- *Corresponding Author:
- Hongmin Liang
Department of Radiology, The First Affiliated Hospital of Kunming Medical University, Kunming, Yunnan Province 650051, China
E-mail: cskmfyy@126.com
| This article was originally published in a special issue,“Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “353-362” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In order to understand the risk factors for prognostic assessment of sodium iodide oral solution in the treatment of hyperthyroidism, we attempted to predict the prognosis by creating nomogram and help clinicians choose more appropriate drug dosages. We retrospectively collected 607 cases who were hospitalized for hyperthyroidism from 2016-2022 and finally included in the study, and collected 25 clinical and personal related indicators to correlate the prognosis of the patients, and followed up the patients at least 1 y after treatment. We further divided patients into training cohort and validation cohort. A nomogram model was developed in the training set to predict the sodium iodide oral solution to hyperthyroidism. Univariate analysis and logistic regression were applied to select the important factors. Receiver operating characteristic curves, calibration curves and the Hosmer-Lemeshow test evaluate the discriminative performance. Five clinical indicators including thyroid weight, technetium-99 uptake, complications, free thyroxine, and anti-thyroid peroxidase were screened to establish a prognostic model for the prognostic relevance of sodium iodide oral solution in hyperthyroid patients, which had an area under the curve >0.7. Calibration plots demonstrated good agreement between predictions of the nomogram and the actual observed values in both the training and validation cohorts. We developed a prognostic model using the nomogram as an indicator to predict the prognosis of patients, which can be used as a reference for clinicians when formulating drug dosages and prognostic assessment.
Keywords
Hyperthyroidism, validation, sodium iodide, Graves’ disease, microbiota
Hyperthyroidism is characterized by excessive synthesis and secretion of thyroid hormones throughout the body, and its most prominent cause is Grave’s disease (Basedow disease) or diffuse toxic goiter[1], Grave’s disease is an autoimmune thyroid disease, with a global prevalence ranging from 0.2 % to 1.3 %[2]. Approximately 0.2 % of males and 2 % of females globally suffer from Grave’s disease[3,4]. Enlarged thyroid gland and generalized hypermetabolic symptoms are the main clinical manifestations, and complications often include proptosis, atrial fibrillation, periodic paralysis, osteoporosis, and reproductive abnormalities[1]. Studies have shown that the pathogenesis of hyperthyroidism is associated with genetic susceptibility, gender, pregnancy, stress, infection, iodine intake, radiation, immunomodulatory, microbiota, geography and ethnicity etc.,[5].
Currently, there are three main treatment methods for hyperthyroidism; antithyroid drug, sodium iodide oral solution for radioactive treatment, and surgery[6]. Antithyroid drug has side effects such as unsatisfactory control, multiple relapses, and many complications, while surgery is an invasive operation with an unpredictable risk of surgical complications, and there are also patients with other risk factors that are unsuitable for surgery, which often results in a long course of the disease and more complications, and brings great disturbance to the patient’s life. These patients tend to have a longer course of disease and more complications, which bring great trouble to their lives. Sodium iodide oral solution is preferred in North America for the treatment of hyperthyroidism because of its side effects and low incidence of hyperthyroidism crisis[7]. Although the efficacy and safety of sodium iodide oral solution for hyperthyroidism have been demonstrated, there are still different recommendations for the dose of sodium iodide oral solution, with the American and the European guideline of thyroid association recommending a single dose of radioactivity sufficient to cause patients to become hypothyroid (usually between 370 and 555 MBq)[6,8], and the European society of radiology suggested that post-treatment hypothyroidism is a side effect of sodium iodide oral solution, so more precise and individualized dose is needed[9-11]. In order to understand the therapeutic dose of sodium iodide oral solution to the patient, nuclear medicine scholars have proposed fixed-metered and calculated-metered approaches. Fixed-metered approaches use 10-15 mCi for internal radiation therapy while calculated-metered approaches assess the dose of the drug based on the weight of the patient’s thyroid gland and iodine uptake rate in combination with a fixed coefficient chosen by the clinician[12], and both approaches allow for the use of high doses without significant differences in the patient’s benefit[13]. Therefore, the question of how to find an individualized and precise dosage regimen remains to be addressed by scholars.
Some current studies have shown that higher thyroid weight[14], higher iodine uptake in male patients, longer medical history, and more complications may be influential factors in increasing the dose of sodium iodide oral solution[15]. In order to understand the prognosis of the patients in our center and to formulate a suitable treatment plan for our patients, we investigated the prognosis-related influencing factors of the patients by retrospective analysis with the aim of establishing a clinical prediction model to assess the clinical factors related to patients before treatment, to understand the dedication of patient’s prognosis, and to assist the clinic in formulating a more accurate drug dosage for patients.
Materials and Methods
Study design and subject matter:
The design of this retrospective cohort study was approved by the Ethics Committee of the First Affiliated Hospital of Kunming Medical University (2023-L-175), and because of the retrospective design of this study, telephone informed consent was obtained from all patients by telephone. This study followed the guidelines of the Declaration of Helsinki and the reporting of multivariate predictive modeling for prognostic guidelines.
General information:
Our patients were those who were clinically diagnosed with primary hyperthyroidism; hyperthy-roidism from December 2016 to June 2022 at the Department of Nuclear Medicine, the First Affili-ated Hospital of Kunming Medical University, where they underwent radioactive iodine therapy by taking sodium iodide oral solution. The inclusion criteria includes patients whose thyroid function monitoring showed elevated Free Thyroxine (FT4), Free Triiodothyronine (FT3) with Thyroid Stimulating Hormone (TSH) suppression, or patients with normal thyroid function due to medication but higher-than-normal iodine uptake, and patients who had palpitations, trembling of the hands, excessive sweating, weight loss with or without thyroid dysfunction protuberance, goiter, anterior tibial mucous edema, periodic paralysis or atrial fibrillation. In exclusion criteria, patients refuse to take sodium iodide oral solution, loss of visits, missing information, other treatments and age <18 y or <75 y old were excluded. We followed all patients for <1 y and documented the patient’s status after taking the drug. Informed consent was obtained from the patients by telephone.
We collected the following information about the patients; age, sex, duration of the disease, family history, complications, medication, dose of sodium iodide oral solution, medication after treatment, number of sodium iodide oral solution, thyroid ultrasound with or without Hashimoto’s disease, thyroid ultrasound with or without nodules, thyroid weight on thyroid static radiography, Thyroid technetium-99 uptake on thyroid static radiography, thyroid 2 h iodine uptake, thyroid 4 h iodine uptake, thyroid 24 h iodine uptake, and 9 thyroid function tests including; T4, T3, FT4, FT3, anti-Thyroid Peroxidase (anti-TPO), Thyroglobulin Antibodies (ANTITG), Thyreoglobulin (TG), TSH and Reverse Triiodothyronine (RT3).
Treatment and follow up:
All patients were given sodium iodide oral solution and the dose of the drug was calculated according to the formula,
Drug dose=Estimated weight of the thyroid gland/ (maximum value of thyroid iodine uptake rate in 2 h/4 h/24 h)/10×(0.7-1.5)
The value of (0.7-1.5) was based on the clinician’s judgment of the patient’s condition. Patients were followed up and their thyroid function was known after treatment 2-3 mo. Post-treatment and the outcome of sodium iodide oral solution was categorized according to the 2016 American thyroid association guidelines for the hyperthyroidism and other thyrotoxic conditions and clinical guideline for 131I treatment of Grave’s hyperthyroidism (2021 edition)[4]. The outcome of sodium iodide oral treatment was complete remission, partial remission, or hypothyroidism occurred over 1 y follow-up. Complete remission was defined if the patient maintained normal thyroid function in the absence of Antithyroid Drug (ATD) or levothyroxine and had no signs or symptoms of hyperthyroidism and hypothyroidism; partial remission was defined if the patient had a decrease in serum FT4 and an improvement in the signs and symptoms associated with hyperthyroidism, but none of them returned to full normal.
Statistical analysis:
Continuous variables were expressed as means with standard deviation; categorical variables were expressed as number and percentage and evaluated using oneway logistic regression analysis. Variable selection took into account predictors including demographic and laboratory data measured at baseline. The data was randomly assigned to the training cohort and the validation cohort in a ratio of 7:3. Predictive modeling was performed in training group by multi-factor logistic regression analysis, regression datasets were generated, and then the estimated parameters were merged into the final model using the R language. A nomogram graph was plotted based on the predictors included in the final model. We applied the final model to the validation cohort. The performance of the model was evaluated using subject work Receiver Operating Characteristic (ROC) curves, Area Under the Curve (AUC), Hosmer- Lemeshow test, and calibration curves of the model, with goodness-of-fit curves as performance indicators. All analyses use Statistical Package for the Social Sciences (SPSS) 26.0, prism, R4.3.2 was performed.
Results and Discussion
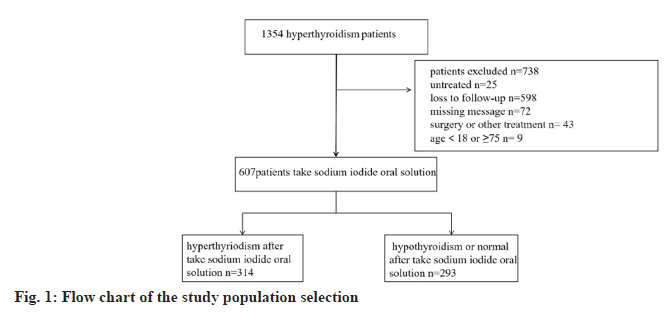
By collecting patients who were hospitalized by taking sodium iodide oral solution for treatment of hyperthyroidism in our hospital from 2016 to 2022, we followed up 1354 cases among them, 738 were excluded due to incomplete information or loss of visits, etc. And finally 607 patients were included in the study, with the age range of 18 y-75 y old, and the average age was about (39.16±12.83) y. Most of these patients used medications before taking sodium iodide oral solution (n=507). Among them 314 patients were hyperthyroid after taking sodium iodide oral solution and 293 patients showed remission of hyperthyroidism (hypothyroidism n=140, normal or partial remission of thyroid function n=153). Due to our geographical characteristics, 140 (22.1 %) of our patients were from ethnic minorities, of which 467 (76.9 %) were Han Chinese, Han Chinese and other ethnic groups (p=0.296) showed no significant difference in the efficacy of sodium iodide oral solution treatment. Among the patients, 183 (30.2 %) cases were male and 424 (69.8 %) cases were female, and the gender also have no significant difference in the outcome (p=0.953). However, the younger the age, the greater the likelihood of post-treatment hyperthyroidism (p=0.011) (Table 1 and fig. 1).
| Factor | n | Remission | Non-remission | p | Exp (B) |
|---|---|---|---|---|---|
| Total | 607 | 293 (48.3 %) | 314 (51.8 %) | ||
| Gender | 0.962 | 0.992 | |||
| Male | 183 (30.2 %) | 88 (30.0 %) | 95 (30.2 %) | ||
| Female | 424 (69.8 %) | 205 (70.0 %) | 219 (69.8 %) | ||
| Nation | 0.296 | 1.223 | |||
| Han | 467 (76.9 %) | 220 (75.0 %) | 247 (78.6 %) | ||
| Other | 140 (22.1 %) | 73 (25.0 %) | 67 (21.4 %) | ||
| Age | 39.16±12.833 | 40.54±12.676 | 37.88±12.864 | 0.011 | 0.984 |
| Disease history | 3.73±5.19 | 3.44±5.22 | 3.99±5.15 | 0.194 | 1.021 |
| missing | 1 | ||||
| Iodine dose (mCi) | 5.40±2.43 | 4.65±1.64 | 6.10±2.80 | 0 | 1.353 |
| Missing | 5 | ||||
| Medicine | 0.001 | 0.674 | |||
| No | 100 (16.5 %) | 63 (22.5 %) | 37 (11.8 %) | ||
| Yes | 507 (83.5 %) | 230 (78.5 %) | 277 (88.2 %) | ||
| Medicine after taking sodium iodide oral solution | 0.251 | 1.24 | |||
| No | 454 (67.8 %) | 213 (72.7 %) | 241 (76.7 %) | ||
| Yes | 153 (32.2 %) | 80 (27.3 %) | 73 (23.3 %) | ||
| Family history | 0.043 | 1.746 | |||
| No | 546 (89.9 %) | 256 (87.3 %) | 290 (92.3 %) | ||
| Yes | 61 (10.1 %) | 37 (13.7 %) | 24 (8.7 %) | ||
| Complication | 0.021 | 0.713 | |||
| No | 388 (64.0 %) | 201 (68.6 %) | 187 (59.9 %) | ||
| Yes | 219 (36.0 %) | 92 (31.4 %) | 127 (40.1 %) | ||
| Ultrasound nodule | 0.514 | 0.897 | |||
| No | 370 (60.9 %) | 182 (62.3 %) | 188 (59.8 %) | ||
| Yes | 236 (39.1 %) | 110 (37.7 %) | 126 (40.2 %) | ||
| Missing | 1 | ||||
| Ultrasound HT | 0.45 | 0.832 | |||
| No | 528 (87.1 %) | 258 (48.8 %) | 270 (51.2 %) | ||
| Yes | 79 (13.9 %) | 35 (44.3 %) | 44 (55.7 %) | ||
| Missing | 1 | ||||
| RAIU | |||||
| 2 h (%) | 58.32±23.12 | 52.09±21.49 | 64.14±23.12 | 0 | 1.024 |
| 4 h | 72.02±39.30 | 68.11±52.05 | 75.67±20.86 | 0.004 | 1.011 |
| 24 h | 79.99±14.87 | 78.22±15.11 | 81.65±14.48 | 0.005 | 1.016 |
| SPECT | |||||
| Weight | 46.46±33.37 | 35.21±17.52 | 56.95±40.50 | 0 | 1.032 |
| technetium-99 uptake | 603 (14.81±10.55) | 291 (11.55±8.00) | 312 (17.84±11.70) | 0 | 1.069 |
| Missing | 4 | ||||
| Thyroid function test (before RAI treatment) | |||||
| TT4 | 228.13±70.09 | 228.98±61.10 | 227.33±77.63 | 0.771 | 1 |
| TT3 | 6.13±3.79 | 292 (5.67±2.90) | 314 (6.56±4.42) | 0.005 | 1.073 |
| Missing | 1 | ||||
| FT4 | 64.61±29.75 | 64.72±27.84 | 64.50±31.47 | 0.928 | 1 |
| FT3 | 27.77±18.50 | 26.15±16.54 | 29.28±20.08 | 0.041 | 1.01 |
| TSH | 0.19±2.78 | 0.34±3.98 | 0.05±0.36 | 0.351 | 0.88 |
| Anti-TG | 535.22±1014.19 | 506.79±951.82 | 561.56±1069.00 | 0.507 | 1 |
| Anti-TPO | 243.48±200.27 | 244.24±196.21 | 242.78±204.28 | 0.929 | 1 |
| TGAg | 124.36±165.85 | 93.43±139.88 | 153.23±182.41 | 0.000 | 1.002 |
| RT3 | 564 (4.42±22.20) | 277 (3.65±16.68) | 287 (5.16±26.47) | 0.443 | 1.003 |
| Missing | 43 | ||||
Note: Exp (B): Exponentiation of the B coefficient
Table 1: Baseline Characteristics and Risk Factors Affecting Taking Sodium Iodide Oral Solution
Patients with complications (n=219) had a higher rate of hyperthyroidism than patients without complications (n=388) (p=0.021), also patients who had used medication before taking sodium iodide oral solution (n=507) were likely to have a higher rate of hyperthyroidism post-treatment than those who did not (n=100) (p=0.001); sodium iodide oral solution dosage and hyperthyroidism incidence were significantly correlated (p=0.000), and patients with a family history (n=61) were also likely to have a higher rate of hyperthyroidism (p=0.043) compared to those without a family history (n=546). Thus complications, use of medication before sodium iodide oral solution, sodium iodide oral solution dose and patients with family history were independent risk factors after taking sodium iodide oral solution. Antithyroid medication after taking sodium iodide oral solution was no significant difference between patients who did not use (n=454) and who used after treatment (n=153) (p=0.251). Longer duration of illness was not associated with hyperthyroidism after treatment (p=0.194).
In our comparison of thyroid static imaging, thyroid iodine uptake rate and nine items of thyroid function in the routine examination of patients with hyperthyroidism, we found that thyroid iodine uptake rate at 2 h (p=0.000), 4 h (p=0.004), 24 h (p=0.005), thyroid weight (p=0.000), technetium 99 uptake rate (p=0.000) and TT3 in the thyroid function ( p=0.005), FT3 (p=0.041), and TGAg (p=0.000) in thyroid function with the outcome of hyperthyroidism were independent risk factors for hyperthyroidism after sodium iodide oral solution, and higher thyroid weight and 2 h, 4 h, and 24 h thyroid iodine uptake rates were positively associated with persistence of hyperthyroidism after treatment.
We analyzed the patients with persistent hyperthyroidism after taking sodium iodide oral solution (n=314), patients who taking sodium iodide oral solution once (number of treatments=1, n=60) vs. patients who taking sodium iodide oral solution on multiple occasions (number of treatments ≥2, n=252), and we analyzed the risk factors associated with multiple treatments. By univariate logstic regression analysis age (p=0.017), comorbidities (p=0.005), were found to be risk factors for multiple treatments in patients with persistent hyperthyroidism.
Among the patients who remitted after taking sodium iodide oral solution (n=293),males (n=88), females (n=205), among those who developed hypothyroidism (n=140), and partial remission (n=153) we also performed subgroup analysis, in which medical history (p=0.047), medication after taking sodium iodide oral solution (p=0.001), and TGAg (p=0.044) were the risk factors for developing hypothyroidism after taking sodium iodide oral solution. In patients who developed hypothyroidism within 3 mo (n=52) compare those with >3 mo (n=98), there was no significant difference.
In order to find the correlation between ultrasound evaluation of patients with hyperthyroidism and their prognosis, we studied the ultrasound findings of the patients included in the study, There was no incidence of hyperthyroidism in patients with Hashimoto’s disease (n=79) than in those whose without Hashimoto’s disease (n=528) (p=0.450), and there was no significant difference in patients with co-morbid thyroid nodules (n=236) than in those without nodules (n=370) (p=0.514).
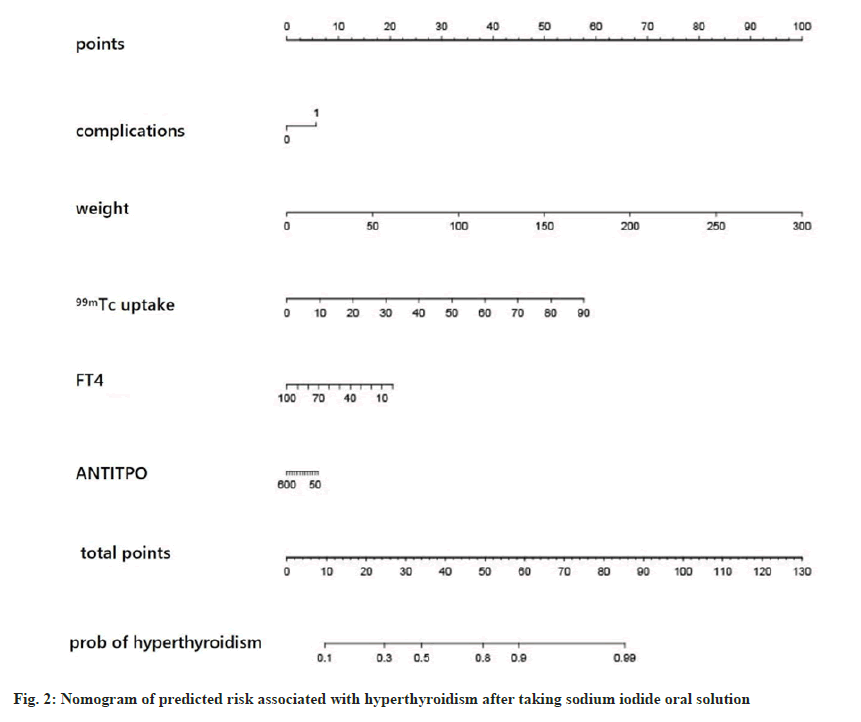
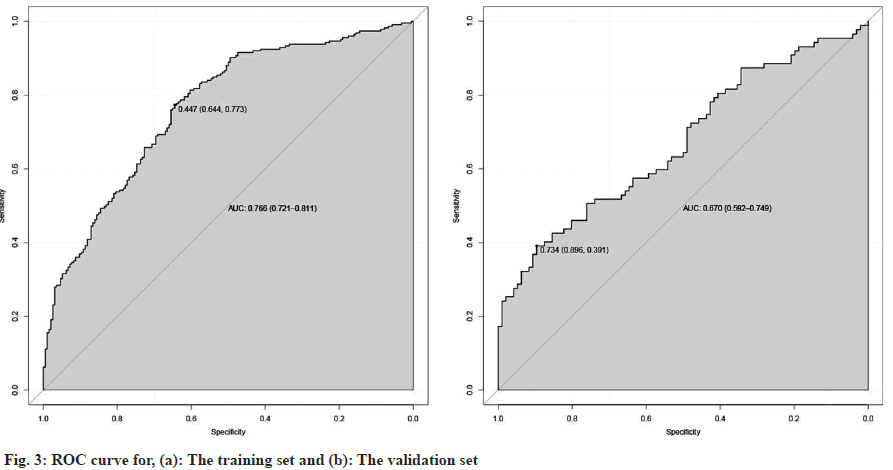
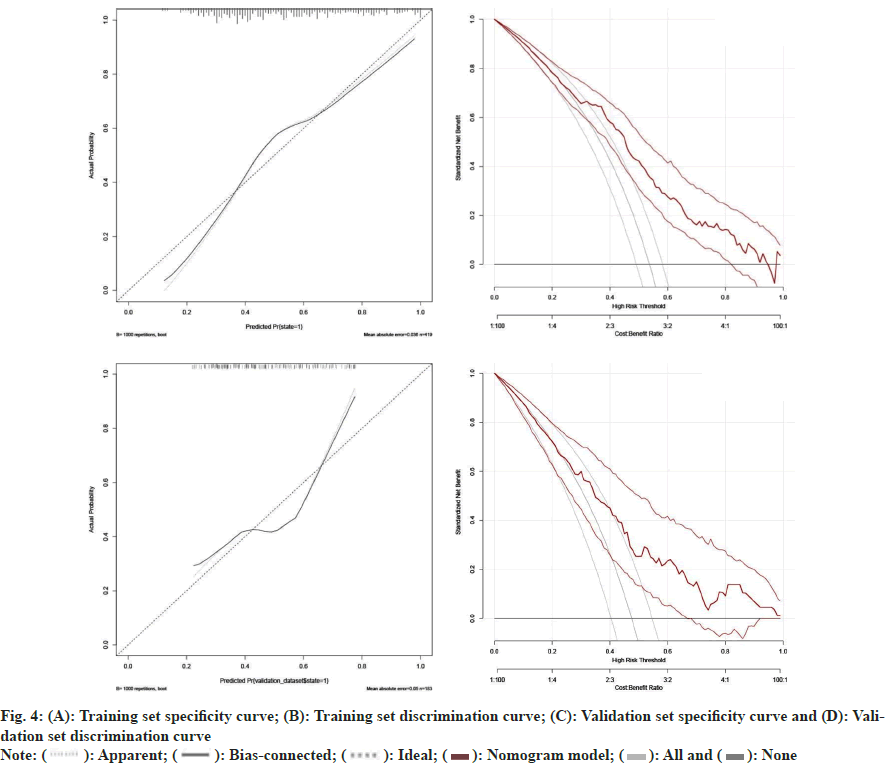
To further correlate the influencing factors obtained from our analysis with hyperthyroidism after taking sodium iodide oral solution, we used multivariate logistic regression analysis. We modeled the nomogram (fig. 2) with the persistence of hyperthyroidism after taking sodium iodide oral solution for hyperthyroidism by adding the thyroid weight, FT4, anti TPO, technetium 99 uptake, complications (Table 2). The ROC curves showed that training group AUC=0.766, 95 % Confidence Interval (CI): 0.865-0.988 and the validation group AUC=0.670, 95 % CI: 0.592–0.749 (fig. 3). The C-index of nomogram was 0.765 (95 % CI: 0.720 0.811), which showed good discrimination. The goodness-of-fit analysis Hosmer-Leimer test showed a significance of 0.102. The calibration curves and Decision Curve Analysis (DCA) were performed to determine the clinical utility of the nomogram. The nomogram related to hyperthyroidism after taking sodium iodide oral solution for hyperthyroidism shows complications, higher uptake of technetium 99 in the thyroid, higher estimation of thyroid static visualization, and lower pre-treatment FT4, anti- TPO may have higher hyperthyroidism rate after taking sodium iodide oral solution (fig. 4).
| B | p | OR (95 % CI) | |
|---|---|---|---|
| Complication | 0.506 | 0.029 | 1.659 (1.053-2.016) |
| Weight | 0.030 | 0.000 | 1.03 (1.053-2.016) |
| Technetium-99 uptake | 0.057 | 0.001 | 1.059 (1.053-2.016) |
| FT4 | -0.018 | 0.000 | 0.982 (1.053-2.016) |
| ANTI-TPO | -0.001 | 0.119 | 0.999 (1.053-2.016) |
Table 2: Multifactor Regression Analysis
The treatment of hyperthyroidism and patient management remains a vexing problem for endocrinologists; sodium iodide oral solution has a clear efficacy in the treatment of hyperthyroidism, but the precise dose and factors affecting the individual remain unclear. In our retrospective analysis, we found that nearly half of our patients had persistent hyperthyroidism after the first dose or took another dose of medication, which may be related to the population included in the study. While the gender and ethnicity was not observed to be associated with the outcome of hyperthyroidism.
Among the risk factors associated with the outcome of sodium iodide oral solution we found that age, dose of radioactive iodine, family history, complications, iodine uptake rate, thyroid weight, technetium 99 uptake, and thyroglobulin (TT3, FT3, TGAg) were independent risk factors for hyperthyroidism after treatment. Thyroid weight, 2 h, 4 h, 24 h iodine uptake rate’s in previous studies opinions were shown to be positively correlated with the persistence of hyperthyroidism after radioiodine treatment of hyperthyroidism[16]. This is consistent with our study. Technetium 99 uptake cannot be used to assess thyroid function, but in our study we found that the level of technetium 99 uptake correlated with the severity of hyperthyroidism[17]. In the study by Hou et al.[18] it was also shown that technetium 99 uptake was positively related to the outcome of hyperthyroidism. In a meta-analysis on the effect of methimazole on the efficacy of radioactive iodine it was shown that patients who were pretreated with methimazole prior to radioiodine had a higher posttreatment outcome. Patients had a higher risk of post-treatment hyperthyroidism (1.02-fold), while the length of discontinuation was not statistically significant for the persistence of hyperthyroidism[19]. In the study by Okosieme et al.[20] it was shown that treatment with radiopharmaceuticals within the previous week resulted in a failure of radioiodine therapy. In another meta-analysis of multiple drugs included in the study, medications were used within the 14 d period before and after the administration of radioiodine. Treatment of patients with toxic nodular goiter who were treated (and discontinued within 7 d prior to treatment) was significantly less successful than treatment of patients who discontinued medications within 14 d prior to treatment, which was not significantly altered in Grave’s disease[21]. While another study noted treatment success in patients who did not discontinue medications compared to those who discontinued antithyroid medications (carbimazole, methimazole, or propylthiouracil) 3 d-7 d prior to sodium iodide oral solution (fewer patients had normal thyroid function or hypothyroidism)[22,23]. Therefore, longer withdrawal times or other measures may be needed to ensure the efficacy of sodium iodide oral solution in patients who are on preoperative medications at the time of treatment.
Ultrasound can assist in the evaluation of thyroid size, nodules, and blood flow, and some studies have shown that ultrasound suggests that increased blood flow within the thyroid gland correlates with the degree of hyperthyroidism. It can also identify the cause of the thyroid gland (e.g. subacute thyroiditis, etc.,). Ultrasound detection of thyroid volume was shown to correlate with the outcome of hyperthyroidism in a study by Wang et al.[24]. However, we did not find a correlation between ultrasound and the prognosis of hyperthyroidism treatment.
The presence or absence of complications, thyroid weight, thyroid uptake rate and FT4, anti-TPO among the clinical indicators before sodium iodide oral solution were correlated with the persistence of hyperthyroidism after radioiodine treatment, and based on these indicators, a column-line graph model was developed to predict the patient’s pretreatment clinical indicators for the persistence of hyperthyroidism after radioiodine treatment. It is intended to assist clinicians in evaluating patient’s drug dosages. However, our results may not necessarily be applicable to other centers due to the presence of a large number of ethnic minorities in our center as well as highland areas.
Sodium iodide oral solution for hyperthyroidism is widely used worldwide, as it has the advantages of fewer side effects and fewer thyroid crises[25-27]. When compared with ATD and surgery, the overall recurrence rate was 52.7 % for ATD, 15 % for sodium iodide oral solution, and 10 % for surgery[16]. The recurrence rates of sodium iodide oral solution and surgery converged. However, surgery still carries risks such as postoperative scarring and intraoperative complications. The choice of subsequent treatment for patients with persistent hyperthyroidism after sodium iodide oral solution is still controversial. A study by Kim et al.[28] showed that a second RAI treatment showed a higher remission rate and shorter duration of remission. Persistent hyperthyroidism after the second treatment also reduces the dose of ATD required by the patient. Repeat sodium iodide oral solution may be an effective treatment option for GD patients with small goiters, long intervals between first time taking sodium iodide oral solution treatments, and a history of ATD discontinuation after the first taking sodium iodide oral solution treatment[29,30].
We have found a way to assess the prognosis for the treatment of patients with hyperthyroidism, but, there are limitations in our retrospective research. First, the majority of patients miss visits resulting in an inability to describe the full situation. Second, lack of an external validation cohort and multicenter matched cohort to prove utility and validation of the model. Third, as the prediction model was built in the context of Chinese population, hence its usefulness is limited. We suggested that for patients who are at high risk to have persistent hyperthyroidism after sodium iodide oral solution, in addition to raising the initial radioactive iodine therapy dose, the patient’s wishes need to be taken into account, and for patients who are very mindful of postoperative hypothyroidism, the patient’s dosage may need to be further considered in the patient-physician communication.
Conflict of interests:
The authors declared no conflict of interests.
References
- De LS, Lee SY, Braverman LE. Hyperthyroidism. Lancet 2016;388(10047):906-18.
- Taylor PN, Albrecht D, Scholz A, Gutierrez-Buey G, Lazarus JH, Dayan CM, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol 2018;14(5):301-16.
[Crossref] [Google Scholar] [PubMed]
- Kwon H, Jung JH, Han KD, Park YG, Cho JH, Han JM, et al. Prevalence and annual incidence of thyroid disease in Korea from 2006 to 2015: A nationwide population-based cohort study. Endocrinol Metab 2018;33(2):260.
[Crossref] [Google Scholar] [PubMed]
- Li Y, Teng D, Ba J, Chen B, Du J, He L, et al. Efficacy and safety of long-term universal salt iodization on thyroid disorders: Epidemiological evidence from 31 provinces of mainland China. Thyroid 2020;30(4):568-79.
[Crossref] [Google Scholar] [PubMed]
- Smith TJ, Hegedus L. Grave?s disease. N Engl J Med 2016;375(16):1552-65.
- Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 2016;26(10):1343-421.
[Crossref] [Google Scholar] [PubMed]
- Brito JP, Payne S, Singh Ospina N, Rodriguez-Gutierrez R, Maraka S, Sangaralingham LR, et al. Patterns of use, efficacy, and safety of treatment options for patients with Graves' disease: A nationwide population-based study. Thyroid 2020;30(3):357-64.
[Crossref] [Google Scholar] [PubMed]
- Kahaly GJ, Bartalena L, Hegedüs L, Leenhardt L, Poppe K, Pearce SH. 2018 European Thyroid Association guideline for the management of Graves? hyperthyroidism. Eur Thyroid J 2018;7(4):167-86.
[Crossref] [Google Scholar] [PubMed]
- Lassmann M, Hanscheid H, Chiesa C, Hindorf C, Flux G, Luster M. EANM Dosimetry Committee series on standard operational procedures for pre-therapeutic dosimetry I: Blood and bone marrow dosimetry in differentiated thyroid cancer therapy. Eur J Nucl Med Mol Imag 2008;35:1405-12.
[Crossref] [Google Scholar] [PubMed]
- Stokkel MP, Handkiewicz Junak D, Lassmann M, Dietlein M, Luster M. EANM procedure guidelines for therapy of benign thyroid disease. Eur J Nucl Med Mol Imag 2010;37(11):2218-28.
[Crossref] [Google Scholar] [PubMed]
- Taprogge J, Gape PM, Carnegie-Peake L, Murray I, Gear JI, Leek F, et al. A systematic review and meta-analysis of the relationship between the radiation absorbed dose to the thyroid and response in patients treated with radioiodine for grave?s disease. Thyroid 2021;31(12):1829-38.
[Crossref] [Google Scholar] [PubMed]
- Silberstein EB, Alavi A, Balon HR, Clarke SE, Divgi C, Gelfand MJ, et al. The SNMMI practice guideline for therapy of thyroid disease with 131I 3.0. J Nucl Med 2012;53(10):1633-51.
[Crossref] [Google Scholar] [PubMed]
- Bartalena L. Diagnosis and management of Graves? disease: A global overview. Nat Rev Endocrinol 2013;9(12):724-34.
[Crossref] [Google Scholar] [PubMed]
- Aung ET, Zammitt NN, Dover AR, Strachan MW, Seckl JR, Gibb FW. Predicting outcomes and complications following radioiodine therapy in Graves? thyrotoxicosis. Clin Endocrinol 2019;90(1):192-9.
[Crossref] [Google Scholar] [PubMed]
- Kaplowitz PB, Jiang J, Vaidyanathan P. Radioactive iodine therapy for pediatric Graves? disease: A single-center experience over a 10 y period. J Pediatr Endocrinol Metab 2020;33(3):383-9.
[Crossref] [Google Scholar] [PubMed]
- Sundaresh V, Brito JP, Wang Z, Prokop LJ, Stan MN, Murad MH, et al. Comparative effectiveness of therapies for Graves' hyperthyroidism: A systematic review and network meta-analysis. J Clin Endocrinol Metab 2013;98(9):3671-7.
[Crossref] [Google Scholar] [PubMed]
- Mariani G, Tonacchera M, Grosso M, Orsolini F, Vitti P, Strauss HW. The role of nuclear medicine in the clinical management of benign thyroid disorders, part 1: Hyperthyroidism. J Nucl Med 2021;62(3):304-12.
[Crossref] [Google Scholar] [PubMed]
- Hou H, Hu S, Fan R, Sun W, Zhang X, Tian M. Prognostic value of-pertechnetate thyroid scintigraphy in radioiodine therapy in a cohort of Chinese grave?s disease patients: A pilot clinical study. Biomed Res Int 2015;2015:974689.
[Crossref] [Google Scholar] [PubMed]
- Bolakale-Rufai IK, Abioro I, Ngene SO, Woldeamanuel Y. Efficacy of methimazole before the administration of radioactive iodine in the management of Graves? disease: A systematic review and meta-analysis. Sao Paulo Med J 2023;141(5):e2022225.
[Crossref] [Google Scholar] [PubMed]
- Okosieme OE, Chan D, Price SA, Lazarus JH, Premawardhana LD. The utility of radioiodine uptake and thyroid scintigraphy in the diagnosis and management of hyperthyroidism. Clin Endocrinol 2010;72(1):122-7.
[Crossref] [Google Scholar] [PubMed]
- Karyampudi A, Hamide A, Halanaik D, Sahoo JP, Kamalanathan S. Radioiodine therapy in patients with Graves? disease and the effects of prior carbimazole therapy. Indian J Endocrinol Metab 2014;18(5):688-93.
[Crossref] [Google Scholar] [PubMed]
- Eschmann SM, Thelen MH, Dittmann H, Bares R. Influence of short-term interruption of antithyroid drugs on the outcome of radioiodine therapy of Grave?s disease: Results of a prospective study. Exp Clin Endocrinol Diabetes. 2006;114(05):222-6.
[Crossref] [Google Scholar] [PubMed]
- Zannat R, Lee J, Muzaffar J, Read ML. The potential interaction between medical treatment and radioiodine treatment success: A systematic review. Front Endocrinol 2023;13:1061555.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Hong L, Lu G, Yang S, Li L, Huang X, et al. Ultrasound combined with Ki67 detection for analyzing contributing factors of failure to cure and recurrence of hyperthyroidism in patients with Grave?s disease after 131I treatment. Nan Fang Yi Ke Da Xue Xue Bao 2022;42(12):1902-6.
[Crossref] [Google Scholar] [PubMed]
- McDermott MT, Kidd GS, Dodson Jr LE, Hofeldt FD. Radioiodine-induced thyroid storm: Case report and literature review. Am J Med 1983;75(2):353-9.
[Crossref] [Google Scholar] [PubMed]
- Akamizu T, Satoh T, Isozaki O, Suzuki A, Wakino S, Iburi T, et al. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid 2012;22(7):661-79.
[Crossref] [Google Scholar] [PubMed]
- Walter MA, Briel M, Christ-Crain M, Bonnema SJ, Connell J, Cooper DS, et al. Effects of antithyroid drugs on radioiodine treatment: Systematic review and meta-analysis of randomised controlled trials. BMJ 2007;334(7592):514.
[Crossref] [Google Scholar] [PubMed]
- Kim MJ, Cho SW, Kim YA, Choi HS, Park YJ, Park DJ, et al. Clinical outcomes of repeated radioactive iodine therapy for Graves? disease. Endocrinol Metab 2022;37(3):524.
[Crossref] [Google Scholar] [PubMed]
- Saadat N, Azizi F, Abdi H, Amouzegar A. Treatment of post-radioactive iodine relapse of hyperthyroidism: Comparison of long-term methimazole and radioactive iodine treatment. J Endocrinol Invest 2022;45(10):1919-24.
[Crossref] [Google Scholar] [PubMed]
- Lin L, Sijin L. Clinical guideline for 131I treatment of Grave?s hyperthyroidism (2021 edition) (in Chinese). Chin J Nucl Med Mol Imag 2021;41(4):242-53.