon isotopic ratio analysis: Novel perspectives regarding forensic investigation and intelligence issues. Anal Bioanal Chem 2011;400(4):1105-12.
[Crossref] [Google Scholar] [PubMed]
- Dillon P, Degenhardt L. Ketamine and GHB: New trends in club drug use? J Subst Use 2001;6(1):11-5.
- Sherry JM, Hazi A, Hale MW, Milsome SL, Crowe SF. Gamma-Butyrolactone (GBL) disruption of passive avoidance learning in the day-old chick appears to be due to its effect on GABAB not Gamma-Hydroxybutric acid (GHB) receptors. Behav Brain Res 2009;197(2):347-55.
[Crossref] [Google Scholar] [PubMed]
- Bay T, Eghorn LF, Klein AB, Wellendorph P. GHB receptor targets in the CNS: Focus on high-affinity binding sites. Biochem Pharmacol 2014;87(2):220-8.
[Crossref] [Google Scholar] [PubMed]
- van Amsterdam JG, Brunt TM, McMaster MT, Niesink RJ. Possible long-term effects of γ-Hydroxybutyric acid (GHB) due to neurotoxicity and overdose. Neurosci Biobehav Rev 2012;36(4):1217-27.
[Crossref] [Google Scholar] [PubMed]
- Jarsiah P, Roehrich J, Kueting T, Martz W, Hess C. GHB related acids are useful in routine casework of suspected GHB intoxication cases. Forensic Sci Int 2021;324:1-6.
[Crossref] [Google Scholar] [PubMed]
- Ingels AS, Wille SM, Samyn N, Lambert WE, Stove CP. Screening and confirmation methods for GHB determination in biological fluids. Anal Bioanal Chem 2014;406(15):3553-77.
[Crossref] [Google Scholar] [PubMed]
- Mason PE, Kerns WP. Gamma Hydroxybutyric acid (GHB) intoxication. Acad Emerg Med 2002;9(7):730-9.
[Crossref] [Google Scholar] [PubMed]
- Tunnicliff G. Significance of γ-hydroxybutyric acid in the brain. Gen Pharmacol 1992;23(6):1027-34.
[Crossref] [Google Scholar] [PubMed]
- Santaniello E, Manzocchi A, Tosi L. Evaluation of gamma-hydroxybutyrate formed from L-glutamate by mouse brain homogenate. J Neurochem 1978;31(4):1117-8.
[Crossref] [Google Scholar] [PubMed]
- Tillakaratne NJ, Medina-Kauwe L, Gibson KM. Gamma-Aminobutyric Acid (GABA) metabolism in mammalian neural and nonneural tissues. Comp Biochem Physiol A Physiol 1995;112(2):247-63.
[Crossref] [Google Scholar] [PubMed]
- Ravasz D, Kacso G, Fodor V, Adam-Vizi V, Chinopoulos C. Catabolism of GABA, succinic semialdehyde or gamma-hydroxybutyrate through the GABA shunt impair mitochondrial substrate-level phosphorylation. Neurochem Int 2017;109:41-53.
[Crossref] [Google Scholar] [PubMed]
- Medina-Kauwe LK, Tobin AJ, de Meirleir L, Jaeken J, Jakobs CA, Nyhan WL, et al. 4-Aminobutyrate aminotransferase (GABA-transaminase) deficiency. J Inherit Metab Dis 1999;22(4):414-27.
[Crossref] [Google Scholar] [PubMed]
- Bessman SP, Rossen J, Layne EC. γ-Aminobutyric acid-glutamic acid transamination in brain. J Biol Chem 1953;201(1):385-91.
[Crossref] [Google Scholar] [PubMed]
- Anderson RA, Ritzmann RF, Tabakoff B. Formation of gamma-hydroxybutyrate in brain1. J Neurochem 1977;28(3):633-9.
[Crossref] [Google Scholar] [PubMed]
- Nelson T, Kaufman E, Kline J, Sokoloff L. The extraneural distribution of γ?hydroxybutyrate. J Neurochem 1981;37(5):1345-8.
[Crossref] [Google Scholar] [PubMed]
- Feigenbaum JJ, Howard SG. Gamma hydroxybutyrate is not a GABA agonist. Prog Neurobiol 1996;50(1):1-7.
[Crossref] [Google Scholar] [PubMed]
- Snead III OC, Furner R, Liu CC. In vivo conversion of γ-aminobutyric acid and 1,4-butanediol to γ-hydroxybutyric acid in rat brain: Studies using stable isotopes. Biochem Pharmacol 1989;38(24):4375-80.
[Crossref] [Google Scholar] [PubMed]
- Bergelson LD, Vaver VA, Prokazova NV, Ushakov AN, Popkova GA. Diol lipids. Biochim Biophys Acta 1966;116(3):511-20.
[Crossref] [Google Scholar] [PubMed]
- Liakoni E, Gugelmann H, Dempsey DA, Wiegand TJ, Havel C, Jacob P, et al. Butanediol conversion to gamma?hydroxybutyrate markedly reduced by the alcohol dehydrogenase blocker fomepizole. Clin Pharmacol Ther 2019;105(5):1196-203.
[Crossref] [Google Scholar] [PubMed]
- Lenz D, Jübner M, Bender K, Wintermeyer A, Beike J, Rothschild MA, et al. Inhibition of 1, 4-butanediol metabolism in human liver in vitro. Naunyn Schmiedebergs Arch Pharmacol 2011;383(6):647-54.
[Crossref] [Google Scholar] [PubMed]
- Poldrugo F, Snead III OC. 1,4 Butanediol and ethanol compete for degradation in rat brain and liver in vitro. Alcohol 1986;3(6):367-70.
[Crossref] [Google Scholar] [PubMed]
- Carai MA, Colombo G, Reali R, Serra S, Mocci I, Castelli MP, et al. Central effects of 1,4-butanediol are mediated by GABAB receptors via its conversion into γ-hydroxybutyric acid. Eur J Pharmacol 2002;441(3):157-63.
[Crossref] [Google Scholar] [PubMed]
- Kapadia R, Böhlke M, Maher TJ. Detection of γ-hydroxybutyrate in striatal microdialysates following peripheral 1,4-butanediol administration in rats. Life Sci 2007;80(11):1046-50.
[Crossref] [Google Scholar] [PubMed]
- Fishbein WN, Bessman SP. Purification and properties of an enzyme in human blood and rat liver microsomes catalyzing the formation and hydrolysis of γ-lactones: I. Tissue localization, stoichiometry, specificity, distinction from esterase. J Biol Chem 1966;241(21):4835-41.
[Crossref] [Google Scholar] [PubMed]
- Arena C, Fung HL. Absorption of sodium γ-hydroxybutyrate and its prodrug γ-butyrolactone: Relationship between in vitro transport and in vivo absorption. J Pharm Sci 1980;69(3):356-8.
[Crossref] [Google Scholar] [PubMed]
- Roth RH, Delgado JM, Giarman NJ. γ-Butyrolactone and γ-hydroxybutyric acid-II. The pharmacologically active form. Int J Neuropharmacol 1966;5(6):421-8.
[Crossref] [Google Scholar] [PubMed]
- Carter LP, Koek W, France CP. Lack of effects of GHB precursors GBL and 1,4?BD following i.c.v administration in rats. Eur J Neurosci 2006;24(9):2595-600.
[Crossref] [Google Scholar] [PubMed]
- Trombley TA, Capstick RA, Lindsley CW. DARK classics in chemical neuroscience: Gamma-Hydroxybutyrate (GHB). ACS Chem Neurosci 2019;11(23):3850-9.
[Crossref] [Google Scholar] [PubMed]
- Brailsford AD, Cowan DA, Kicman AT. Pharmacokinetic properties of γ-Hydroxybutyrate (GHB) in whole blood, serum, and urine. J Anal Toxicol 2012;36(2):88-95.
[Crossref] [Google Scholar] [PubMed]
- Bhattacharya I, Boje KM. GHB (γ-hydroxybutyrate) carrier-mediated transport across the blood-brain barrier. J Pharmacol Exp Ther 2004;311(1):92-8.
[Crossref] [Google Scholar] [PubMed]
- Struys EA, Verhoeven NM, Jansen EE, Ten Brink HJ, Gupta M, Burlingame TG, et al. Metabolism of γ-hydroxybutyrate to D-2-hydroxyglutarate in mammals: Further evidence for D-2-hydroxyglutarate transhydrogenase. Metabolism 2006;55(3):353-8.
[Crossref] [Google Scholar] [PubMed]
- Zhang GF, Sadhukhan S, Ibarra RA, Lauden SM, Chuang CY, Sushailo S, et al. Metabolism of γ-hydroxybutyrate in perfused rat livers. Biochem J 2012;444(2):333-41.
[Crossref] [Google Scholar] [PubMed]
- Sadhukhan S, Zhang GF, Tochtrop GP. Modular isotopomer synthesis of γ-hydroxybutyric acid for a quantitative analysis of metabolic fates. ACS Chem Biol 2014;9(8):1706-11.
[Crossref] [Google Scholar] [PubMed]
- Vayer P, Schmitt M, Bourguignon JJ, Mandel P, Maitre M. Evidence for a role of high Km aldehyde reductase in the degradation of endogenous γ-hydroxybutyrate from rat brain. FEBS Lett 1985;190(1):55-60.
[Crossref] [Google Scholar] [PubMed]
- Schep LJ, Knudsen K, Slaughter RJ, Vale JA, Mégarbane B. The clinical toxicology of gamma-hydroxybutyrate, gamma-butyrolactone and 1,4-butanediol. Clin Toxicol 2012;50(6):458-70.
[Crossref] [Google Scholar] [PubMed]
- Alzeer S, Ellis EM. Metabolism of gamma hydroxybutyrate in human hepatoma HepG2 cells by the aldo-keto reductase AKR1A1. Biochem Pharmacol 2014;92(3):499-505.
[Crossref] [Google Scholar] [PubMed]
- Kaufman EE, Relkin N, Nelson T. Regulation and properties of an NADP+ oxidoreductase which functions as a γ-hydroxybutyrate dehydrogenase. J Neurochem 1983;40(6):1639-46.
[Crossref] [Google Scholar] [PubMed]
- Kaufman EE, Nelson T. Evidence for the participation of a cytosolic NADP+?dependent oxidoreductase in the catabolism of γ?hydroxybutyrate in vivo. J Neurochem 1987;48(6):1935-41.
[Crossref] [Google Scholar] [PubMed]
- Kaufman EE, Nelson T, Miller D, Stadlan N. Oxidation of γ?hydroxybutyrate to succinic semialdehyde by a mitochondrial pyridine nucleotide-independent enzyme. J Neurochem 1988;51(4):1079-84.
[Crossref] [Google Scholar] [PubMed]
- Kaufman EE, Nelson T, Fales HM, Levin DM. Isolation and characterization of a hydroxyacid-oxoacid transhydrogenase from rat kidney mitochondria. J Biol Chem 1988;263(32):16872-9.
[Crossref] [Google Scholar] [PubMed]
- Struys EA, Verhoeven NM, Ten Brink HJ, Wickenhagen WV, Gibson KM, Jakobs CA. Kinetic characterization of human hydroxyacid-oxoacid transhydrogenase: Relevance tod?2?hydroxyglutaric and γ?hydroxybutyric acidurias. J Inherit Metab Dis 2005;28(6):921-30.
[Crossref] [Google Scholar] [PubMed]
- Vayer P, Mandel P, Maitre M. Conversion of γ?hydroxybutyrate to γ?aminobutyrate in vitro. J Neurochem 1985;45(3):810-4.
[Crossref] [Google Scholar] [PubMed]
- Godin Y, Mark J, Mandel P. The effects of 4-hydroxybutyric acid on the biosynthesis of amino acids in the central nervous system. J Neurochem 1968;15(10):1085-91.
[Crossref] [Google Scholar] [PubMed]
- Sherif FM, Ahmed SS. Basic aspects of GABA-transaminase in neuropsychiatric disorders. Clin Biochem 1995;28(2):145-54.
[Crossref] [Google Scholar] [PubMed]
- Kim KJ, Pearl PL, Jensen K, Snead OC, Malaspina P, Jakobs C, et al. Succinic semialdehyde dehydrogenase: Biochemical-molecular-clinical disease mechanisms, redox regulation, and functional significance. Antioxid Redox Signal 2011;15(3):691-718.
[Crossref] [Google Scholar] [PubMed]
- Maitre M. The γ-hydroxybutyrate signalling system in brain: Organization and functional implications. Prog Neurobiol 1997;51(3):337-61.
[Crossref] [Google Scholar] [PubMed]
- Doherty JD, Roth RH. Metabolism of γ?hydroxy?[1?14C] butyrate by rat brain: Relationship to the Krebs cycle and metabolic compartmentation of amino acids. J Neurochem 1978;30(6):1305-9.
[Crossref] [Google Scholar] [PubMed]
- Lee HH, McGinty GE, Pearl PL, Rotenberg A. Understanding the molecular mechanisms of Succinic Semialdehyde Dehydrogenase Deficiency (SSADHD): Towards the development of SSADH-targeted medicine. Int J Mol Sci 2022;23(5):1-15.
[Crossref] [Google Scholar] [PubMed]
- Lee CR. Evidence for the β-oxidation of orally administered 4-hydroxybutyrate in humans. Biochem Med 1977;17(3):284-91.
[Crossref] [Google Scholar] [PubMed]
- Fell V, Lee CR, Pollitt RJ. The occurrence of (S)-3,4-dihydroxybutyrate in human blood and urine. Biochem Med 1975;13(1):40-5.
[Crossref] [Google Scholar] [PubMed]
- Patterson AD, Gonzalez FJ, Idle JR. Xenobiotic metabolism: A view through the metabolometer. Chem Res Toxicol 2010;23(5):851-60.
[Crossref] [Google Scholar] [PubMed]
- Walsham NE, Sherwood RA. Ethyl glucuronide and ethyl sulfate. Adv Clin Chem 2014;67:47-71.
[Crossref] [Google Scholar] [PubMed]
- Petersen IN, Tortzen C, Kristensen JL, Pedersen DS, Breindahl T. Identification of a new metabolite of GHB: Gamma-hydroxybutyric acid glucuronide. J Anal Toxicol 2013;37(5):291-7.
[Crossref] [Google Scholar] [PubMed]
- Harrington RD, Woodward JA, Hooton TM, Horn JR. Life-threatening interactions between HIV-1 protease inhibitors and the illicit drugs MDMA and γ-hydroxybutyrate. Arch Intern Med 1999;159(18):2221-4.
[Crossref] [Google Scholar] [PubMed]
- Jung S, Kim S, Seo Y, Lee S. Metabolic alterations associated with γ-hydroxybutyric acid and the potential of metabolites as biomarkers of its exposure. Metabolites 2021;11(2):1-15.
[Crossref] [Google Scholar] [PubMed]
- Steuer AE, Sutter L, Steuer C, Kraemer T. New Gamma-Hydroxybutyric acid (GHB) biomarkers: Development and validation of a liquid chromatography-tandem mass spectrometry method for the determination of GHB amino acid, carnitine, and fatty acid conjugates in urine. Drug Test Anal 2023;15(4):426-43.
[Crossref] [Google Scholar] [PubMed]
- Mehling LM, Piper T, Spottke A, Heidbreder A, Young P, Madea B, et al. GHB-O-β-glucuronide in blood and urine is not a suitable tool for the extension of the detection window after GHB intake. Forensic Toxicol 2017;35(2):263-74.
- Piper T, Mehling LM, Spottke A, Heidbreder A, Young P, Madea B, et al. Potential of GHB phase-II-metabolites to complement current approaches in GHB post administration detection. Forensic Sci Int 2017;279:157-64.
[Crossref] [Google Scholar] [PubMed]
- Beck R, Mimica Matanovi? S, Zibar L. Gamma-hydroxybutyric acid, gamma-butyrolactone, and 1,4-butanediol addiction: A serious health threat. Arh Hig Rada Toksikol 2019;70(2):149-50.
[Crossref] [Google Scholar] [PubMed]
- Marchei E, Tini A, Pirani F, Af LF, Marinelli S. Is GHB-glucuronide useful as a biomarker for the exogenous use of GHB? Eur Rev Med Pharmacol Sci 2019;23(6):2311-3.
[Crossref] [Google Scholar] [PubMed]
- Steuer AE, Raeber J, Steuer C, Boxler MI, Dornbierer DA, Bosch OG, et al. Identification of new urinary gamma?hydroxybutyric acid markers applying untargeted metabolomics analysis following placebo?controlled administration to humans. Drug Test Anal 2019;11(6):813-23.
[Crossref] [Google Scholar] [PubMed]
- Steuer AE, Raeber J, Simbuerger F, Dornbierer DA, Bosch OG, Quednow BB, et al. Towards extending the detection window of gamma-hydroxybutyric acid-an untargeted metabolomics study in serum and urine following controlled administration in healthy men. Metabolites 2021;11(3):1-16.
[Crossref] [Google Scholar] [PubMed]
- Kraemer M, Broecker S, Kueting T, Madea B, Maas A. Fatty acid esters as novel metabolites of γ?hydroxybutyric acid: A preliminary investigation. Drug Test Anal 2022;14(4):690-700.
[Crossref] [Google Scholar] [PubMed]
- Thimm JS, Hofmann V, Bartel M, Sundermann TR. Phospholipid metabolites of GHB as potential biomarkers in whole blood: Synthesis, analytics, and in vitro formation of homolog 16:0/18:1. Drug Test Anal 2023;15(2):192-203.
[Crossref] [Google Scholar] [PubMed]
- Jarsiah P, Roehrich J, Wyczynski M, Hess C. Phase I metabolites (organic acids) of gamma?hydroxybutyric acid-validated quantification using GC-MS and description of endogenous concentration ranges. Drug Test Anal 2020;12(8):1135-43.
[Crossref] [Google Scholar] [PubMed]
- Jarsiah P, Kueting T, Roehrich J, Germerott T, Remane D, Toennes SW, et al. GHB related acids (dihydroxy butyric acids, glycolic acid) can help in the interpretation of post mortem GHB results. Forensic Sci Int 2020;316:1-9.
[Crossref] [Google Scholar] [PubMed]
- Küting T, Schneider B, Heidbreder A, Krämer M, Jarsiah P, Madea B, et al. Detection of γ?hydroxybutyric acid?related acids in blood plasma and urine: Extending the detection window of an exogenous γ?hydroxybutyric acid intake? Drug Test Anal 2021;13(9):1635-49.
[Crossref] [Google Scholar] [PubMed]
- Palomino-Schatzlein M, Wang Y, Brailsford AD, Parella T, Cowan DA, Legido-Quigley C, et al. Direct monitoring of exogenous γ-hydroxybutyric acid in body fluids by NMR spectroscopy. Anal Chem 2017;89(16):8343-50.
[Crossref] [Google Scholar] [PubMed]
- Seo C, Park M, Choi B, Lee S, Paik MJ. Metabolomic analysis of urinary organic acids following intraperitoneal injection with γ-hydroxybutyric acid in rats. Metabolomics 2016;12(12):1-9.
- Seo C, Na M, Jang J, Park M, Choi B, Lee S, et al. Monitoring of altered amino acid metabolic pattern in rat urine following intraperitoneal injection with γ-hydroxybutyric acid. Metabolomics 2018;14(9):1-8.
[Crossref] [Google Scholar] [PubMed]
- Lee HS, Seo C, Kim YA, Park M, Choi B, Ji M, et al. Metabolomic study of polyamines in rat urine following intraperitoneal injection of γ-hydroxybutyric acid. Metabolomics 2019;15(4):1-10.
[Crossref] [Google Scholar] [PubMed]
- Saracino MA, Catapano MC, Iezzi R, Somaini L, Gerra G, Mercolini L. Analysis of γ-hydroxy butyrate by combining capillary electrophoresis-indirect detection and wall dynamic coating: Application to dried matrices. Anal Bioanal Chem 2015;407(29):8893-901.
[Crossref] [Google Scholar] [PubMed]
- Schröck A, Hari Y, König S, Auwärter V, Schürch S, Weinmann W. Pharmacokinetics of GHB and detection window in serum and urine after single uptake of a low dose of GBL-an experiment with two volunteers. Drug Test Anal 2014;6(4):363-6.
[Crossref] [Google Scholar] [PubMed]
- Andresen H, Aydin BE, Mueller A, Iwersen?Bergmann S. An overview of gamma?hydroxybutyric acid: Pharmacodynamics, pharmacokinetics, toxic effects, addiction, analytical methods, and interpretation of results. Drug Test Anal 2011;3(9):560-8.
[Crossref] [Google Scholar] [PubMed]
- Kankaanpää A, Liukkonen R, Ariniemi K. Determination of γ-Hydroxybutyrate (GHB) and its precursors in blood and urine samples: A salting-out approach. Forensic Sci Int 2007;170(2-3):133-8.
[Crossref] [Google Scholar] [PubMed]
- Elliott SP. Gamma Hydroxybutyric acid (GHB) concentrations in humans and factors affecting endogenous production. Forensic Sci Int 2003;133(1-2):9-16.
[Crossref] [Google Scholar] [PubMed]
- Lott S, Piper T, Mehling LM, Spottke A, Maas A, Thevis M, et al. Measurement of exogenous Gamma-Hydroxybutyric Acid (GHB) in urine using Isotope Ratio Mass Spectrometry (IRMS). Toxichem Krimtech 2015;82:264-7.
- Guthery B, Shuker DE, Pillinger CT, Gilmour MA, Valussi S, Wicks J, et al. Stable isotopes (δ13C): A proposed means of identifying the source of Gamma-Hydroxybutyric acid (GHB). 2007.
- Shi Y, Cui X, Shen M, Xiang P. Quantitative analysis of the endogenous GHB level in the hair of the Chinese population using GC/MS/MS. J Forensic Leg Med 2016;39:10-15.
[Crossref] [Google Scholar] [PubMed]
- Bertol E, Mari F, Lachi A, Tespio G, Vaiano F. Determination of endogenous GHB levels in chest and pubic hair. Forensic Sci Int 2021;325:1-6.
[Crossref] [Google Scholar] [PubMed]
- Vaiano F, Serpelloni G, Furlanetto S, Palumbo D, Mari F, Fioravanti A, et al. Determination of endogenous concentration of γ-Hydroxybutyric acid (GHB) in hair through an ad hoc GC-MS analysis: A study on a wide population and influence of gender and age. J Pharm Biomed Anal 2016;118:161-6.
[Crossref] [Google Scholar] [PubMed]
- Kim S, Lee MS, Kim M, Ko BJ, Lee HS, Lee S. Derivatization-assisted LC-MS/MS method for simultaneous quantification of endogenous gamma-hydroxybutyric acid and its metabolic precursors and products in human urine. Anal Chim Acta 2022;1194:339401.
[Crossref] [Google Scholar] [PubMed]