- *Corresponding Author:
- D. Madamwar
Department of biosciences, Sardar patel university, Vallabh vidyanagar - 388 120, India
E-mail: datta_madamwar@yahoo.com
| Date of Submission | 27 October 2006 |
| Date of Revision | 7 September 2007 |
| Date of Acceptance | 3 December 2007 |
| Indian J Pharm Sci, 2007, 69 (6): 784-790 |
Abstract
Alternate copolymers of n-butyl methacrylate and maleic anhydride were prepared and characterized. Amoxycillin was bound on copolymers through pendent anhydride groups by chemical bonding. In vitro release rates were investigated in 0.1 M phosphate buffer solution with varying pH at 37 0 as well as in biological media. The release rates of the investigated model drug molecules followed first order kinetics and were dependent on buffer concentration and pH. It was observed that molecular weight of copolymers varied from 8000 to 22,000 g/mol and duration of the delay period prior to release increased from 6 to 9 d. P(BMA-MA-1) showed increase in cumulative release rate with increase in pH unit from 3.8 to 7.2, which further decreased from pH 7.8 and 8.0, respectively. With P(BMA-MA-2) gradual increase in release activity was observed in acidic range and in neutral to alkaline condition (pH 7.0 to 8.0) the release activity lasted for 7 to 9 d respectively. Similar condition for P(BMA-MA- 3) showed cumulative release activity for 9 d in acidic pH, which further decreased to 7 d from pH 7.0 to 8.0. P(BMA-MA-1, 2 and 3) showed maximum inhibition of 40%, 59.3% and 72.8% towards Bacillus subtilis, 40%, 44% and 65.4% inhibition against Staphylococcus aureus and 41.2%, 51.7% and 69.1% inhibition against Escherichia coli at 48 h of incubation.
Keywords
Acrylate polymers, Maleic anhydride, Copolymer, Antimicrobial, Amoxycillin
Vinyl polymers, methacrylate polymers, polyamides and poly(ethylene oxide) polymers have been widely studied as drug carriers due to their diversity, multifunctionality and biocompatible nature [1-6]. They do not form toxic byproducts during their biodegradation and which have tendency to swell, when they come in contact with biological environment [7]. Amoxycillin possesses a broad antibacterial spectrum, but exerts short half-life values, which demand frequent drug administration. Therefore, continuous infusion has been suggested as the most beneficial mode of β-lactams [8-10]. Amoxycillin has been reported to be successfully used in various infections [11] including in septic absorptions, urinary tract infections, upper and lower respiratory infections, skin and soft tissue and GI tract infections.
The present studies describe controlled release of amoxycillin from the poly (butyl methacrylate-comaleic anhydride)-amoxycillin [P(BMA-MA)AMOX] controlled-release system. In this system, the drug is covalently bound to polymeric matrix in the presence of triethylamine (TEA) as a catalyst. This conjugate can be made to release drugs at controlled rates over extended periods of time. The specific goal of this investigation was to develop an improved oral dosage form for amoxycillin, a representative beta-lactam antibiotic.
Materials and Methods
Amoxycillin was obtained from Vitara Chemicals Ltd., (Nandesari, Vadodara). Butylmethacrylate was obtained from Gujarat State Fertilizers Ltd., (Vadodara, Gujarat). Maleic anhydride (MA) and 2,2’-azobisisobutyronitrile were purchased from Fluka. Butylmethacrylate was purified using an inhibitor remover for hydroquinone and monomethylether. AIBN (azobisisobutyrilnitrile) was purified as described by Szajnecki et al [12]. All other chemicals used were of analytical grade.
Synthesis and characterization of copolymers
N-butylmethacrylate and maleic anhydride (at different molar ratios) along with AIBN was dissolved in distilled toluene. The solution was degassed by bubbling nitrogen gas for 10 min. Polymerization occurred as the solution was heated under constant stirring at 75o for 4 h. Polymers were recovered by precipitation in methanol (yield 80-85%). The homopolymer of BMA formed was removed from the copolymer by Soxhlet extraction (which is non-solvent for copolymer).
The average molecular weights were determined by gel permeation chromatography in THF. Monodispersed polystyrene standards were used for calibration. Copolymer composition was determined by estimating molar proportion of any one of the monomer in the copolymer. The MA content of the copolymer was determined by esterification-hydrolysis method [13]. Viscosity measurement of copolymers was carried out in dimethylformamide (DMF) at 30°. An Ubbelohde suspended level viscometer was used to measure viscosity [14] by measuring the time (±0.05 s) of flow of known amount of liquid (solvent and solution of different concentration) at constant temperature maintained in thermostat with accuracy of ±0.1°.
Incorporation of amoxycillin
The butylmethacrylate-maleic anhydride copolymer with different mol % of anhydride was ground to obtain 200 mesh cut. The copolymer was cycled through washes with methanol and cold distilled water to remove unreacted anhydride group [15] and was dried at 400. In the method, 1 g (constant in all synthesis) of butylmethacrylate-maleic anhydride copolymer was dissolved in dry N,N-dimethyl formamide (DMF, 25 ml) along with catalyst triethyl amine (0.3 ml). A calculated amount of amoxycillin in dry DMF (12.5 ml) was added drop wise at 20-25°. The reaction mixture was stirred for 1 h and for variable times, depending on the experimental program at room temperature. On finishing the coupling process, 250 ml cold distilled water with 0.3 ml hydrochloric acid was introduced in order to precipitate the reaction product and the mixture was stirred for half an hour before filtration. The precipitates were filtered off, washed twice with distilled water. The polymer bound amoxycillin samples were treated with (0.1 M) aqueous NaHCO3 solution for the hydrolysis of unreacted anhydride group. The washed precipitate was collected and dried over calcium chloride in desiccator. Similar procedure was followed for coupling of amoxycillin on copolymers P(BMAMA- 2) and P(BMA-MA-3) respectively. All the washings were collected quantitatively. The amount of amoxycillin in the entire washed material was estimated colorimetrically using p-dimethyl aminobenzaldehyde reagent at 410 nm using HP− 8452 Diode Array UV/Vis spectrophotometer as described by Sethi [16-17]. The results are mentioned in Table 1. Similar method was followed for copolymer 2 and 3, respectively. The concentration of bound amoxycillin was calculated by considering the initial amoxycillin taken for binding and the amount released in subsequent washings as unbound amoxycillin.
| Polymer system | Mole fraction in feed | Mole fraction in copolymer | Number average molecular weight (g/mol) | Weight average Molecular weight (g/mol) | Poly dispersity | Content of BAC in mg/g of copolymer | Intrinsic Viscosity (IV) (dl/g) | ||
|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | m1 | m2 | Mncx103 | Mwcx103 | ||||
| P (BMA-MA-1) | 0.676 | 0.676 | 0.713 | 0.287 | 5. | 23. | 4.60 | 246 | 0.187 |
| P (BMA-MA-2) | 0.406 | 0.406 | 0.589 | 0.411 | 4. | 17. | 4. | 418 | 0.168 |
| P (BMA-MA-3) | 0.186 | 0.186 | 0.512 | 0.488 | 3. | 8. | 3. | 618 | 0.121 |
Where, M2= mole fraction of BMA in feed, m1 = mole fraction of BMA in copolymer, M2 = mole fraction of MA in Feed, m2 = mole fraction of MA in copolymer
Table 1: Characterization of N-butyl methacrylate-maleic anhydride copolymer
In Vitro drug release study
In an experiment, 25 mg of polymer bound amoxycillin sample was suspended with 5 ml of the 0.1 M buffer (Tris-pH 3.8, citrate-pH 4.6, 5.8 and phosphate-pH 7.0, 7.2, 8.0) of varying pH as described for a fixed time of 24 h in triplicate. The released amoxycillin in buffer was separated by centrifugation at 3000 g for 15 min and amount of amoxycillin in supernatant, was determined spectrophotometrically as described above. The residues after centrifugation were again equilibrated with a fresh 5 ml portion of buffer. The process was repeated after every 24 h until no more elution of amoxycillin in the supernatant was observed. This method was adopted for all the three systems. The data was subjected to analysis by calculating the fractional release at different intervals of time (Mt/Mα) and plotting the graph of these values against time, square root of time and log of time. From the linearity of the graph, the order of drug release was ascertained. The rate of release was obtained from the graph and correlated with the physicochemical characteristics of polymer and drug.
Antimicrobial activity
The antimicrobial activity was studied on both gram positive (Staphylococcus aureus and Bacillus subtilis) and gram negative (Escherichia coli) bacteria. The cultures were inoculated in nutrient broth and incubated for 12 h at 37°. Optical density of all the three cultures was adjusted to 0.3 by diluting with sterile nutrient broth. For antibacterial activity 50 ml of individual liquid culture in 250 ml flask with an initial O.D660 of 0.3 was spiked with 25 mg of prodrug and increase in growth (absorbance at 660 nm) was monitored at every 8 hours interval up to 48 h.
Statistical analysis
Values reported are averages of the release activity data in triplicate. The Microsoft Excel (Release 2000) under Windows 98 operating system (ICON Software Technologies, Baroda, Gujarat, India) was used to carry out the statistical calculations. The values represented in the tables are the limits (3 SD Limits) within which the observed data lies. The ±3 SD from the mean covers 95% of the observed data. The observed mean effect is significant with a P value less than 0.035.
Results and Discussion
Copolymers of n-butyl methacrylate and maleic anhydride comparatively of low average molecular weight (8113 to 22638) was prepared by solution polymerization technique using AIBN as initiator and characterized by viscosity to use as a drug carrier, the results are described in Table 1. Weight average molecular weights were 8113 to 22638 g/mol with a poly-dispersity index (Mw/Mn) of 2.67 to 4.60. The feed proportions of each co-monomer are mentioned in Table 1, with the corresponding alternate copolymers referred to as P(BMA-MA-1), P(BMAMA- 2) and P(BMA-MA-3), respectively. MA contents determined by titration were 0.28, 0.41 and 0.48 mole % for the three copolymers. Reactivity ratio of copolymer of BMA and MA was determined by using linear graphical method of Finemann and Ross (data not shown). The reactive ratio observed was 0.83 and 0.04, respectively.
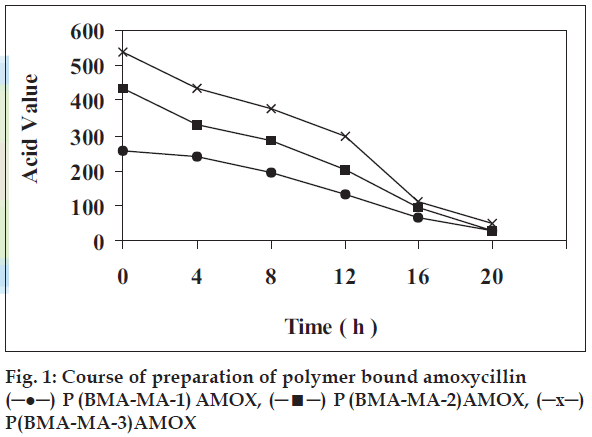
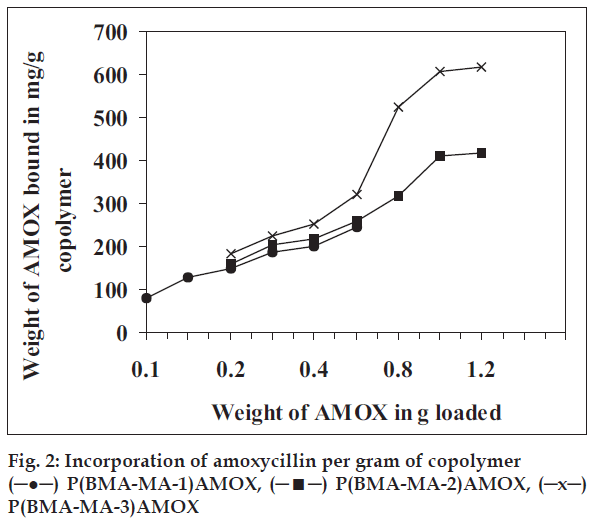
Amoxycillin was bound with each of these three copolymers and the study of sustained release of the drug from each system was investigated by monitoring released amoxycillin in buffer by spectrophotometric estimation method described under materials and methods. Coupling of the amoxycillin to the copolymer carriers in the presence of a TEA was carried out (Table 1). Amongst the solvents, DMF was found to be more suitable as amount of amoxycillin, coupled was about 80-85% compared to amount of ~45-50% in other solvent. To determine minimum time for maximum loading of drug the reactions were carried for various times (fig. 1). It indicates that maximum coupling accomplished at room temperature at constant stirring nearly with in 20 h indicated by minimum and constant acid value. Similarly to optimize mole ratio of anhydride, biological active compound and catalyst the coupling reaction, was carried out by varying mole ratios. The results given in fig. 2 reveals that amount of amoxycillin coupled to polymer increases with increase in anhydride content. Data in fig. 2 also reveals that the maximum loading of amoxycillin is 1 g to 1 g because the sites for coupling (anhydride) are not available. Thus, amount is leveled off beyond this point. The amount of amoxycillin covalently bound to copolymer was determined spectroscopically at 410 nm. Amoxycillin (24.6 mg), (41.8 mg) and (61.8 mg) was bound respectively to 100 mg each of copolymer P(BMAMA- 1), P(BMA-MA-2) and P(BMA-MA-3) in 20 h (Table 1).
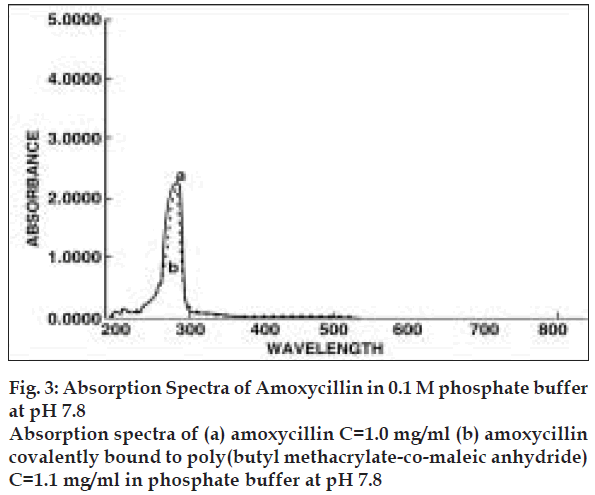
The content of amoxycillin in the copolymer was determined via their characteristic UV absorption. After proper adjustment of concentration of amoxycillin, UV absorptions measurement were carried out at 286 nm (fig. 3). It was observed that copolymer does not absorb in this range and hence it did not interfere with the absorption of drug. The measurements were carried out using aqueous buffer solution of pH 7.8. Similar observation, were also noticed by Azori et al [18] who worked on the model drug p-nitroaniline bound to polyanionic carrier.
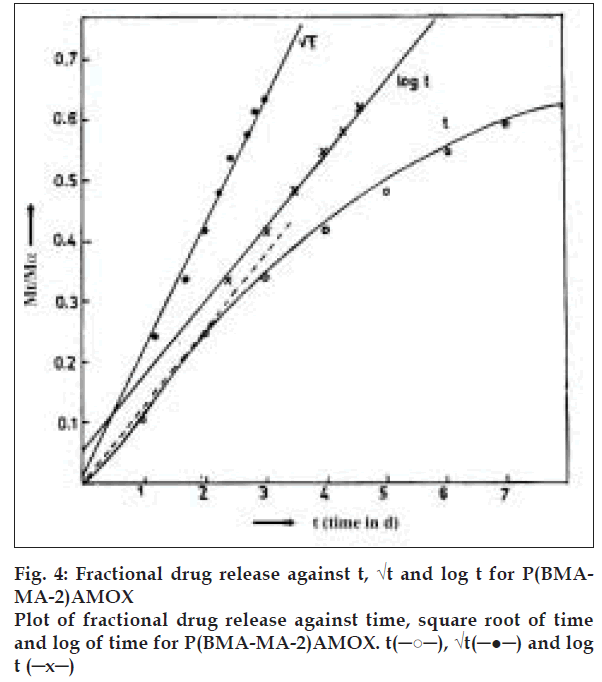
Amoxycillin covalently bound to polymeric matrix P(BMA-MA) represents insoluble inert matrix. These matrices act as a retardant either by forming insoluble matrix or by formation of a skeleton. The rate determining step in controlling release rate from this formulation is the diffusion of liquid into the matrix. Drug release is triggered by penetration of eluting media into the matrices thereby releasing drug by hydrolysis, which is channelised by diffusion. The depletion zone gradually extends into the core of the matrix. In the absence of additive, drug release is prolonged and nonlinear. Plot of fractional drug release Mt/Mα against time, square root of time and log of time are shown in fig. 4, for the system P(BMA-MA-2)AMOX. From the plot it is clear that the systems behave as described above with the release profile of Mt/Mα vs. Square root of time being linear. Thus, these systems can be classified as mentioned above i.e. insoluble, inert matrices. Similar plot of the other polymer system can be expected to yield similar results.
The release activity of the drug depends on the, kind of polymeric system and the characteristics of the drug (molecular weight, solubility) and in every case the amount of drug release gradually decreases with time. Release activity studies for all the three polymeric prodrugs, was also studied at different pH taking into consideration the pH existing in digestive and circulatory systems. The experimental sets were kept in triplicate for each of the polymeric systems and incubated at 37° taking into consideration the normal body temperature.
We studied the effect of ionic strength on release activity of amoxycillin results indicates that, increasing the ionic strength of buffer medium decreases the rate of release activity (data not shown). It was observed that at buffer concentration of 0.1 M the drug was totally released in 9 and 7 d, respectively. Observation explains that during drug release equivalent acid groups are formed which can be compensated easily by higher concentration of buffering ion which in turn favors easy and higher rate of release in comparison to lower buffering ion concentration.
Further experiments were conducted in 0.1 M concentration of citrate buffer (pH 3.8, 4.6 and 5.8) and phosphate buffer (pH 7.0, 7.2 and 8.0). Results revealed that P(BMA-MA-1) showed cumulative release of 48%, which lasted for maximum 8 d in acidic pH and in alkaline range 53% of cumulative release was observed which lasted for 9 d (Table 2). It was observed that on increasing the payload of amoxycillin i.e. in P(BMA-MA-2), 50-55% cumulative release was observed in acidic pH and 60-65% in alkaline pH. The release activity lasted for 9 d with this polymeric prodrug system (Table 3).
| Days (d) | pH 3.8 Cumulative release |
pH 4.6 % Cumulative release |
pH 5.8 % Cumulative release |
pH 7.0 % Cumulative release |
pH 7.2 % Cumulative release |
pH 8.0 % Cumulative release |
|---|---|---|---|---|---|---|
| 1 | 7.75±0.01 | 5.60±0.01 | 15.50±0.02 | 12.16±0.01 | 13.27±0.15 | 14.37±0.10 |
| 2 | 17.69±0.01 | 7.75±0.01 | 18.80±0.01 | 23.21±0.01 | 22.66±0.15 | 15.48±0.10 |
| 3 | 18.80±0.02 | 12.74±0.03 | 27.14±0.15 | 30.40±0.02 | 30.96±0.03 | 30.96±0.15 |
| 4 | 19.35±0.01 | 16.61±0.03 | 36.72±0.02 | 31.51±0.02 | 38.21±0.01 | 37.59±0.10 |
| 5 | 21.56±0.03 | 21.60±0.12 | 38.12±0.02 | 33.72±0.15 | 40.91±0.01 | 46.99±0.10 |
| 6 | 24.89±0.10 | 28.24±0.15 | 43.54±0.01 | 39.25±0.01 | 46.99±0.02 | 49.76±0.20 |
| 7 | - | 33.25±0.15 | 47.12±0.15 | 42.01±0.15 | 49.20±0.02 | 53.07±0.20 |
| 8 | - | 36.10±0.20 | 47.89±0.04 | 45.88±0.01 | 51.31±0.10 | - |
| 9 | 46.99±0. 03 | 51.86±0.10 | - |
P (BMA-MA-1) containing 6.14 mg of Amoxycillin in 25 mg of copolymer at temperature 370± 0.50.
Table 2: In vitro release of amoxycillin from P (bma-ma-1) amox preparation
| Days (d) | pH 3.8 % Cumulative release |
pH 4.6 % Cumulative release |
pH 5.8 % Cumulative release |
pH 7.0 % Cumulative release |
pH 7.2 % Cumulative release |
pH 8.0 % Cumulative release |
|---|---|---|---|---|---|---|
| 1 | 6.50±0.01 | 9.40±0.22 | 11.06±0.01 | 11.06±0.01 | 9.76±0.00 | 9.76±0.01 |
| 2 | 12.36±0.11 | 13.99±0.21 | 19.52±0.01 | 20.82±0.01 | 20.17±0.10 | 18.22±0.01 |
| 3 | 15.62±0.15 | 16.27±0.02 | 26.68±0.02 | 31.23±0.17 | 27.98±0.10 | 25.38±0.03 |
| 4 | 16.92±0.02 | 18.88±0.02 | 33.19±0.01 | 40.99±0.11 | 37.52±0.15 | 33.84±0.02 |
| 5 | 18.87±0.02 | 22.89±0.15 | 40.32±0.15 | 50.11±0.21 | 43.97±0.02 | 40.34±0.02 |
| 6 | 19.52±0.01 | 25.70±0.16 | 46.53±0.02 | 54.01±0.21 | 47.22±0.03 | 48.48±0.16 |
| 7 | - | 27.33±0.11 | 51.41±0.02 | 55.12±0.02 | 48.20±0.11 | 52.71±0.15 |
| 8 | - | - | 54.33±0.15 | - | - | 57.92±0.15 |
| 9 | - | - | 55.64±0.15 | - | - | 61.82±0.01 |
P (BMA-MA-2) containing 10.45 mg of Amoxycillin in 25 mg of copolymer at temperature 37°± 0.5°.
Table 3: In vitro release of amoxycillin from P (bma-ma-2) amox preparation
With P(BMA-MA-3) the release activity lasted for 9 d in acidic range with maximum cumulative release of 60%, where as in alkaline range 80% cumulative release was observed which lasted for maximum 7 d (Table 4). From the results it can be concluded that increasing percentage of maleic anhydride increases drug release. Strong swelling was observed during the release of the drug from the copolymer in case of P(BMA-MA-2) and P(BMA-MA-3) after 2 d. The high rate of swelling was due to high carboxylic content, which also increased drug release. Increase in pH also increased the release activity.
| Days (d) | pH 3.8 Cumulative release |
pH 4.6 % Cumulative release |
pH 5.8 % Cumulative release |
pH 7.0 % Cumulative release |
pH 7.2 % Cumulative release |
pH 8.0 % Cumulative release |
|---|---|---|---|---|---|---|
| 1 | 6.20±0.11 | 15.41±0.10 | 16.51±0.00 | 14.74±0.03 | 26.63±0.22 | 28.52±0.23 |
| 2 | 12.76±0.15 | 39.83±0.10 | 32.57±0.10 | 31.47±0.01 | 43.79±0.04 | 56.80±0.11 |
| 3 | 18.05±0.10 | 56.12±0.15 | 34.55±0.04 | 47.53±0.01 | 60.52±0.04 | 76.95±0.10 |
| 4 | 22.45±0.10 | 59.86±0.05 | 40.71±0.04 | 59.19±0.02 | 73.28±0.12 | 88.95±0.10 |
| 5 | 24.43±0.05 | 60.96±0.05 | 48.19±0.01 | 66.24±0.03 | 76.58±0.12 | 91.25±0.03 |
| 6 | 29.71±0.02 | 61.18±0.22 | 53.92±0.01 | 72.40±0.03 | 78.34±0.03 | 93.26±0.01 |
| 7 | 33.33±0.16 | 61.62±0.05 | 58.54±0.01 | 73.50±0.03 | 80.54±0.15 | - |
| 8 | 35.41±0.01 | 63.81±0.05 | 60.74±0.02 | - | - | - |
| 9 | - | - | 60.96±0.02 | - | - | - |
P (BMA-MA-3) containing 15.45 mg of Amoxycillin in 25 mg of copolymer at temperature 37°± 0.5°.
Table 4: In vitro release of amoxycillin from P (bma-ma-3) amox preparation
Antimicrobial activity of amoxycillin in free and polymer bound form was determined. Experiments on antimicrobial activity of polymer bound amoxycillin samples were conducted in nutrient broth. To determine antimicrobial activity, optical density was measured as a function of time in each case. It is known that as bacterial growth increases optical density of the medium increases. It may be noted that dead bacteria contributes to optical density. Data in Tables 5 to 7 clearly indicate that after initial induction period, the antimicrobial activity is observed by constant optical density in all cases, which is attributed by dead bacteria. Further the results were confirmed by withdrawing samples from each of the systems at time intervals indicated in Tables 5 to 7 and plating on nutrient agar medium. Initial and final colony counts were performed (results not provided). Observations showed the activity profile in the form: P(BMA-MA-3) > P(BMA-MA-2) > P(BMA-MA-1) > Pure polymer. In case of unbound amoxycillin low induction period as well as high inhibition (low optical density) was observed.
| Time (h) | Control O.D. | Copoly-mer O.D. | P (BMA-MA-1) AMOX | P (BMA-MA-2) AMOX | P (BMA-MA-3) AMOX | Amoxycillin | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| O.D. | % Inh | O.D. | %Inh | O.D. | %Inh | 0.D. | %Inh | |||
| 0 | 0.0060±0.00 | 0.0050±0.00 | 0.0060±0.00 | 0.0050±0.00 | 0.0360±0.00 | 0.0020±0.00 | ||||
| 8 | 0.1440±0.0020 | 0.1438±0.005 | 0.128±0.001 | 10.6±0.1 | 0.1300±0.0015 | 9.7±0.1 | 0.0970±0.0010 | 32.7±0.10 | 0.0026±0.0002 | 98.2±0.3 |
| 16 | 0.2640±0.0010 | 0.2642±0.0015 | 0.2199±0.002 | 16.7±0.2 | 0.2120±0.0020 | 19.7±0.1 | 0.1730±0.0010 | 34.5±0.20 | 0.0069±0.0005 | 97.4±0.7 |
| 24 | 0.6680±0.0030 | 0.6684±0.0015 | 0.4916±0.0015 | 26.6±0.1 | 0.4670±0.0015 | 30.1±0.1 | 0.2550±0.0010 | 61.8±0.20 | 0.0154±0.0001 | 97.7±0.5 |
| 32 | 0.8970±0.002 | 0.8975±0.001 | 0.6055±0.0017 | 32.5±0.1 | 0.5040±0.0010 | 43.8±0.2 | 0.2890±0.0025 | 67.8±0.14 | 0.0188±0.001 | 97.9±0.5 |
| 40 | 1.2410±0.002 | 1.2409±0.002 | 0.7992±0.0012 | 35.6±0.5 | 0.5690±0.0021 | 54.1±0.2 | 0.3810±0.0020 | 69.3±0.10 | 0.0310±0.0015 | 97.5±0.2 |
| 48 | 1.4110±0.001 | 1.4114±0.002 | 0.8452±0.002 | 40.1±0.5 | 0.5740±0.0010 | 59.3±0.1 | 0.3840±0.0010 | 72.8±0.15 | 0.0296±0.0012 | 97.9±0.2 |
P (BMA-MA-3) containing 15.45 mg of Amoxycillin in 25 mg of copolymer at temperature 37°± 0.5°.
Table 5: In vitro release of amoxycillin from P (bma-ma-3) amox preparation
| Time (h) | Control O.D. | Copoly-mer 0.D. | P (BMA-MA-1) AMOX | P (BMA-MA-2) AliOX | P (BMA-MA-3) AMOX | Amoxyallin | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.D. | %Inh | O.D. | %Inh | O.D. | %Inh | 0.D. | %Inh | |||
| 0 | 0.0040±0.00 | 0.0030±0.000 | 0.0040±0.03 | - | 0.0040±0.00 | - | 0.0040±0.00 | - | 0.00201±0.00 | - |
| 8 | 0.8770±0.002 | 0.8774±0.006 | 0.8010±0.001 | 8.7±0.2 | 0.686±0.003 | 21.8±0.15 | 0.4410±0.025 | 49.7±0.4 | 0.0161±0.002 | 98.2±0.5 |
| 16 | 1.2000±0.015 | 1.2100±0.005 | 0.9984±0.005 | 16.8±0.4 | 0.818±0.004 | 31.8±0.20 | 0.5981±0.021 | 50.2±0.2 | 0.0410±0.001 | 96.6±0.2 |
| 24 | 1.3510±0.020 | 1.3509±0.005 | 0.9065±0.002 | 32.9±0.3 | 0.8201±0.002 | 39.3±0.01 | 0.6152±0.030 | 54.5±0.2 | 0.0337±0.002 | 97.5±0.15 |
| 32 | 1.5400±0.020 | 1.5411±0.010 | 0.9871±0.003 | 35.9±0.5 | 0.8360±0.003 | 45.9±0.03 | 0.6312±0.005 | 59.1±0.5 | 0.0389±0.005 | 97.5±0.15 |
| 40 | 1.6200±0.011 | 1.6212±0.010 | 0.8995±0.006 | 36.8±0.4 | 0.8410±0.0015 | 48.1±0.5 | 0.6401±0.004 | 60.5±0.3 | 0.0412±0.001 | 97.4±0.22 |
| 48 | 1.7600±0.012 | 1.7653±0.015 | 0.8932±0.002 | 41.2±0.1 | 0.8510±0.0015 | 51.7±0.6 | 0.6563±0.035 | 69.1±0.3 | 0.0418±0.002 | 97.7±0.25 |
% Inh = Inhibition; O.D. = Optical density at 660 nm
Table 6: Antimicrobial acitivity of polymer bound amoxycillin as a function of time and increasing ma content against E. coli
| Time (h) | Control O.D. | Copoly-mer 0.D. | P (BMA-MA-1) AMOX | P (BMA-MA-2) AMOX | P (BMA-MA-3) AMOX | Amoxycillin | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| O.D. | % lnh | 0.D. | % lnh | O.D. | % Inh | O.D. | % lnh | |||
| 0 | 0.0040±0.00 | 0.0030±0.00 | 0.0040±0.00 | 0.0040±0.00 | 0.0030±0.00 | 0.0020±0.00 | ||||
| 8 | 0.2710±0.07 | 0.2714±0.010 | 0.2351±0.002 | 12.6±0.1 | 0.2281±0.004 | 15.9±0.5 | 0.159140.003 | 43.3±0.5 | 0.0024±0.00 | 99.1±0.75 |
| 16 | 0.4621±0.004 | 0.4622±0.002 | 0.4043±0.001 | 13.3±0.1 | 0.3694±0.002 | 20.1±0.1 | 0.2374±0.002 | 48.7±1.0 | 0.0065±0.0001 | 98.6±0.30 |
| 24 | 0.7781±0.002 | 0.7773±0.004 | 0.6115±0.001 | 21.5±0.2 | 0.5812±0.011 | 25.3±1.1 | 0.3971±0.006 | 49.0±1.0 | 0.0147±0.001 | 98.1±0.12 |
| 32 | 0.8942±0.004 | 0.8960±0.003 | 0.6824±0.001 | 23.7±0.2 | 0.6421±0.002 | 28.2±1.2 | 0.4102±0.001 | 54.1±0.75 | 0.0197±0.002 | 97.8±0.22 |
| 40 | 1.1913±0.001 | 1.1911±0.001 | 0.8512±0.003 | 28.5±0.3 | 0.8093±0.015 | 32.1±0.8 | 0.4563 ±0.003 | 61.740.4 | 0.0214±0.002 | 98.2±0.25 |
| 48 | 1.4482±0.002 | 1.4486±0.002 | 0.8670±0.002 | 40.1±0.1 | 0.8110±0.015 | 44.0±1.0 | 0.5018±0.002 | 65.4±0.4 | 0.0261±0.001 | 98.2±0.30 |
% Inh = Inhibition; O.D.= Optical density at 660 nm
Table 7: Antimicrobial acitivity of polymer bound amoxycillin as a function of time and increasing ma content against ST. aureus
For various polymers bound amoxycillin (AMOX) samples, antimicrobial activity was observed in all the cases and found to increase with increase in maleic anhydride content in polymeric system. Table 5 to 7 shows antimicrobial activity of polymer bound amoxcillin as a function of time and increase in MA content towards each bacterial strain. P(BMA-MA- 1, 2 and 3) showed maximum inhibition of 40%, 59.3% and 72.8% towards Bacillus subtilis, 40%, 44% and 65.4% against Staphylococcus aureus and 41.2%, 51.7% and 69.1% against Escherichia coli at 48 h of incubation.
This study provides a concept of charging a therapeutic level of active agent in the target site for a long duration and permits to manipulate the pharmacokinetic behavior of a drug. As a matter of fact, the bound drug is wholly released after 7 to 9 d as against the time of 30 min required for the unbound drug. This evidences its retarding action is due to the binding of the drug with the polymer matrix. The release behavior of drug depends on the kind of polymer system and the characteristics of the drug i.e. molecular weight and solubility. The release study clearly indicates that the amount of amoxycillin should be released in the range of minimum inhibitory concentration of the drug. In view of this the copolymer system with higher anhydride content are most suitable for releasing drug equivalent to MIC at constant rate for an extended period i.e. 6 to 9 d at a particular pH.
References
- Cornforth JW, Plotts KT, Morgan ED, Rees RJ. Preparation of antituberculous polyoxyethylene ethers of homogeneous structure. Tetrahedron 1973;29:1659-67.
- Ottenbrite RM, Kaplan AM. Some biologically active copolymers of maleic anhydride. Ann N Y AcadSci 1985;446:160-8.
- Dumitriu S, Popa M, Dumitriu M. Polymeric drug carrier systems. J Bioact Compatible Polym 1989;4:151-97.
- Puglisi L, Caruso V, Paoletti R, Ferruti P, Tanzi MC. Macromolecular drugs I. Long-lasting antilipolytic activity of nicotinic acid bound to polymer. Pharm Res Commun 1976;8:379-86.
- Kenawy E, Bowlin GL, Mansfield K, Layman J, Simpson DG, Sanders EH, et al . Release of tetracycline hydrochloride from electrospunpoly(ethylene-co-vinyl acetate), poly(lactic acid) and a blend. J Control Release 2002;81:57-67.
- Dumitiu S, Butnaru R, Simionescu C. Bioektivepolymere, I: Bioaktive derivate de zellulose. Cellulose ChemTechnol 1973;7:553-9.
- Khan MA, Reddy IK. Controlled drug delivery: Development of solid oral dosage forms with acrylate polymers. S T P Pharm Sci 1997;7:483-90.
- Craig W, Ebert S. Continuous infusion of beta-lactam antibiotics. Antimicrob Agent Chemother 1992;36:2577-83.
- Drusano G. Role of pharmacokinetics in the outcome of infections. Antimicrob Agent Chemother 1988;32:289-97.
- Vogelman B, Dudmundsson S, Leggett J, Turnidge J, Ebert S, Craig W. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infec Dis 1988;158:831-47.
- Brogen RN, Speight TM, Avery GS. Amoxycillin: A review of its antimicrobial and pharmacokinetic properties and therapeutic use. Drugs 1975;9:88-140.
- Szajnecki L, Pasieczna S, Ryczkopwski J. The influence of the copolymerization condition on the polymer structure. J Phys IV France 2006;137:357-61.
- Loucheux MH, Banderet A. Reactivite des groupmementfounctionalsporterspr des macro molecules. Bull SocChim France 1964;6:1216-20.
- Csakvari E, Azori M, Tudos F. Physico-chemical studies of polymeric carriers. Polymer Bull 1981;5:413-6.
- Dumitriu S, Popa M. Bioactive polymers, 51a synthesis and characterization of a macromolecular prodrug of ampicillin. MakromolChem 1988;189:103-10.
- Sethi PD. Quantative analysis of drugs in pharmaceutical formulations. 2nd ed. Delhi: CBS Publication; 1993.
- Singh M, Bala K, Vasudevan P. Cellulosic microcapsules for drug delivery, 1 hydrocortisone release. MakromolChem 1982;183:1897-903.
- Azori M, Pato J, Fehervari F, Tudos F. Polymeric prodrugs: In vitro study of the drug release on a model p-nitroanilide bound onto polyanionic carrier. MakromolChem 1986;187:303-9.