- Corresponding Author:
- N. G. N. Swamy
Department of Pharmaceutics, Government College of Pharmacy, Subbaiah Circle, Bangalore–560 027, India
E-mail: ngnswami@yahoo.co.in
| Date of Submission | 06 April 2011 |
| Date of Revision | 05 November 2011 |
| Date of Acceptance | 12 November 2011 |
| Indian J Pharm Sci, 2011, 73 (6): 608-61412-Mar-2013 |
Abstract
Amlodipine besylate microspheres for intranasal administration were prepared with an aim to avoid first-pass metabolism, to achieve controlled blood level profiles and to improve therapeutic efficacy. Hydroxypropyl Guar, a biodegradable polymer, was used in the preparation of microspheres by employing water in oil emulsification solvent evaporation technique. The formulation variables were drug concentration, emulsifier concentration, temperature, agitation speed and polymer concentration. All the formulations were evaluated for particle size, particle shape and surface morphology by scanning electron microscopy, percentage yield, drug entrapment efficiency, in vitro mucoadhesion test, degree of swelling and in vitro drug diffusion through sheep nasal mucosa. The microspheres obtained were free flowing, spherical and the particles ranged in size from 13.4±2.38 μm to 43.4±1.92 μm very much suitable for nasal delivery. Increasing polymer concentration resulted in increased drug entrapment efficiency and increased particle size. Amlodipine besylate was entrapped into the microspheres with an efficiency of 67.2±1.18 % to 81.8±0.64 %. The prepared microspheres showed good mucoadhesion properties, swellability and sustained the release of the drug over a period of 8 h. The data obtained were analysed by fitment into various kinetic models; it was observed that the drug release was matrix diffusion controlled and the release mechanism was found to be non- Fickian. Stability studies were carried out on selected formulations at 5±3°, 25±2°/60±5% RH and 40±2°/75±5% RH for 90 days. The drug content was observed to be within permissible limits and there were no significant deviations in the in vitro mucoadhesion and in vitro drug diffusion characteristics.
Keywords
Amlodipine besylate, degree of swelling, drug entrapment efficiency, in vitro mucoadhesion studies, mucoadhesive microspheres, nasal drug delivery, water in oil emulsification solvent evaporation technique
The nasal route is conventionally used for drug delivery for treatment of local diseases [1]. In the recent years, this route has received special attention as a convenient and reliable method for the systemic delivery of drugs, especially those that are ineffective by oral route due to their metabolism in the gastrointestinal tract subject to first-pass effect and must be administered by injection [2].
The nasal cavity as a site for systemic absorption of drugs has advantages such as relatively large surface area, porous endothelial membrane, highly vascularized epithelial layer, enhanced blood flow, avoidance of first-pass metabolism due to lack of gastric and pancreatic enzymatic activity, neutral pH of the nasal mucus and ready accessibility [3,4]. However, the major limitation of the nasal drug delivery is the mucociliary clearance that determines a limited time available for adsorption within the nasal cavity [5].
Nasal mucociliary clearance is one of the most important limiting factor for nasal drug delivery [6]. It severely limits the time allowed for drug absorption to occur and effectively rules out sustained nasal drug administration. However, mucoadhesive preparations have been developed to achieve the increased contact time of the dosage form with mucosal layers of nasal cavities resulting in enhanced drug absorption [7].
Amongst the various approaches available to enhance the transnasal delivery of drugs, the mucoadhesive microsphere drug delivery system is an attractive concept that has the ability to control the rate of drug clearance from the nasal cavity as well as to protect the drug from enzymatic degradation. The microspheres form a gel-like layer, which is cleared relatively slowly from the nasal cavity, resulting in prolonged residence time of the drug formulation, thereby increasing the systemic bioavailability of drugs [8].
Amlodipine besylate (AB), a calcium channel blocker, is a drug of choice in the treatment of hypertension and angina pectoris. AB, on oral administration undergoes first-pass metabolism and exhibits 60-65% bioavailability [9]. The present investigation was aimed at avoidance of first-pass metabolism of AB by preparing hydroxypropyl guar (HPG) microspheres for nasal administration. Guar gum is the refined endosperm of guar (Cyamopsis tetragonalobus) seed. HPG is made by reacting guar gum with propylene oxide [10]. HPG has been investigated for its gelling [11], viscosity enhancing [12], film forming [13] and suspending properties [14].
The purpose of this study is to investigate the suitability of HPG microspheres as nasal drug delivery system and also to study the influence of the process variables in the preparation of the microspheres.
Materials and Methods
AB and HPG were obtained as gift samples from Micro Labs Limited, Bangalore and Encore Polymers, Mumbai, respectively. Liquid Paraffin (light and heavy) were purchased from Qualigens fine chemicals, Span 80 was purchased from Himedia Laboratories Pvt. Ltd., Mumbai, India. All other reagents used were of analytical grade.
Preparation of microspheres:
Mucoadhesive HPG microspheres containing AB were prepared by water in oil emulsification solvent evaporation technique [15]. A 1% w/v aqueous HPG dispersion was prepared using a magnetic stirrer. Pure AB was added to the aqueous polymeric dispersion and agitated for 15 min. The resultant dispersion was extruded through a syringe (Needle no. 20) into 100 ml of liquid paraffin (heavy and light 1:1 ratio) containing 0.5% w/v span 80 as emulsifying agent. The aqueous phase was emulsified into the oily phase by agitating the system at a constant agitation speed of 2000 rpm. While stirring, the flask and its contents were heated to 80º. Stirring and heating were maintained for about 4.5 h until aqueous phase was completely removed by evaporation. The mineral oil was decanted and the microspheres obtained were washed three times with 100 ml aliquots of n-hexane, filtered through Whatman filter paper and then dried in a hot air oven at 500 for 2 h and preserved in a desiccator at room temperature.
Effect of process variables on microsphere properties:
HPG microspheres were prepared with different drug to polymer ratio (0.5:3, 1:3, 1.5:3 and 2:3) at emulsifier concentrations of 0.2, 0.3, 0.4 and 0.5% w/v, at temperatures of 60, 70, 80 and 90º, at agitation speeds of 1400, 1600, 1800 and 2000 rpm and with varying HPG to drug ratios of 1:1, 2:1, 3:1 and 4:1. The effect of process variables on the properties of the resulting microspheres is depicted in Tables 1-3.
Determination of percentage yield:
The practical percentage yield [16] was calculated from the weight of dried microspheres recovered from each batch in relation to the sum of the initial weight of starting materials. The percentage yield was calculated using the following formula: % Yield= [Practical mass (Microspheres) / Theoretical mass (Polymer + Drug)]×100.
Drug entrapment efficiency:
Microspheres equivalent to 5 mg of AB were crushed in a glass mortar and pestle and the powdered microspheres were suspended in 25 ml of phosphate buffer pH 6.4. After 24 h, the solution was filtered, 1 ml of the filtrate was pipetted out and diluted to 10 ml and analyzed for the drug content using Elico SL-159 UV/Vis spectrophotometer at 366 nm [17]. The drug entrapment efficiency [18] was calculated using the following formula: %Drug entrapment efficiency = [Practical drug content/Theoretical drug content]×100.
Particle size analysis:
Particle size of the microspheres was determined by optical microscopy. The eye piece micrometer was calibrated with the help of a stage micrometer. The particle diameters of more than 300 microspheres were measured randomly. The average particle size [19] was determined by using Edmondson’s equation. Dmean=Σnd/Σn, Where, n=number of microspheres checked; d=mean size range.
Shape and Surface morphology:
The shape and surface characteristics [20] of the microspheres were evaluated by means of scanning electron microscopy (Jeol–JSM-840A, Japan). The samples were prepared by gently sprinkling the microspheres on a double adhesive tape, which is stuck to an aluminium stub. The stubs were then coated with gold using a sputter coater (Jeol Fine coat JFC 1100E, ion sputtering device) under high vacuum and high voltage to achieve a film thickness of 30 nm. The samples were then imaged using a 20 KV electron beam.
| Formulation code | Drug to polymer ratio | Emulsifierconcentration(% w/v) | Temperature(°) | % Drug entrapment efficiency* | Particle size(µm) |
|---|---|---|---|---|---|
| HM-1 | 0.5:3 | 0.5 | 80 | 82.7±1.62 | 21.92±2.03 |
| HM-2 | 1:3 | 0.5 | 80 | 80.3±1.34 | 39.67±2.45 |
| HM-3 | 1.5:3 | 0.5 | 80 | 74.6±2.19 | 45.88±3.04 |
| HM-4 | 2:3 | 0.5 | 80 | 69.2±2.54 | 54.95±2.94 |
Table 1: Effect Of Drug To Polymer Ratio On % Drug Entrapment Efficiency And Particle Size Of Microspheres.
| Formulation code | Emulsifier concentration(% w/v) | Temperature(°) | Agitation speed (rpm) | Particle size(µm)* |
|---|---|---|---|---|
| HM-5 | 0.2 | 80 | 2000 | 131.6±2.52 |
| HM-6 | 0.3 | 80 | 2000 | 86.3±3.79 |
| HM-7 | 0.4 | 80 | 2000 | 63.5±3.94 |
| HM-8 | 0.5 | 80 | 2000 | 41.2±2.50 |
| HM-9 | 0.5 | 60 | 2000 | 63.4±2.80 |
| HM-10 | 0.5 | 70 | 2000 | 48.6±3.14 |
| HM-11 | 0.5 | 80 | 2000 | 35.6±4.67 |
| HM-12 | 0.5 | 90 | 2000 | 19.1±4.74 |
| HM-13 | 0.5 | 80 | 1400 | 85.9±5.09 |
| HM-14 | 0.5 | 80 | 1600 | 68.7±5.01 |
| HM-15 | 0.5 | 80 | 1800 | 53.1±4.22 |
| HM-16 | 0.5 | 80 | 2000 | 38.6±2.93 |
Table 2: Effect Of Emulsifier Concentration, Temperature And Agitation Speed On Particle Size Of Microspheres.
| HAM-1 | HAM-2 | HAM-3 | HAM-4 | ||
|---|---|---|---|---|---|
| Zero order | R | 0.7523 | 0.863 | 0.8861 | 0.8762 |
| k | 0.1489 | 0.1378 | 0.126 | 0.1148 | |
| First order | R | 0.7542 | 0.8641 | 0.887 | -8771 |
| k | -0.0015 | -0.0014 | -0.0013 | -0.0012 | |
| Matrix | R | 0.9821 | 0.9938 | 0.9959 | 0.9937 |
| k | 0.3657 | 0.3347 | 0.3049 | 0.2783 | |
| Hixon-Crowell | R | 0.7536 | 0.8638 | 0.8867 | 0.8768 |
| k | -0.0005 | -0.0005 | -0.0004 | -0.0004 | |
| Peppas | R | 0.9706 | 0.9818 | 0.9829 | 0.9743 |
| k | 0.3966 | 0.3015 | 0.2678 | 0.2404 | |
| n | 0.4572 | 0.5749 | 0.5895 | 0.6021 |
Table 3: Influence Of Polymer To Drug Ratio On % Yield, % Drug Entrapment Efficiency, Particle Size, Degree Of Swelling And % Mucoadhesion.
Degree of swelling:
The swellability [21] of microspheres in physiological media was determined by allowing the microspheres to swell in the phosphate buffer saline pH 6.4. 100 mg of accurately weighed microspheres were immersed in little excess of phosphate buffer saline of pH 6.4 for 24 h and washed thoroughly with deionised water. The degree of swelling was arrived at using the following formula: α=Ws–Wo/Wo, Where, α is the degree of swelling; Wo is the weight of microspheres before swelling and Ws is the weight of microspheres after swelling.
In vitro mucoadhesion studies:
The in vitro mucoadhesion study of microspheres was assessed using Falling liquid film technique [22]. A strip of sheep nasal mucosa was mounted on a glass slide and 50 mg of accurately weighed microspheres were sprinkled on the nasal mucosa. This glass slide was incubated for 15 min in a desiccator at 90% relative humidity to allow the polymer to interact with the membrane and finally placed on the stand at an angle of 45º. Phosphate buffered saline of pH 6.4 previously warmed to 37±0.5° was allowed to flow over the microspheres and membrane at the rate of 1 ml/min for 5 min with the help of a peristaltic pump. At the end of this process, the detached particles were collected and weighed. % Mucoadhesion=[(weight of sampleweight of detached particles)/weight of sample]×100.
In vitro drug diffusion studies:
Fresh sheep nasal mucosa was collected from a nearby slaughter house. The nasal mucosa of sheep was separated from sub layer bony tissues and stored in distilled water containing few drops of gentamycin injection [23]. After complete removal of blood from mucosal surface, it was attached to the donor chamber tube.
In vitro nasal diffusion study [24] was done using nasal diffusion cell, having three openings each for sampling, thermometer and donor tube chamber. The receptor compartment has a capacity of 60 ml in which phosphate buffer of pH 6.4 was taken. Within 80 min of removal, the nasal mucosa measuring an area of 3 cm2 was carefully cut with a scalpel and tied to the donor tube chamber and it was placed establishing contact with the diffusion medium in the recipient chamber. Microspheres equivalent to 5 mg of AB were spread on the sheep nasal mucosa. At hourly intervals, 1 ml of the diffusion sample was withdrawn with the help of a hypodermic syringe, diluted to 10 ml and absorbance was read at 366 nm. Each time, the sample withdrawn was replaced with 1 ml of pre-warmed buffer solution (pH 6.4) to maintain a constant volume of the receptor compartment vehicle.
In vitro drug release kinetics:
For understanding the mechanism of drug release and release rate kinetics [25] of the drug from the dosage form, the data obtained was analysed with software (PCP-Disso V2.08) [26] equipped with zero order, first order, Higuchi matrix and Korsmeyer–Peppas model kinetics. By analyzing the R values, the best fit model was arrived at.
Stability studies:
Stability studies were carried out at 5±3º, 25±2º/60±5% RH and 40±2º/75±5% RH for three months using programmable environmental test chambers [27] (Remi Instruments Ltd.). The selected formulations were packed in amber coloured glass containers and closed with air tight closures and stored for 90 days. Samples were analyzed at the end of 30, 60 and 90 days and they were evaluated for % drug entrapment efficiency, in vitro mucoadhesion test and in vitro drug diffusion studies.
Results and Discussion
The mucoadhesive microspheres of HPG were prepared by water in oil solvent evaporation technique. Preliminary trials were carried out to optimize the process of preparation. Batches HM-1 to HM-16 were prepared to study the effect of drug to polymer ratio, emulsifier concentration, temperature and agitation speed on the % drug entrapment efficiency and particle size.
As the drug concentration was varied from 0.5:3 to 2:3, it was observed that the particle size increased, whereas, entrapment efficiency decreased in the same concentration range. The increase in particle size could be attributed to the increased drug content of the emulsion droplet at higher drug concentration. The decrease in entrapment efficiency with increase in drug concentration could be related to the increased extent of drug diffusion to the external phase due to greater flux at higher drug content during the emulsification and microsphere formation process. Hence, further trials were carried out with a drug to polymer ratio of 1:3.
Increasing the surfactant concentration from 0.2 to 0.5% w/v exhibited a reversal in trend between particle sizes. Microspheres fabricated with 0.2% w/v Span 80 showed the largest particle size while those fabricated with 0.5% w/v showed lowest particle size. When the surfactant is added in small concentrations, it may not have been able to cover the entire droplet surface. Thus some of the droplets would tend to aggregate till the surface area was decreased to such a point that the available amount of surfactant was able to coat the entire surface of the agglomerate and form a stable emulsion resulting in a larger microparticle size. When the concentration of emulsifier is increased, it will allow the emulsion to stabilize to a greater interfacial surface area, thus leading to smaller particle size. Based on these observations concentration of emulsifier was optimized to 0.5% w/v.
Increase in the temperature from 60º to 90º led to a decrease in the mean particle size. However, further increase in temperature above 90º did not produce any significant change in mean particle size. Increase in temperature from 60º to 90º increases the degree of congealing or rigidization of the polymer, which ultimately results in shrinking of the particles, leading to decrease in particle size. Hence for the final formulation design, a temperature of 80º was optimized.
The results were in general agreement with the general theory of microspheres that the particle size of microspheres prepared at 2000 rpm were smaller than those prepared at 1400, 1600 and 1800 rpm. Since the microspheres obtained at 2000 rpm were in the size range of 30-40 μm, which are suitable for nasal delivery, 2000 rpm was chosen to obtain microspheres.
It was observed that as the polymer to drug ratio increases, the product yield also increases. The low percentage yield in some formulations may be due to microspheres lost during the washing process. The percentage yield was found to be in the range of 64.30 to 84.26%. Percent drug entrapment efficiency of AB ranged from 67.2±1.18% to 81.8±0.64% for HPG microspheres. Increase in the polymer concentration resulted in increased viscosity of the dispersed phase. The particle size increases exponentially with viscosity. The higher viscosity of the polymer solution at the highest polymer concentration would be expected to decrease the diffusion of the drug into the external phase which would result in higher entrapment efficiency.
The prepared microspheres were in a size range suitable for nasal drug delivery. The mean microsphere size increased with increasing polymer concentration due to a significant increase in the viscosity, thus leading to an increased aqueous droplet size leading to an increase in the size of the microsphere. HPG-AB microspheres in the size range of 13.4±2.38 μm to 43.4±1.92 μm were obtained.
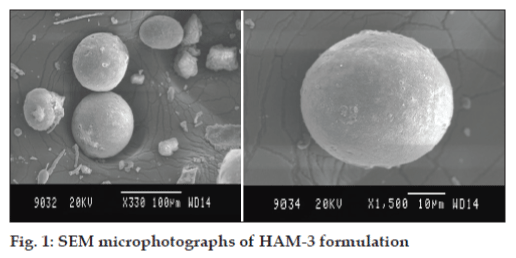
The photographs of the optimized formulation taken by scanning electron microscope are depicted in the fig. 1. The SEM photographs revealed that the microspheres of HPG (HAM-3) were discrete and spherical in shape with a rough outer surface morphology which could be because of the surface association of the drug with the polymer. The pores on microsphere surface could help in drug release by diffusion mechanism.
Swellability is an indicative parameter for rapid availability of drug solution for diffusion with greater flux. Swellability data revealed that amount of polymer plays an important role in solvent transfer. It can be concluded from the data shown in Table 3 that with an increase in polymer concentration, the degree of swelling also increases ranging from 0.743±0.015 to 1.086±0.012. Thus we can say that amount of polymer directly affects the degree of swelling.
As the polymer to drug ratio is increased, HPG microspheres exhibited an increase in percent mucoadhesion ranging from 75.94±0.076 to 80.70±0.210; the results of in vitro mucoadhesion test are compiled in Table 3.
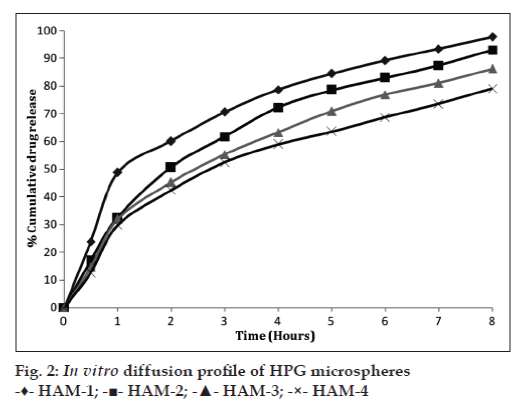
The in vitro diffusion of AB from the prepared microspheres exhibited a biphasic mechanism. The release of AB from the microspheres was characterized by an initial phase of burst effect due to the presence of drug particles on the surface of the microspheres followed by a second phase of moderate release. The initial burst effect is a desired effect to achieve initial therapeutic plasma concentration of the drug.
The initial burst effect was considerably reduced with increase in polymer concentration. The increase in the polymer concentration resulting in better entrapment efficiency could be the reason for the observed decrease in burst effect.
As the polymer to drug ratio (HAM-1 to HAM-4) was increased, the extent of drug release decreased from 97.73–79.11%. A significant decrease in the rate and extent of drug release is attributed to the increase in density of polymer matrix that results in increased diffusion path length which the drug molecules have to traverse. The release of the drug has been controlled by swelling control release mechanism. Additionally, the larger particle size at higher polymer concentration also restricts the total surface area thus resulting in slower drug release over a span of 8 h. The comparative in vitro drug diffusion profile from the HPG microspheres is depicted in fig. 2.
| HAM-1 | HAM-2 | HAM-3 | HAM-4 | ||
|---|---|---|---|---|---|
| Zero order | R | 0.7523 | 0.863 | 0.8861 | 0.8762 |
| k | 0.1489 | 0.1378 | 0.126 | 0.1148 | |
| First order | R | 0.7542 | 0.8641 | 0.887 | -8771 |
| k | -0.0015 | -0.0014 | -0.0013 | -0.0012 | |
| Matrix | R | 0.9821 | 0.9938 | 0.9959 | 0.9937 |
| k | 0.3657 | 0.3347 | 0.3049 | 0.2783 | |
| Hixon-Crowell | R | 0.7536 | 0.8638 | 0.8867 | 0.8768 |
| k | -0.0005 | -0.0005 | -0.0004 | -0.0004 | |
| Peppas | R | 0.9706 | 0.9818 | 0.9829 | 0.9743 |
| k | 0.3966 | 0.3015 | 0.2678 | 0.2404 | |
| n | 0.4572 | 0.5749 | 0.5895 | 0.6021 |
Table 4: In Vitro Release Data Fitting Into Various Mathematical Models.
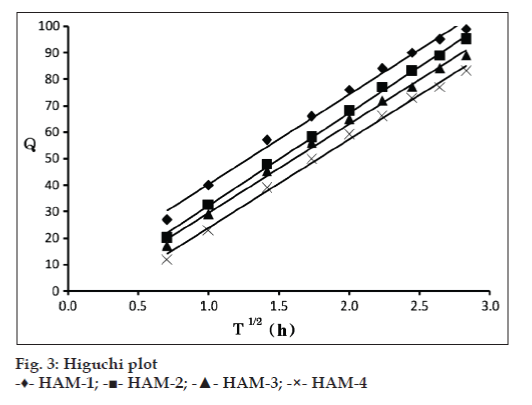
The drug release kinetic data is compiled in Table 4. In all the cases, the R values of Higuchi matrix model were close to 1. The diffusion coefficient (n) values ranged between 0.457 to 0.602. Since the R values of Higuchi matrix were close to 1, the drug release follows matrix diffusion kinetics and the plot shown in fig. 3 revealed linearity; hence it was concluded that diffusion was the main mechanism of drug release from the mucoadhesive microspheres. Further, the observed diffusion coefficient values are indicative of the fact that the drug release from the formulation follows non-Fickian transport mechanism.
The stability data showed that there was no change in the appearance of the microspheres indicating that the formulations were stable at different conditions of storage. It was observed that there was slight reduction in the drug content of the microspheres which were stored at 40±2º/75±5% RH at the end of 90 days and no significant change in drug content were observed for formulations stored at room temperature and at 5±3º. The extent of mucoadhesion of the formulations did not show any significant change after the microspheres were subjected to stability studies. In vitro drug diffusion studies for all the four formulations were carried out at the end of 90 days and did not reveal any significant change in drug release from all the formulations. Thus, we may conclude that, the drug does not undergo degradation on storage.
The water in oil solvent evaporation technique for obtaining HPG microspheres has proved to be a useful tool in the preparation of microspheres for nasal drug delivery. By virtue of prolonged drug residence at the site of absorption, improved bioavailability can be achieved in contrast to oral dosage form prone for first-pass metabolism.
References
- Gavini E, Hegge AB, Rassu G, Sanna V, Testa C, Pirisino G, et al. Nasal administration of Carbamazepine using chitosan microspheres: In vitro/in vivo studies. Int J Pharm 2006;307:9-15.
- Mainardes RM, Urban MC, Cinto PO, Chaud MV, Evangelista RC, Gremião MP. Liposomes and micro / nanoparticles as colloidal carriers for nasal drug delivery. Curr Drug Deliv 2006;3:275-85.
- Jadhav KR, Gambhire MN, Shaikh IM, Kadam VJ, Pisal SS. Nasal drug delivery system – factors affecting and applications. Curr Drug Ther 2007;2:27-38.
- Krishnamoorthy R, Mitra AK. Prodrugs for nasal drug delivery. Adv Drug Deliv Rev 1998;29:135-46.
- Soane RJ, Frier M, Perkins AC, Jones NS, Davis SS, Illum L.Evaluation of the clearance characteristics of bioadhesive systems in humans. Int J Pharm 1999;178:55-65.
- Hasçiçek C, Gönül N, Erk N. Mucoadhesive microspheres containing gentamicin sulfate for nasal administration: Preparation and in vitrocharacterization. IL Farmaco 2003;58:11-6.
- Vidgren P, Vidgren M, Vainio P, Nuutinen J, Paronen P. Double-labelling technique in the evaluation of disodium cromoglycatemicrospheres. Int J Pharm 1991;73:131-6.
- Chein YW. Nasal drug delivery and drug delivery systems. In: Novel Drug Delivery Systems. 2nd ed. New York: Marcel Dekker Inc.; 1992. p. 229-68.
- Patil SB, Murthy RS. Preparation and in vitroevaluation ofmucoadhesive chitosan microspheres of Amlodipine Besylate for Nasal
- Administration. Indian J Pharm Sci 2006;68:64-7.
- Swamy NGN, Dharmarajan TS, Paranjothi KL. Derivatization of Guar to various Hydroxyalkyl derivatives and their characterization. Indian Drugs 2006;43:756-9.
- Swamy NGN, Dharmarajan TS, Paranjothi KL. Study of HydroxypropylGuar derivative for its gelling property and its use in the formulation of Tenoxicam Gels. Pak J Pharm Sci 2007;20:61-6.
- Swamy NGN, Dharmarajan TS, Paranjothi KL. Study of thickening properties of hydroxypropyl Guar and its utilization in the formulation and evaluation of Ibuprofen suppositories. Indian Drugs 2007;44:925-9.
- Swamy NGN, Dharmarajan TS, Paranjothi KL. Study of film forming properties of Hydroxypropyl Guar and its use in the preparation of medicated transdermal patches. Indian J Pharm Educ Res 2008;42:147-53.
- Swamy NGN, Dharmarajan TS, Paranjothi KL. Study of the Rheological behavior of hydroxypropyl guar and its use in the formulation and evaluation of Sulphamethoxazole suspensions. Indian Drugs 2009;46:947-53.
- Abd El-Hameed MD, Kellaway IW. Preparation and in vitro characterization of mucoadhesive polymeric microspheres as intra-nasal delivery systems. Eur J Pharm Biopharm 1997;44:53-60.
- Mahajan HS, Gattani SG. Gellan gum based microparticles of Metoclopromide hydrochloride for Intranasal delivery: Development and Evaluation. Chem Pharm Bull 2009;57:388-92.
- British Pharmacopoeia. Medicinal and Health care products. Vol. 2. London: Regulatory Agency (MHRA); 2005. p. 126-8.
- Dubey RR, Parikh RH. Two-stage optimization process for formulation of Chitosan microspheres. AAPS PharmSciTech 2004;5:1-9.
- Dandagi PM, Mastiholimath VS, Gadad AP, Iliger SR. Mucoadhesive microspheres of Propranolol Hydrochloride for Nasal Delivery. Indian J Pharm Sci 2007;69:402-7.
- Chaurasia M, Chourasia MK, Jain NK, Jain A, Soni V, Gupta Y, et al.Cross-linked guar gum microspheres: A viable approach for improved delivery of anticancer drugs for the treatment of colorectal cancer. AAPS PharmSciTech 2006;7:E1-9.
- Jain SK, Jain NK, Gupta Y, Jain A, Jain D, Chourasia MK. Mucoadhesive chitosan microspheres for non-invasive and improved nasal delivery of Insulin. Indian J Pharm Sci 2007;69:498-504.
- Ascentiis AD, Grazia JL, Bowman CN, Colombo P, Peppas NA. Mucoadhesion of poly (2-hydroxyethyl methacrylate) is improved when linear poly (ethylene oxide) chains are added to the polymer network. J Control Release 1995;33:197-201.
- Chaudhari PD, Kolsure P, Ajab A, Vari N. Recent trends in nasal drug delivery systems - An overview. Pharm Rev 2006;4. Available from: http://www.pharmainfo.net/reviews/recent-trends-nasal-drug-delivery-system-overview. [Last Accessed on 2011 Nov 5].
- Pisal S, Shelke V, Mahadik K, Kadam S. Effect of Organogelcomponents on in vitro nasal delivery of Propranolol hydrochloride. AAPS PharmSciTech 2004;4:1-9.
- Costa P, Lobo JM. Modeling and Comparision of dissolution profiles. Eur J Pharm Sci 2001;13:123-33.
- Swamy NGN, Rupa V, Abbas Z, Dasankoppa FS. Formulation and evaluation of Nanosuspensions for enhancing the dissolution of poorly soluble Mebendazole. Indian Drugs 2010;47:47-54.
- Tamizharasi S, Rathi JC, Rathi V. Formulation and evaluation of Pentoxifylline-loaded poly (e-caprolactone) microspheres. Indian J Pharm Sci 2008;70:333-7.