- Corresponding Author:
- Mahalaxmi rathananand
Department at Yale Schol of Medicine Yale University, New Haven, CT 06519, USA
E-mail: dawn.foster@yale.edu
| Date of Submission | 2 September 2006 |
| Date of Revision | 3 July 2007 |
| Date of Acceptance | 22 September 2007 |
| Indian J. Pharm. Sci., 2007, 69 (5): 651-657 |
Abstract
In this study the suitability of spray drying as a method for the formulation of mucoadhesive microspheres for nasal delivery was evaluated. The microspheres were produced from mucoadhesive polymers including chitosan salt, hydroxypropylmethylcellulose, hydroxypropylcellulose, sodium alginate and contained levocetirizine dihydrochloride as the model drug. The microspheres formed were evaluated for particle size distribution, drug loading, production yield, in vitro release characteristics and suitability for nasal drug delivery in terms of particle size and release properties. The conditions of the spray dryer were optimized for production yield and particle size.
Keywords
Microspheres, nasal delivery, spray drying, levocetirizine.
There has been increasing interest in using the nose as a route for administration of systemically active drugs. One of the reasons for the low degree of absorption of peptides and proteins via the nasal route is rapid movement away from the absorption site in the nasal cavity due to the mucociliary clearance mechanism. In order to overcome the barriers to nasal absorption of drugs two main approaches have been utilized: modification of the permeability of the nasal membrane by employment of absorption enhancers (such as surfactants, bile salts, cyclodextrins, phospholipids, and fatty acids), which can promote the absorption of poorly absorbable drugs and the use of mucoadhesive systems such as bioadhesive liquid formulations, microspheres, powders, and liquid gelling formulations that decrease the mucociliary clearance of the drug formulation and thereby increase the contact time between the drug and the site of absorption. Nasal drug delivery is a useful delivery method for drugs that are active in low doses and show no or minimal oral bioavailability such as proteins and peptides [1-5]. Microsphere technology is one of the specialized systems becoming popular for designing nasal products, as it may provide prolonged contact with the nasal mucosa and thus enhances absorption and bioavailability. In the presence of microspheres, the nasal mucosa is dehydrated due to moisture uptake by the microspheres. This result in reversible shrinkage of the cells, providing a temporary physical separation of the tight (intercellular) junctions that increases the absorption of the drugs [6-12]. Particularly important for the nasal drug absorption is the respiratory region, which contains three nasal turbinates and the deposition of the particles in this region will depend on their size. Classically, larger particles including droplets (>10 µm), are deposited in the nasal cavity after inhalation; the larger the particles, the more anterior the deposition. For smaller particles the site of deposition depends on the velocity at which the particles are inhaled and the turbulence in the air flow, however the particles of size smaller than 1 µm are not normally deposited in the nasal cavity but travel down to the trachea to reach the lung. The rationale behind the use of a microsphere system is that, the application of bioadhesive microspheres (in the powder form) with good bioadhesive properties would permit such microspheres to swell in contact with nasal mucosa to form a gel and control the rate of clearance from the nasal cavity, thereby giving poorly absorbed drugs a longer time to be available at the absorptive surface. Microspheres have been reported to be present up to 3-5 h in the nasal cavity depending upon the bioadhesive material used for formulation. The ideal microsphere particle size requirement for nasal delivery should range from 10 to 50 µm as smaller particles than this will enter the lungs. However, studies have reported that a significant portion of small particles are also deposited in the nose. The capacity of removal for the upper respiratory tract is 100% for the particles with size larger than 10 µm, and approximately 80% for the particles of 5 µm. Clearance capacity drops progressively with further reduction in size and approaches zero for particles at 1-2 µm. Studies have also reported that 4 µm is the sufficient size for the nasal delivery.
As far as nasal microspheres are concerned, spray drying [13-17] is an important method for its preparation. It will give rise to microspheres in which active drug will be in the matrix of the polymer. Although the drying air temperature may be relatively high (e.g. >100°), the actual temperature of the evaporating droplets is significantly lower due to cooling by latent heat of vaporization. Thus, thermal degradation of the active ingredient is not so much a concern as it first appears [18],[19]. The properties of the spray-dried microspheres can be controlled by both the process and formulation parameters. The advantages of using this process for nasal delivery is that it can be operated in aseptic conditions, because as per US FDA regulations the nasal products should be manufactured as sterile or preserved. Microspheres formed by this method have a very high drug loading. Moreover the process is more feasible for scaling up compared to other microsphere fabrication methods, for instance emulsion solvent extraction [20-21].
Vidgren et al.[20], prepared microspheres of disodium cromoglycate (DSCG) with either polyacrylic acid (Carbopol 934) or sodium carboxymethylcellulose (NaCMC) by the spray drying technique and evaluated for in vitro characteristics. The current study aimed to observe the suitability of the spray drying as a technique for the formulation of the mucoadhesive microspheres for nasal delivery. As this route is limited by the particle size of the microsphere system, this factor was chosen as the primary objective of the study. The model drug chosen was levocetirizine dihydrochloride. The drug is water soluble and is used for the seasonal allergic rhinitis, perennial allergic rhinitis, chronic urticaria, unlabeled uses are in pollen induced asthma (alone or as an adjunct) and atopic dermatitis including atopic eczema.
Materials and Methods
Levocetirizine dihydrochloride (m.p. 225°) manufactured by Symed Labs Ltd., India, was acquired from Orchid Chemicals and Pharmaceuticals Ltd., Chennai, India. Hydroxypropylmethylcellulose (Methocel E5) was obtained from Colorcon Asia Pvt. Ltd. Verna, India. Sodium alginate was obtained from ISP, Alginates, San Diego, UK; Chitosan salt was a product from Signet Chemical Corporation, Mumbai, India. Eudragit was obtained from Degussa, Germany and HPC obtained from Signet Chemical Corporation, Mumbai, India. Spray dryer (SD-05) was from Lab Plant, G. B. R, stereomicroscope (Leica Microsystems, Cambridge, UK), dissolution apparatus, USP Type-3 (Vankel Technology Group N.C.), UV Spectrophotometer (Varian), magnetic stirrer (Remi, Mumbai).
Preparation of the microspheres
Table 1 shows the various formulae used for the production of the microspheres using spray drying technique. Feed solutions were prepared by dissolving the polymer(s) in the solvent/solvent mixture by vortexing (using magnetic stirrer) and then adding the drug to this polymer solution and stirred to dissolve it. In case of formulation-II, the slurry of polymers was prepared in half of the volume of water using mechanical stirrer, then the solution of the drug in water was added. The remaining water was added and stirred till a clear solution was formed. These solutions were used as the feed solutions for spray dryer. The conditions optimized for the different formulae are shown in the Table 2. The flow rate of 2.472 ml/min was executed at 2 rpm pump speed; air flow of 30 m 3/h was used for all the formulations.
Drug loading in microspheres
Aqueous solutions of levocetirizine dihydrochloride were prepared at concentrations of 1, 2, 5, 8, 10, 12, 15 µg/ml. The solutions were assayed using a UV Spectrophotometer at a wavelength of 230.1 nm to generate a calibration plot. The actual loading of the drug in microspheres was determined by suspending 25 mg of microspheres in 100 ml of pH 6.4 buffer and stirred on a magnetic stirrer for about 8 h to ensure the complete dissolution of the drug. This solution was filtered through the 0.45 µm nylon filter and absorbance of the solution at 230.1 nm was taken after suitable dilution, taking a placebo microspheres batch processed in the same way as the blank. An approximation of the amount of un entrapped drug was determined by stirring 25 mg of microspheres in 5 ml of water for about 1 minute and filtering the solution through a 0.45 µm nylon filter, and measuring the absorbance at 230.1 nm taking a blank of the placebo batch processed in the same way. The loading values were reported as percent of the theoretical loading, which is calculated as (Actual loading)–(nonentrapped drug)×100/ Theoretical loading.
Particle size distribution
The microspheres were evaluated for particle size and shape. A stereomicroscope was used for the purpose, which was calibrated using calibration micrometers. The microscope was equipped with the software, Image Manager through a camera. A suspension of the microspheres was prepared on a slide using Nujol and covered with the cover slip to form a specimen. This slide was observed under the microscope. An image was clicked and used for the particle size and shape analysis. The magnification of the microscope used for observations was 115X. Size of around 250 particles was measured for each batch on the different portions of the slide. From the size distribution the average particle size and standard deviation σ were calculated for each batch of microspheres.
Measurement of the in vitro release rate
A modification of the USP-III (reciprocating cylinder type) was used. The media used in this method was phosphate buffer of pH 6.4, the volume of media taken was 25 ml that will just touch the surface of the reciprocating cylinder’s mesh # 400. The temperature of the media was maintained at 37±0.5°. The media was kept covered throughout the experiment and the dipping mechanism was stopped i.e. the dissolution profiles were developed in static conditions. Periodical samples of 3 ml were withdrawn and replaced after uplifting the assembly. The samples were taken after stirring the media. Samples were filtered through a 0.45 µm nylon filter and, after suitable dilution; absorbances were taken at 230.1 nm. The experiment was performed in triplicate and average values reported.
Stability of the microspheres
The microsphere batches were stored at 25o and 65% RH for 30 d and observed for the size and shape at 10 d interval. The drug content was determined after the study using the method of determination of drug loading.
Results and Discussion
The yield obtained from these three formulations was 20.02±1.088% (formulation-I), 45.86±1.009% (formulation-II) and 63.44±1.109% (formulation- III). The primary reason for low yields is the high viscosity of the feed solution, which requires a longer drying time that can be applied by changing drying parameters such as temperature, air flow rate. But these parameters are interlinked so it was not possible to optimize the root cause. As the yield was very low in formulation-I, this preparation was not subjected to further evaluation.
The low polymer concentration was not able to give the desired particle size as the droplet size was small and most of the part of drop consisted of solvent which would evaporate leaving the small particles. Therefore, the polymer concentration was increased and optimized for at 3-6% w/v for different formulations.
The particle size range in both the formulations (II and III) was 5-55 µm with an average of 15.48±6.979 and 15.08±7.427 µm, respectively with a moderate drug loading of 20%. All formulations were spherical in particle shape with a few elongated particles as exceptions. The increment in the drug loading appeared to have an enhancing effect on the size of the microspheres, but this effect was not significant. The results clearly show that in all the batches the particle size range is suitable for nasal delivery, as more than 75% particles are in the range 10-40 µm. Previous studies showed that 4-5 µm size was sufficient, but we have presented a superior particle size range in comparison.
Drug loading was notably high in this method whilst generally low in the other methods. The reason for a high loading in the microspheres prepared by spray drying is the evaporation of the liquid phase and the remaining solid phase will entrap the drug with little drug contained outside the matrix. The percent loading is decreasing as the theoretical loading is increased because more drug remains unentrapped and hence the percent loading decreases (Table 3).
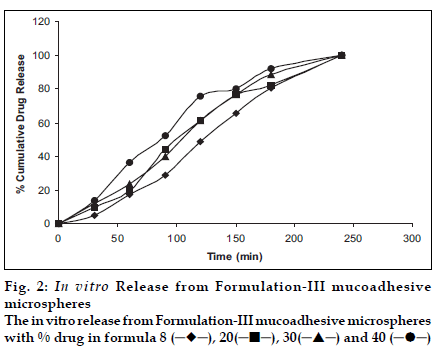
Figs. 1 and 2 show the release profiles of levocetirizine dihydrochloride from the microspheres prepared with formulation-II and III, respectively. The microspheres prepared in the formulation design were free flowing, and showed a great deal of agglomeration upon observation under the stereomicroscope.
The release pattern obtained shows an extended release up to 2 h in formulation-II and up to 4 h in formulation-III. The burst effect observed in formulation-II is due to rapid dissolution initially but the effect was absent in formulation-III possibly due to slow dissolution as Eudragit is a pH dependent polymer, that slowly dissolves above pH 6.0. Moreover the conditions are static and media volume is lower. The method is therefore discriminatory for different formulations. The release of the drug almost at every interval is higher for formulations with higher drug loading. Chitosan is more soluble in aqueous media and gave a faster release. The method shows a release profile that will be easy to correlate with in vivo data as the mucoadhesive microspheres are reported to reside for a period of 2-4 h in the nasal cavity; depending on its mucoadhesivity. Moreover the method seems to simulate the conditions as following delivery, the microspheres stick to the mucous membrane and will be almost static (a very little agitation of mucocilliay clearance) to give a drug release in very low volumes of nasal fluids. Sink conditions will be due to the nasal blood flow only and not due to nasal fluid.
Levocetirizine dihydrochloride is a water soluble drug that dissolves almost immediately (about 5 min). In the microsphere the drug is dispersed in the polymer matrix and dissolves in water by diffusion through and erosion of the polymer matrix. Drug release was observed to be slower with increased polymer concentrations i.e. higher loading batches gave faster release.
On storing at 25°and 65% RH, microspheres were stable as far as morphology is concerned. The average particle size remained relatively unchanged. Drug loss was minor as observed after a month study. There was 0.07±0.0032% drug loss in formulation-II and 0.05±0.003% in formulation-III. Formulation- III contained the polymer Eudragit L-100 which shows change in physical appearance i.e. it is getting elongated at higher temperature so conditions could not be altered so much for this polymer and except the temperature other conditions were kept same as these were optimized for the formulation-II. On the basis of various trials taken, the effect of the flow rate of the feed solution (controlled by pump speed), inlet temperature and nozzle size used on particle size and yield of the product were studied for the formulation- II (Table 4).
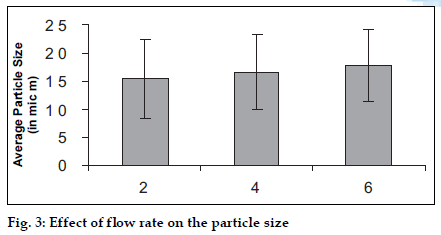
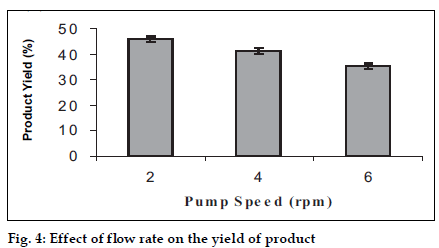
A study was carried out using three levels of the pump speed i.e. 2, 4, 6 rpm and effects were observed (figs. 3 and 4).The increase in flow rate causes an increase in the average particle size, which is due to the formation of the bigger droplets as more amount of the liquid is available for the spraying. But the yield of the product decreases with increase in the flow rate, the reason for which may be the loss of the product in the un-dried form due to deposition on the drying chamber walls since bigger droplets require more drying time to form microspheres.
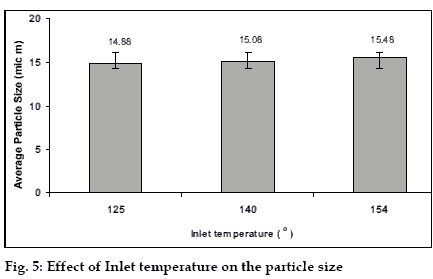
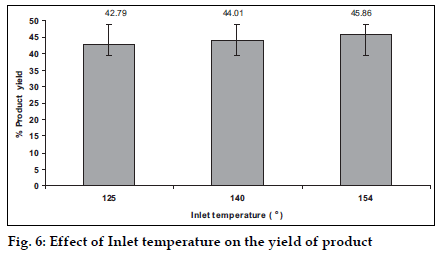
A change in the inlet temperature of the equipment can influence the drying rate. So a study was carried out using three levels of the inlet temperature i.e. 125, 140, 155° and the effects were observed (figs. 5 and 6). The increase in the inlet temperature of the equipment shows a very small, (which may be insignificant) increment of the average particle size. The increase in the temperature leads to the fast drying of the droplets and increases the proportion of the larger particles in the product, as the bigger droplets will dry faster with high temperature. The increase was small due to the reason that the increment of inlet temperature leads to a little increase in the temperature of the surface of the droplets because of the latent heat of evaporation playing a role. It leads to an increase in the yield of the product due to the faster drying of the droplets.
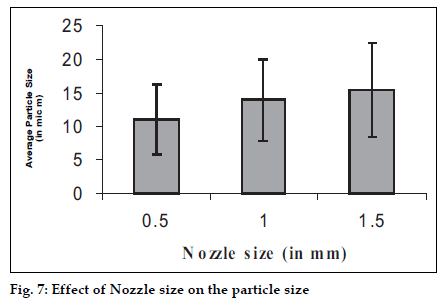
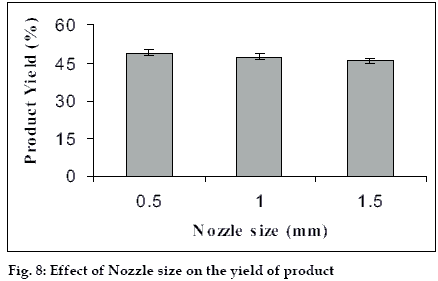
The size of the microspheres depended upon the type and size of the nozzle used and it can influence the yield of the microspheres hence the study was carried out with three different nozzle sizes i.e. 0.5, 1.0, 1.5 mm and the effects were observed (figs. 7 and 8). The bigger nozzle produce bigger droplets which upon drying form larger spheres and hence the proportion of bigger particle increases in the product. Whereas this increase in the droplets size has also caused reduction in the yield of the product as the bigger droplets take more time to get dried.
This study shows that it is possible to prepare mucoadhesive microspheres with good morphological characteristics using spray-drying technique. The microspheres can be formed with relatively high drug loading [94-98%], which is generally very less in other methods. The type and amount of polymer can be changed to achieve the objectives as change in polymer can alter the dissolution properties of the microspheres. The conditions for spray drying can be optimized to achieve the particular objectives like particle size and yield of product. The water proportion in the feed solution, its viscosity and the dimensions of the equipment are very important for a particular product. Size of the microspheres and yield can be altered using the different nozzle sizes, temperature and flow rate of the feed solution. The microspheres prepared are suitable for the delivery to nasal mucosa as the size distribution achieved was in the desired range. The dissolution profiles developed have shown probable candidature for in vitro and in vivo correlation (IVIVC). Stability studies carried out show that there was no change in the particle morphology and drug content batches were kept at 25o and 65% RH for 30 days. So the drug is well protected inside the polymer matrix.
References
- Chien YW, Kenneth SE, Chang SF. In : Nasal systemic delivery. Inc. Marcel Dekker: New York;1989. p. 1-297.
- Chein YW, Chang SF. Intranasal drug delivery for systemic medications. Crit Rev Ther Drug Carrier Syst 1987;4:67-194.
- Chein YW, Chang SF. In :Chein YW. editors. Transnasal systemic medications: Fundamentals concepts and biomedical assessment. Elsevier Science Publishers: Amsterdam; 1985.
- Chang SF, Chein YW. Intranasal drug administration for systemic medication. Pharm Int 1984;5:287.
- Illum L. Nasal drug delivery-possibilities, problems and solutions. J Control Release 2003;87:187-98.
- Nagai T, Machida Y. Mucosal adhesive dosage forms. Pharm Int 1985;6:196-200.
- Kamath KR, Park K. Mucosal Adhesive preparations. In :Swabrick J. Boylan JC. editors. Encyclopedia of Pharmaceutical Technology. Marcel Dekker: New York; 1994. p. 133.
- Jimenez-Castellanous MR, Zia H, Rhodes CT. Mucoadhesive drug delivery. Drug DevInd Pharm 1993;19:143-94.
- Morimoto K, Yamaguchi H, Iwakura Y, Morisaka K, Ohashi Y, Nakai Y. Effects of viscous hyaluronate sodium solutions on the nasal absorption of vasopressin and an analogue. Pharm Res 1991;8:471-4.
- Ikeda K, Murata K, Kobayashi M, Noda K. Enhancement of bioavailability of dopamine via nasal route in beagle dogs. Chem Pharm Bull 1992;40:2155-8.
- Nagai T, Nishimoto Y, Nambu N, Sekine K. Powder dosage form of insulin for nasal administration. J Control Release 1984;1:15-22.
- Illum L, Farraj NF, Davis SS. Nasal administration of Gentamicin using a novel Microsphere delivery system. Int J Pharm 1988;46:261-5.
- van der Lubben IM, van Opdorp FA, Hengeveld MR, Onderwater JJ, Koerten HK, Verhoef JC, et al. Transport of chitosan microparticles for mucosal Vaccine delivery in a Human intestinal M-cell model. J Drug Target 2002;10:449-56.
- Ozbas-Turan S, Akbuga J, Aral C. Controlled release of interleukin-2 from chitosan microspheres. J Pharm Sci 2002;91:1245-51.
- Hasiek C, G fnül N, Erk N. Mucoadhesive microspheres containing gentamicin sulfate for nasal administration: Preparation and in vitro characterization. Farmaco 2003;58:11-6.
- He P, Davis SS, Illum L. In vitro evaluation of the mucoadhesive properties of chitosan microspheres. Int J Pharm 1998;166:75-88.
- Bruschi ML, Cardoso ML, Lucchesi MB, Gremio MP. Gelatinmicroparticles containing propolis obtained by spray-drying technique: Preparation and characterization. Int J Pharm 2003;264:45-55.
- Lim ST, Martin GP, Berry DJ, Brown MB. Preparation and evaluation of the in vitro drug release properties and mucoadhesion of novel microspheres of hyaluronic acid and chitosan. J Control Release 2000;66:281-92.
- Morimoto K, Katsumata H, Yabuta T, Iwanaga K, Kakemi M, Tabata Y, et al . Evaluation of gelatin microspheres for nasal and intramuscular administrations of salmon calcitonin. Eur J Pharm Sci 2001;13:179-85.
- Vidgren P, Vidgren M, Arppe J, Hakuli T, Laine E, Paronen P. In vitro evaluation of spray-dried mucoadhesive microspheres for nasal administration. Drug Develop Ind Pharm 1992;18:581-97.
- Huang YC, Yeh MK, Chiang CH. Formulation factors in preparing BTM-chitosan microspheres by spray drying method. Int J Pharm 2002;242:239-42.
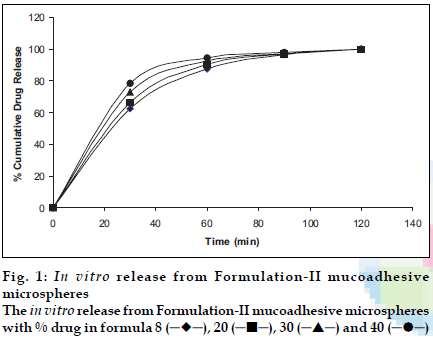
 ), 20 (
), 20 ( ), 30 (
), 30 ( ) and 40 (
) and 40 ( )
)