- Corresponding Author:
- H. V. Chavda
Shri Sarvajanik Pharmacy College, Hemchandracharya North Gujarat University, Mehsana - 384 001, India
E-mail: hvchavda@sspcmsn.org
Chavda and Patel: NaCMC-SPHC based Drug Delivery System
| Date of Submission | 1 December 2009 |
| Date of Revision | 29 December 2010 |
| Date of Acceptance | 2 January 2011 |
| Indian J Pharm Sci, 2011, 73 (1): 30-37 |
Abstract
This study discusses efforts made to design drug-delivery system based on superporous hydrogel composite for sustained delivery of ranitidine hydrochloride. The characterization studies involve measurement of apparent density, porosity, swelling studies, mechanical strength studies, and scanning electron microscopy. Scanning electron microscopic images clearly showed the formation of interconnected pores, capillary channels, and the cross-linked sodium carboxymethylcellulose molecules around the peripheries of pores. The prepared system floated and delivered the ranitidine hydrochloride for about 17 h. The release profile of ranitidine hydrochloride was studies by changing the retardant polymer in the system. To ascertain the drug release kinetics, the dissolution profiles were fitted to different mathematical models that include zero-order, first-order, Higuchi, Hixson-Crowell, Korsmeyer-Peppas, Weibull, and Hopfenberg models. The in vitro dissolution from system was explained by Korsmeyer-Peppas model. The diffusion exponent values in Korsmeyer-Peppas model range between 0.48±0.01 and 0.70±0.01, which appears to indicate an anomalous non-Fickian transport. It is concluded that the proposed mechanically stable floating drug-delivery system based on superporous hydrogel composite containing sodium carboxymethylcellulose as a composite material is promising for stomach specific delivery of ranitidine hydrochloride.
Keywords
Floating drug-delivery system, kinetic modeling, ranitidine hydrochloride, sodium carboxymethylcellulose, superporous hydrogel composite
Ranitidine is a competitive and reversible inhibitor of the action of histamine at histamine H2-receptors, including receptors on gastric cells with a minimal effect on H1− receptors. It is used in the treatment of active duodenal ulcers, gastric ulcers, Zollinger− Ellison syndrome, erosive esophagitis, and gastro esophageal reflux disease. The recommended oral dosage of ranitidine is 150 mg twice daily or 300 mg once daily. In case of endoscopically−diagnosed erosive esophagitis, the dosage is 150 mg ranitidine four times a day [1,2]. The conventional dose, 150 mg, can inhibit gastric acid secretion up to 5 h but not up to 10 h. An alternative dose, 300 mg, leads to fluctuations in plasma levels. A dosage form that can sustain the release of ranitidine hydrochloride (R−HCl) would be beneficial in such cases [3]. The ranitidine has a short biological half−life of approximately 2−3 h, an absolute bioavailability of 50%, and it is absorbed only in the initial part of the small intestine [4−6]. A gastroretentive drug−delivery system that can be retained in the stomach and increase the local delivery of R−HCl would also be very useful.
The conventional nonporous hydrogels swell slowly and have low loading capacities [7,8], which restrict their use in effective drug delivery. The superporous hydrogels (SPHs) are able to absorb water very rapidly as well as swell to equilibrium size in a very short period of time [9,10]. Synthesis of SPH involves copolymerization/cross linking of co−monomers using a cross linking agent which is a multifunctional comonomer. The polymerization reaction is initiated by a chemical initiator. Generally, gas blowing technique is used to synthesize SPHs. The inorganic carbonates such as sodium carbonate and sodium bicarbonates are used as foaming agents. Cross linking of linear polymers by chemical compounds or by irradiation is another method for its synthesis. SPHs show rapid swelling and large swelling ratio which expand their applications in the development of gastric retention devices [11]. SPH composites (SPHCs) possess improved mechanical properties with composite agents such as chitosan, Ac−Di−Sol and Carbopol [12−16]. The drug−delivery systems based on SPH and/or SPHC for human insulin, octreotide, buserelin, and desmopressin have been reported [15,17−19].
The objectives of the investigation were: to synthesis SPHC containing sodium carboxymethyl cellulose (NaCMC) as a composite material with improved characteristics over CSPH; and to prepare a drugdelivery system based on it. Acrylic acid (AA) and acrylamide (AM) were chosen as the base monomers for their high water affinity and fast copolymerization velocity [20], while NaCMC was selected as the second polymer component for its biocompatibility [21]. In this investigation preparation of a mechanically stable floating drug−delivery system, using low density SPHC that deliver R−HCl in sustained manner in the stomach was tried.
Materials and Methods
R−HCl was a generous gift from Espee Formulation Pvt Ltd., Rajkot, Gujarat, India. AM was obtained from Burgoyne Burbidges and Co. Pvt. Ltd., Mumbai, India. AA, N,N′−methylenebisacrylamide (BIS), Span 80, ammonium persulphate (APS), N,N,N′,N′’tetramethylethylenediamine (TEMED), hydroxypropylmethyl cellulose (HPMC), ethyl cellulose (EC), and NaCMC were purchased from SD Fine Chem. Ltd, Mumbai, India. Double distilled water (DDW) was prepared in laboratory. Simulated gastric fluid (SGF) with pH 1.2 was prepared in laboratory by dissolving 2 g of sodium chloride, 3.2 g pepsin, and 6.8 ml of hydrochloric acid in DDW to produce 1 l. All other chemicals used were of analytical grade and used as obtained.
SPHC synthesis
The synthesis of SPHC of poly(AM−co−AA) was carried out in a test tube at 25°. Solutions of ingredients used were made in DDW. 50% w/v AM, 50% v/v AA, 2.5% w/v BIS, 10% v/v Span 80, 20% v/v TEMED, DDW, and NaCMC (300 µl, 200 µl, 100 µl, 30 µl, 20 µl, 330 µl, and 98 mg, respectively) were added subsequently. SPH of poly (AM−co−AA) was synthesized by same procedure except the addition of NaCMC. 45 µl of APS 20% w/v was added after adjusting the pH of solution to 5.0 with 5M NaOH solution. The polymerization reaction was allowed to continue for 10 min. After addition of each ingredient, the reaction mixture was shaken vigorously. 200 mg of sodium bicarbonate was added to the solution in the last step and mixed, quickly. The SPHCs synthesized were removed, dried in oven at 60° for 4 days. These SPHCs were cut into suitable size pieces and stored in airtight container until further use.
Scanning electron microscopy analysis
For scanning electron microscopic (SEM) studies, the internal structure of dried SPH and SPHC was exposed by cutting them. The internal porous structures and morphology of SPH and SPHC were examined under the Scanning Electron Microscope (ESEM EDAX XL−30, Philips, Netherlands) at operating voltage of 30 kV.
Measurement of density, porosity, swelling parameters and mechanical strength
The apparent density of the dried SPH and SPHC were measured using the solvent displacement method. A piece of the dried polymer, with known mass, was immersed in a known volume of hexane in a graduated cylinder. The increased volume of hexane was measured as the polymer volume. The density was then calculated using, Density = M/V…. Eqn. 1, where, V is the volume of solvent displaced by polymer and M is the polymer mass.
The porosity of dried polymer was measured by immersing it in hexane for over night. The excess hexane from the polymer surface was blotted and polymer was weighed. The porosity was obtained using, Porosity = VP/VT….Eqn. 2, where, VP (= VT − VPOL) is the pore volume of polymer and VT is the total volume of the polymer. The SPH and SPHC were cylindrical in shape, and so VT can be measured from its dimensions.
The equilibrium swelling ratio of polymer was calculated using, Q = (Ms−Md)/Md…. Eqn. 3, where, Q is the equilibrium swelling ratio, Ms and Md are the mass in the swollen and dried state, respectively. The dried gel was immersed in an excess of swelling medium. At regular time intervals, the gel was removed from the medium (excessive medium on the surface was blotted) and reweighed to determine Ms [9].
A bench comparator described by Chen et al. [14] was modified and used for the penetration pressure measurement. The fully swollen hydrogel was put longitudinally under the lower touch and then weights were applied successively to the upper touch until the hydrogel fractured completely. The compressive force was read from the gauge, and the penetration pressure [15] was calculated using, PP = Fu/S….Eqn. 4, where, PP is the penetration pressure, Fu is the ultimate compressive force at complete fracture of the polymer and S is the area of the lower touch.
Preparation of SPHC−based drug delivery systems (SPHC−DDSs)
The drug delivery systems based on SPHC was prepared as a shuttle system as described by Dorkoosh et al. [17] with modifications. The SPHCDDS, as shown in fig. 1, consisted of conveyor system made of SPHC and core containing mixture of R−HCl and release retardant polymer. A hole was made up to specific depth in prepared SPHC to prepare the conveyor system. The surface of fully swollen SPHC was blotted to remove the excess DDW. With the help of borer a hole of suitable size was made at the centre of SPHC, to accommodate core component after drying. The swollen SPHC containing hole was dried in hot air oven below 50°. The core component was made by mixing R−HCl (equivalent to 300 mg of ranitidine) with release retardant polymers as shown in Table 1. The optimized amount of release retardant polymers viz. HPMC, EC, Chitosan, and NaCMC after preliminary studies (data not shown) was 150 mg. For Carbopol it was 100 mg. The core component was sieved through 80# sieve and filled in the hole of conveyer system. A piece of SPHC was used to close the hole of conveyer system using biodegradable glue. Instead of using SPH as reported by Dorkoosh et al. [17], a piece of SPHC was used as a cap. The SPHC−DDS was placed in a hard gelatin capsule (size 000) for further studies. However, the preparation of SPHCDDS was a little bit complex; it represents different aspect of drug delivery system.
| Formulation | R−HCl | HPMC | CBP | EC | CHT | NaCMC | Total weight |
|---|---|---|---|---|---|---|---|
| code | (mg) | (mg) | (mg) | (mg) | (mg) | (mg) | (mg) |
| CM1 | 336 | − | − | − | − | − | 336 |
| CM2 | 336 | 150 | − | − | − | − | 486 |
| CM3 | 336 | − | 100 | − | − | − | 436 |
| CM4 | 336 | − | − | 150 | − | − | 486 |
| CM5 | 336 | − | − | − | 150 | − | 486 |
| CM6 | 336 | − | − | − | − | 150 | 486 |
Table 1: Composition of sphc-ddss
Floating behavior of SPHC−DDSs:
The prepared SPHC−DDS was placed in a 100 ml of SGF. The time required for SPHC−DDS to rise to the surface and float was considered as the floating lag time. Time duration for which SPHC−DDS remains buoyant was taken as a total floating time.
in vitro dissolution studies
The release rate of R−HCl from SPHC−DDSs (n = 3) was determined using United States Pharmacopoeia (USP) XXIV dissolution testing apparatus II (paddle method). The dissolution test was performed using 900 ml SGF, at 37±0.5° and 75 rpm. Volumes of 10 ml were withdrawn at predetermined interval times (hourly) for 18 h from the dissolution medium and were replaced immediately with fresh medium. The samples were passed through 0.45 µm membrane filter and diluted (if needed) to a suitable concentration with SGF. The absorbance of these solutions was measured at 314 nm using a Shimadzu UV−1700 UV/Vis double beam spectrophotometer. The cumulative percentage of drug release was calculated using an equation obtained from a standard curve. The in vitro drug release data were analyzed by fitting them to different kinetic models [22] as shown in Table 2 in order to evaluate release mechanism of drug from SPHCDDSs.
| Mathematical model | Equation |
|---|---|
| Zero-order | Qt = Q0 + K0t |
| First-order | ln Qt = ln Q0 + K1t |
| Higuchi | Qt = KHt1/2 |
| Hixson-Crowell | Q01/3−Qt1/3=Kst |
| Korsmeyer-Peppas | Qt/Q∞ = Kktn |
| Weibull | log [−ln (1 − m)] = β log (t − Ti) − log α |
| Hopfenberg | Qt/Q∞ = 1 − [1 − k0t/C0a0]n2 |
Table 2: Mathematical models used to ascertain drug release [22]
Results and Discussion
In the synthesis procedure of SPHC, AA and AM were used as the monomers, BIS as a cross−linker, Span 80 as a foam stabilizer. In present investigation Span 80 was used instead of Pluronic F127 as reported by Dorkoosh et al. [23] Span 80 contributed to as a surface−active agent to create the porous structure of polymer. APS was polymerization initiator and TEMED catalyzed the reaction. At pH 5 the proper foam formation rate was there and so SPHCs with well−distributed pores were synthesized. Homogeneous SPHC synthesis with large numbers of pores was dependent on NaCMC amount present. Only when amount of NaCMC was up to 98 mg, homogeneous SPHCs with large numbers of interconnected pores could be obtained. The foam generation was fine and uniform during the synthesis. As the NaCMC amount was increased above 98 mg, SPHC was nonhomogeneous with few pores. The presence of NaCMC enhanced the reaction system viscosity, which prevented bubbles from escaping. And so the residual gas bubbles formed interconnected channels. NaCMC along with surfactant was favorable for foam generation as well as foam stabilization. It was readily blended and well−distributed with the solution, yielding a homogeneous SPHC.
Fig. 2 shows the SEM pictures of SPHC and CSPH. Both CSPH and SPHC showed large number of pores, indicating that formation of SPHC would retain the superporous structure. White fibers of NaCMC molecules on the peripheries of the inner pores were primarily observed in SPHC while not in CSPH. The fully swollen CSPH in DDW was found to be invisible and number of bubbles could be observed within the CSPH. By comparison, white fibers could be seen in the swollen SPHC as netlike distribution. It indicated that the NaCMC molecules appeared as the white fibers and they were well distributed in the SPHC. This primarily confirmed the formation of the SPHC of NaCMC.
Density, porosity, swelling parameters and mechanical strength of CSPH and SPHC are shown in Table 3. The presence of NaCMC decreased apparent density and porosity of SPHC. NaCMC prevented the rate of bubble formation and escaping from the solution mixture. The pore size of SPHC decreased due the accumulation of the NaCMC around the pore peripheries.
| Apparent density (g/cc) | % Porosity | Swollen sizea (mm2) in |
Swelling ratio in | Penetration pressure (cm water) in | ||||
|---|---|---|---|---|---|---|---|---|
| DDW | SGF | DDW | SGF | DDW | SGF | |||
| SPH | 0.79±0.03 | 69.30±4.36 | 1658±53 | 394±23 | 151.53±20.81 | 19.38±2.63 | 10 b | 0514±93 |
| SPHC | 0.49±0.04 | 42.38±2.68 | 875±43 | 250±11 | 31.49±07.16 | 06.41±1.84 | 104±13 | 1698±65 |
Table 3: Parameters comparison of SPH and SPHC
The presence of NaCMC led to retard equilibrium swelling ratio and swelling size of SPHC compared to CSPH. Higher content of NaCMC in SPHCs decreased the equilibrium swelling ratio (data nor shown). Through entanglement with the crosslinked NaCMC network, there was restriction of the polymeric chains flexibility. H−bonds between poly (AM−co−AA) and NaCMC reduced the polymer ability to form H−bonds with water molecules, limiting its water absorption. Overall, a dense NaCMC network would restrict the SPHC swelling. However, SPH and SPHC, owing to their porous structures, still possessed faster swelling rate as well as larger equilibrium swelling ratio compared to CSPH.
SPHC should withstand the pressure expected during repeated gastric contractions. The maximum pressure reported during the gastric contraction was from 50−70 cm water [24]. The penetration pressure was dependent on the swelling medium, and showed good penetration pressure when swollen in SGF compared to that of in DDW. DDW swollen SPHC showed satisfactory mechanical strength, but it was too less when compared to that of in SGF. SGF swollen SPHC showed penetration pressure of 1698 cm water. The prepared SPHC could withstand the pressure expected in the stomach. SPH showed penetration pressure less than 10 cm water in DDW, showed too lower mechanical stability.
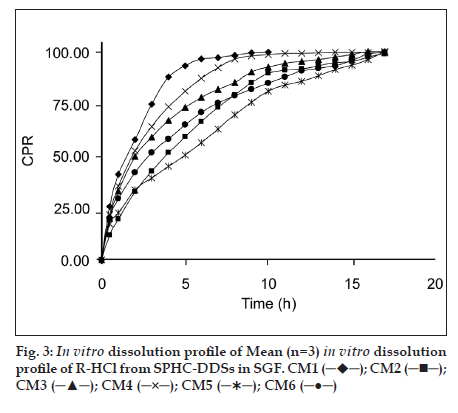
The superporous structure of SPHC−DDS provided density that was lower than that of the release medium. This system floated immediately upon contact with the medium, with zero lag−time. Compared to most of the conventional floating systems including gas−generating ones SPHC−DDS was low dense and so floated without sinking (t=0). In SGF the SPHC−DDS achieved proper in vitro floating behavior at earlier stage with capsule shell and also at subsequent stage for more than 18 h.
All SPHC−DDSs were found floating, swollen retaining their physical integrity till the end of dissolution study for 17 h. Batches F2−F6 were formulated to understand the effect of various release retardant polymers on R−HCl release from SPHCDDSs. Batch F1 was formulated without addition of release retardant polymer to check the drug release in the absence of polymer.
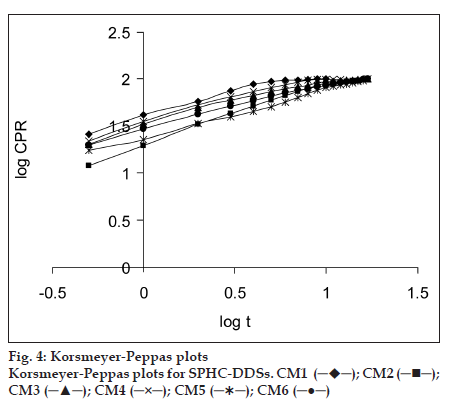
The dissolution data of batches was fitted to zero−order, first−order, Higuchi, Hixson−Crowell, Korsmeyer−Peppas, Weibull, and Hopfenberg models. in vitro dissolution profile of R−HCl in SGF from SPHCs is shown in fig. 3. The zero−order describes the drug release rate is independent of its concentration. The first−order describes the release of the drug from the systems is concentration dependent. Higuchi′s model describes the drug release from an insoluble matrix as a square root of a time−dependent process based on Fickian diffusion. The Hixson−Crowell cube root law model was used to understand the change in SPHC−DDS surface area and diameter with the progressive dissolution as a function of time. Korsmeyer−Peppas model is expected to be valid up to ~60% cumulative drug released, so the data were restricted to that range. Korsmeyer−Peppas plots are shown in fig. 4. Weibull plots can describe the dissolution curve in terms of applicable parameters. The shape parameter, β, characterizes the curve as either exponential (β= 1), S−shaped with upward curvature followed by a turning point (β>1), or with a steeper initial slope than is consistent with the exponential (β<1). The Hopfenberg model was used to ascertain the drug release from surface−eroding devices with cylinder shape displaying heterogeneous erosion.
The curve fitting and plotting was performed in Excel (Microsoft Software Inc., USA) and GraphPad Prism® version 5.02 (GraphPad Software Inc., USA). Linearization of dissolution profiles was carried out using the equations in Table 2. The determination coefficient (r2) was used as to indicate best fit model. The release constants (n) were calculated from the slope of the various plots. The n and r2 for individual batches are shown in Table 4. The average r2 of all batches was used for the selection of best fit model, which indicates the release mechanism of drug from SPHC−DDSs.
| CM1 | CM2 | CM3 | CM4 | CM5 | CM6 | ||
|---|---|---|---|---|---|---|---|
| Zero-order | K0 (h-1) | 7.1480 | 5.0050 | 4.1000 | 6.2510 | 4.8890 | 4.3230 |
| r2 | 0.7799 | 0.8781 | 0.8165 | 0.8383 | 0.9601 | 0.8940 | |
| First-order | K1 (h-1) | 0.1140 | 0.0964 | 0.0667 | 0.1029 | 0.0898 | 0.0731 |
| r2 | 0.6806 | 0.7108 | 0.6567 | 0.7073 | 0.8453 | 0.7447 | |
| Higuchi | KH (h-1/2) | 30.5300 | 26.6700 | 22.3000 | 28.6400 | 25.2500 | 22.9800 |
| r2 | 0.9022 | 0.9676 | 0.9373 | 0.9455 | 0.9934 | 0.9798 | |
| Hixson-Crowell | Ks (h-1) | 0.3851 | 0.2007 | 0.2533 | 0.3146 | 0.2130 | 0.1955 |
| r2 | 0.9674 | 0.9869 | 0.9267 | 0.9844 | 0.8570 | 0.9731 | |
| Korsmeyer-Peppas | n | 0.5786 | 0.6966 | 0.5936 | 0.6111 | 0.4809 | 0.5127 |
| Kk (h-n) | 0.0260 | 0.0518 | 0.0329 | 0.0299 | 0.0417 | 0.0352 | |
| r2 | 0.9943 | 0.9990 | 0.9907 | 0.9967 | 0.9963 | 0.9981 | |
| Weibull | Td (h) | 2.30 | 5.55 | 3.48 | 2.90 | 7.03 | 4.70 |
| β | 1.0230 | 1.0070 | 0.8384 | 0.9833 | 0.8281 | 0.8461 | |
| α | 2.3445 | 5.6170 | 2.8449 | 2.8489 | 5.0276 | 3.7039 | |
| r2 | 0.9871 | 0.9863 | 0.9902 | 0.9806 | 0.9504 | 0.9389 | |
| Hopfenberg | k0 (h-1) | 3.7360 | 2.0430 | 2.1500 | 3.0690 | 1.8750 | 1.9370 |
| r2 | 0.9214 | 0.9702 | 0.9467 | 0.9537 | 0.9921 | 0.9737 |
Table 4: Linearization of R-HCI release from SPHC-DDSs
The in vitro drug release from SPHCDDS was best explained by Korsmeyer−Peppas model, as the plots showed the highest linearity (r2= 0.9959), followed by Weibull (r2= 0.9723), Hopfenberg (r2= 0.9596), Higuchi (r2= 0.9543), and Hixson−Crowell (r2= 0.9493). The magnitude of the release exponent n in Korsmeyer−Peppas′s model indicates that the release mechanism is Fickian diffusion, case II transport, or anomalous transport [25] as shown in Table 5. The prepared SPHCDDS was of cylindrical shape, the limits for n considered were 0.45 and 0.89. Here, the values of n range between 0.48±0.01 and 0.70±0.01, which appears to indicate a coupling of the diffusion and polymer relaxation mechanism (anomalous non− Fickian transport) and may indicate that the release of drug from SPHCDDS could be controlled by more than one process.
| Diffusion exponent (n) | Overall solute diffusion mechanism |
|---|---|
| 0.45 | Fickian diffusion |
| 0.45 < n < 0.89 | Anomalous (non-Fickian) diffusion |
| 0.89 | Case−II transport |
| n > 0.89 | Super case−II transport |
Table 5: Diffusion exponent and solute release mechanism for cylindrical shape[25]
This study discusses the preparation of stomach specific drug−delivery system of R−HCl. SPHC showed improved characteristics than CSPH, especially for sustained release drug−delivery systems. Mechanical stability of SPHC in SGF was improved significantly and depends on the NaCMC content. SPHC−DDS was low dense, having good swelling tendency, mechanically stable and pH dependant. The in vitro drug release from SPHC−DDS was best explained by Korsmeyer−Peppas model. The drug release was found to be controlled by more than one process as Korsmeyer−Peppas plots indicated an anomalous non−Fickian transport. If we keep the little bit complex procedure for the synthesis SPHC aside, prepared SPHC−DDS successfully provided a sustained delivery of R−HCl.
Acknowledgements
The authors acknowledge Espee formulation Pvt. Ltd., Rajkot, Gujarat, India, for generous gift sample of R−HCl.
References
- Histamine H2 antagonists. In: Drug facts and comparisons. St. Louis: Facts and Comparisons; 2002. p. 1192-7.
- McCarty-Dawson D, Sue SO, Morrill B, Murdock RH. Ranitidine versus cimetidine in the healing of erosive esophagitis. ClinTher 1996;18:1150-60.
- Somade S, Singh K. Comparative evaluation of wet granulation and direct compression methods for preparation of controlled release ranitidine HCI tablets. Indian J Pharm Sci 2002;64:285-6.
- Lauritsen K. Clinical pharmacokinetics of drugs used in the treatment of gastrointestinal diseases. ClinPharmacokinet 1990;19:94-125.
- Grant S. Ranitidine: An updated review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in peptic ulcer and other allied diseases. Drugs 1989;37:801-70.
- Gramatté T, El Desoky E, Klotz U. Site-dependent small intestinal absorption of ranitidine. Eur J ClinPharmacol 1994;46:253-9.
- Leonard M, De Boisseson MR, Hubert P, Dalencon F, Dellacherie E. Hydrophobically modified alginate hydrogels as protein carriers with specific controlled release properties. J Control Release 2004;98: 395-405.
- Burmania JA, Stevens KR, Kao WJ. Cell interaction with proteinloaded interpenetrating networks containing modified gelatin and poly(ethylene glycol) diacrylate. Biomaterials 2003;24:3921-30.
- Chen J, Park H, Park K. Synthesis of superporous hydrogels: Hydrogels with fast swelling and superabsorbent properties. J Biomed Mater Res 1999;44:53-62.
- Chavda H, Patel C. Chitosan superporous hydrogel composite-based floating drug delivery system: A newer formulation approach. J Pharm Bioall Sci 2010;2:124-31.
- Chavda HV, Patel CN. Preparation and characterization of swellable polymer-based superporous hydrogel composite of poly (acrylamide-co-acrylic acid). Trends BiomatArtif Organs 2010;24:83-9.
- Yin L, Fei L, Cui F, Tang C, Yin C. Superporous hydrogels containing poly(acrylic acid-co-acrylamide)/O-carboxymethyl chitosan interpenetrating polymer networks. Biomaterials 2007;28:1258-66.
- Chavda HV, Patel CN, Karen HD. Preparation and characterization of chitosan-based superporous hydrogel composite. J Young Pharm 2009;1:199-204.
- Chen J, Park K. Synthesis and characterization of superporous hydrogel composites. J Control Release 2000;65:73-82.
- Polnok A, Verhoef JC, Borchard G, Sarisuta N, Junginger HE. in vitro evaluation of intestinal absorption of desmopressin using drug delivery systems based on superporous hydrogels. Int J Pharm 2004;269:303-10.
- Tang C, Yin CH, Pei YY, Zhang M, Wu LF. New superporous hydrogels composites based on aqueous Carbopols solution (SPHCcs): Synthesis, characterization and in vitrobioadhesive force studies. Eur Polym J 2005;41:557-62.
- Dorkoosh FA, Verhoef JC, Borchard G, Rafiee-Tehrani M, Verheijden JH, Junginger H. Intestinal absorption of human insulin in pigs using delivery systems based on superporous hydrogel polymers. Int J Pharm 2002;247:47-55.
- Dorkoosh FA, Verhoef JC, Ambagts MH, Rafiee-Tehrani M, Borchard G, Junginger HE. Peroral delivery systems based on superporous hydrogel polymers: Release characteristics for the peptide drugs buserelin, octreotide and insulin. Eur J Pharm Sci 2002; 15:433-9.
- Dorkoosh FA, Verhoef JC, Verheijden JH, Rafiee-Tehrani M, Borchard G, Junginger HE. Peroral absorption of octreotide in pigs formulated in delivery systems on the basis ofsuperporous hydrogel polymers. Pharm Res 2002;19:1532-6.
- Park K, Chen J, Park H. Hydrogel composites and superporous hydrogel composites having fast swelling, high mechanical strength and superabsorbent properties. US Patent 6271278. 2001.
- Ichikawa S, Iwamoto S, Watanabe J. Formation of biocompatible nanoparticles by self-assembly of enzymatic hydrolysates of chitosan and carboxymethyl cellulose. Biosci Biotech Biochem 2005;69:1637-42.
- Costa P, Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci 2001;13:123-33.
- Dorkoosh FA, Brussee J, Verhoef JC, Borchard G, Rafiee-Tehrani M, Junginger H. Preparation and NMR Characterization of Superporous Hydrogels (SPH) and SPH Composites. Polymer 2000;41:8213-20.
- Guyton AC. Basic Human Physiology: Normal Function and Mechanisms of Disease. Vol. 2. Philadelphia: W.B. Saunders Co.;1977. p. 662-4.
- Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 1983;15:25-35.


 ; CM2 (
; CM2 ( ); CM3 (
); CM3 ( ); CM4 (
); CM4 ( ); CM5 (
); CM5 ( ); CM6 (
); CM6 ( )
)