- *Corresponding Author:
- G. P. Agrawal
Department of Pharmaceutical Sciences, Dr. Harisingh Gour Vishwavidyalaya, Sagar-470 003
E-mail: pmishra51@rediffmail.com
| Date of Submission | 15 May 2004 |
| Date of Revision | 23 September 2005 |
| Date of Acceptance | 05 July 2006 |
| Indian J Pharm Sci, 2006, 68 (4): 425-431 |
Abstract
Tablets of mutual prodrugs of ibuprofen, i.e, 'ibuprofen with paracetamol' and 'ibuprofen with salicylamide,' were prepared by direct compression method. The preformulation studies such as flow property, solid state stability at elevated temperatures, solid state stability under different humidity conditions, photolytic stability and compatibility studies of prodrugs with excipients were also performed to design and develop tablet formulations of prodrugs. Quality control tests and in vivo studies of prepared tablets of prodrugs were performed. The result of preformulation studies revealed that prodrugs have good flow property, good solid state stability at elevated temperatures and unstable under different humidity conditions. The photolytic stability study showed that prodrugs are quite stable to light; hence prodrugs are nonphotolytic. The compatibility study indicated that there was no incompatibility or interaction between prodrugs and excipients, which were tried. The prepared tablets of prodrugs were found to satisfy all quality control requirements of tablets mentioned in the Indian Pharmacopoeia. In vivo study of tablet formulations of prodrugs confirmed that they possessed the ability of parent drug, i.e., ibuprofen. In vivo study also showed better extent of bioavailability (indicated by AUC0-24) of tablet of prodrugs as compared to tablets of ibuprofen.
Ibuprofen is a well-known nonsteroidal antiinflammatory drug belonging to the family of propionic acid derivatives. It can cause upper gastrointestinal damage, including lesion, peptic ulcers, bleeding and perforation. These side effects are attributed to the presence of free – COOH group and inhibition of endogenous prostaglandins. Therefore, blocking this group by synthesizing functional derivatives of carboxylic acid may reduce these side effects [1]. Earlier reports revealed the most prevalent approach for preparing a prodrug of NSAIDs. In recent years, there has been an increasing interest in the design and development of mutual prodrugs, which involves combining of two different pharmacophores with similar pharmacological activities to give synergistic action [2,3].
The synthesis and characterization of mutual prodrugs of ibuprofen, i.e., ‘ibuprofen with paracetamol (IBU-PA)’ and ‘ibuprofen with salicylamide (IBU-SAL)’ were earlier reported [4]. Drug substances are most frequently administered orally by means of solid dosage forms such as tablets. Solid oral dosage forms are a preferred class of a product. The prime reasons for this popularity include ease of accurate dosage, good physical and chemical stability, competitive unit production cost and an elegant distinctive appearance resulting in high level of patient acceptability [5,6]. The present work was aimed to design and develop the oral dosage forms, i.e., tablets, of the synthesized mutual prodrugs of ibuprofen (IBU-PA and IBU-SAL). The preformulation studies provide a rational basis for the formulation approaches – to maximize the chances of success in formulating an acceptable safe, efficacious, stable product and to ultimately provide a basis for optimizing drug product quality and performance [7]. Therefore, the preformulation studies of prodrugs were performed to design and develop tablet formulations of prodrugs. In vivo studies of developed tablet formulations were also performed.
Materials and Methods
The ibuprofen was obtained from Knoll Pharmaceuticals Ltd., Jejuri, Pune, as a gift sample. Microcrystalline cellulose (Emcocel) and anhydrous lactose were obtained from De-Melkindustrie Ueghel, Netherlands, as gift samples. Dextrose (Emdex), starch 1500 and unmilled dicalcium phosphate (Emcompress) were obtained from Edward Mendell Co. Inc, New York, also as gift samples. All other chemicals used in this study were of IP grade or analytical grade.
Evaluation of flow properties of mutual prodrugs
The mutual prodrugs were evaluated for flow properties like angle of repose and Carr’s compressibility index. The angle of repose of prodrugs powder was determined by the Pilpel method [8] and Carr’s compressibility index was determined by the Carr method [9].
Solid state stability
The solid state reactions are slow and it is customary to use stress conditions in the investigation of stability. This approach is not always straight forward and due care must be exerted in the interpretation of the data. High temperatures can drive moisture out of a sample and render a material apparently stable that would otherwise be prone to hydrolysis. Therefore, the stability studies of prodrugs were performed at elevated temperature and under different humidity conditions and photolytic studies were also performed.
Solid state stability at elevated temperatures
The prodrugs (200 mg) were placed in glass vials. These vials were kept at 40°, 50° and 60° for 3 mo. The samples so stored were examined for caking, liquefaction, discolouration and odour or gas formation [10,11]. The sample (10 mg) was withdrawn from vials after 10 days up to 3 mo and dissolved in acetonitrile and diluted suitably for estimation of prodrugs by HPLC method.
Method of analysis
HPLC was performed on the instrument of M/s Shimadzu, Japan, equipped with dual-piston reciprocating pump (model LC–10 AT vp), Rheodyne injection system (model 7125 with loop capacity of 20 μl), UV/Vis photodiode array detector (model SDP–MIOA vp) and stainless steel column (Luna 5 μ, 250×6.4 mm, C18, Phenomenex Inc, USA). Pure acetonitrile of HPLC grade was used as mobile phase. The flow rate of mobile phase was maintained at 1.0 ml/min and all solutions to be analysed were injected at a volume of 20 μl. The UV/Vis photodiode array detector was set at 240 nm. The retention time of ibuprofen, IBU-PA and IBU-SAL was 3.19, 3.85 and 3.84 min respectively. The amount of ibuprofen and its mutual prodrugs in the sample was calculated and percent of ibuprofen and prodrug was determined. The rate of degradation (k) of prodrugs in solid state at 40°, 50° and 60° temperatures were obtained by plotting percent prodrug remaining versus time. The value of k at 25° was obtained from Arrhenius equation and shelf life (t10%) of prodrugs was calculated [12].
Solid state stability under different humidity conditions
In the presence of moisture, many drug substances hydrolyse, react with other excipients or oxidise. These reactions can be accelerated by exposing the solid drug to different relative humidity conditions. Controlled humidity environments can be readily obtained using laboratory desiccators containing structured solutions of various salts. For making the controlled environments, saturated solutions of CaCl2 6H2O (31% RH), Mg (NO3)2 6H2O (52% RH), NH4Cl (79.3% RH) and Na2CO3 10H2O (87% RH) were prepared [13]. These solutions were transferred into properly labelled desiccators. The prodrugs (100 mg) were placed in open Petri dishes. These Petri dishes were placed in desiccators maintained at different relative humidities. The desiccators were closed with wax and placed at 25°. After every 7 d up to 28 d, the samples of prodrugs (10 mg) were removed from each desiccator. The sample was dissolved in acetonitrile, diluted suitably and filtered through 0.25 μ filter paper. The filtrate was analysed for prodrug content by HPLC method as above. The shelf life of prodrugs in different relative humidities was then calculated. Results are shown in Table 1.
| Prodrug | Shelf life of prodrug at different relative humidity (RH) (day) | Shelf life of prodrug at solid state stability at 25° (day) | |||
|---|---|---|---|---|---|
| 31% | 52% | 79.3% | 87% | ||
| IBU-PA | 175 | 80 | 70 | 48 | 877 |
| IBU-SAL | 93 | 58 | 31 | 26 | 863 |
Table 1: Shelf Life Of Prodrugs At Solid State Stability At Different Temperatures And Different Relative Humidities
Photolytic stability of mutual prodrugs
The photolytic stability of mutual prodrugs was performed by exposing prodrugs at 600 foot-candles (fc) of illumination for a period of 4 w periods [14]. The prodrugs (100 mg) were placed in Petri dishes and exposed to 600 fc of illumination for a period of 4 w. Over this period, the prodrug samples placed in Petri dishes were examined frequently for change in appearance if any. The prodrugs stored under the same conditions but protected from light were used as control for comparison.
Compatibility studies of prodrugs with excipients
The tablet excipients could affect the stability of the drug. Therefore, the knowledge of drug-excipients interaction is very useful to the formulator in selecting appropriate excipients for formulation of new drugs. Carstensen recommended drug/excipients ratios of 20:1 and 1:5 by weight for lubricants and other excipients respectively [15]. In the present work, microcrystalline cellulose, dextrose, anhydrous lactose, dicalcium phosphate, starch 1500, mannitol, magnesium stearate, talc and colloidal silica were selected for compatibility studies. The prodrugs were mixed with excipients in different ratios and placed in vials. These vials were sealed and kept at 55° for a period of 2 w (except for dicalcium phosphate, kept at 45°). During this period, the samples were examined physically for caking, liquefaction, discolouration and odour or gas formation. After 2 w, the samples were then examined for interaction by thin layer chromatography by using benzene: methanol (4:1) solvent system with iodine vapour and UV light as an indicator [16]. Results are shown in Table 2.
| Excipient | Excipient ratio per unit weight of prodrug | Rf- value of IBU-PA | Rf- value of IBU-SAL |
|---|---|---|---|
| Anhydrous lactose | 1:5 | 0.74 | 0.70 |
| Dextrose | 1:5 | 0.74 | 0.71 |
| Mannitol | 1:5 | 0.75 | 0.70 |
| Starch 1500 | 1:5 | 0.73 | 0.71 |
| Micro crystalline cellulose | 1:5 | 0.73 | 0.70 |
| *Di calcium phosphate | 1:5 | 0.74 | 0.69 |
| Magnesium stearate | 20:1 | 0.73 | 0.70 |
| Talc | 20:1 | 0.74 | 0.70 |
| Colloidal silica | 20:1 | 0.73 | 0.71 |
Table 2: TLC Data For Compatibility Studies Of Proudugs With Excipients At 55°
Preparation of tablets of mutual prodrugs by direct compression
The tablet excipients were selected on the basis of preformulation studies and review of literature. The tablets of prodrugs were prepared by direct compression. The seven formulae of each of the prodrugs were designed and are shown in Table 3.
| Ingredient | Quantity of ingredient per tablet (mg) | ||||||
|---|---|---|---|---|---|---|---|
| I | II | III | I V | V | V I | VII | |
| IBU-PA/ IBU-SAL | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| Microcrystalline cellulose (Emcocel) | 148.0 | - | - | 58.0 | - | 74.0 | 74.0 |
| Dextrose (Emdex) | - | - | - | - | 147.5 | - | |
| Anhydrous lactose | - | - | - | 90.0 | - | - | |
| Unmilleddicalcium phosphate (Emcompress) | - | - | 148.0 | - | - | - | 74.0 |
| Starch 1500 | - | 148.0 | - | - | - | 74.0 | |
| Magnesium stearate | 1.5 | 0.5 | 1.5 | 1.5 | 2.0 | 1.0 | 1.0 |
| Colloidal silica | 0.5 | 1.5 | 0.5 | 0.5 | 0.5 | 1.0 | 1.0 |
Table 3: Formulae For Tablets Of Prodrug
The weight of each ingredient was calculated as per weight of the tablet. The formulae for tablets of prodrugs were designed to keep 200 mg as the average weight of tablet. All ingredients were screened through a 40 mesh sieve. The ingredients except magnesium stearate and colloidal silica were mixed thoroughly in a mortar with constant triturating for 20 min. The magnesium stearate and colloidal silica were then mixed with the above powder mixture by triturating for 5 min. This powder mixture was compressed on single-punch power-driven tablet press (KKF3 model) using 5/6 inch standard concave punches. The compressed tablets were transferred into clear amber-coloured bottles and used for further studies.
Quality control tests of formulated tablets of mutual prodrugs
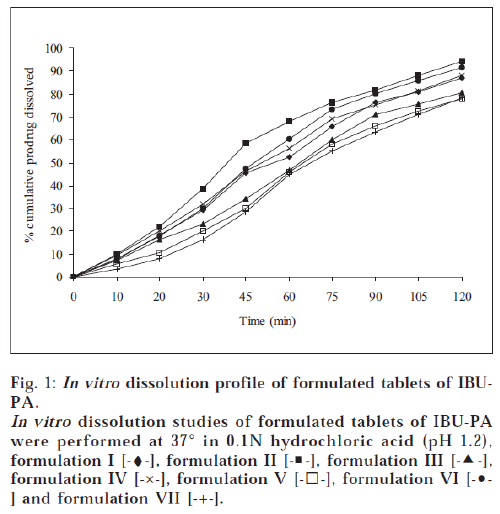
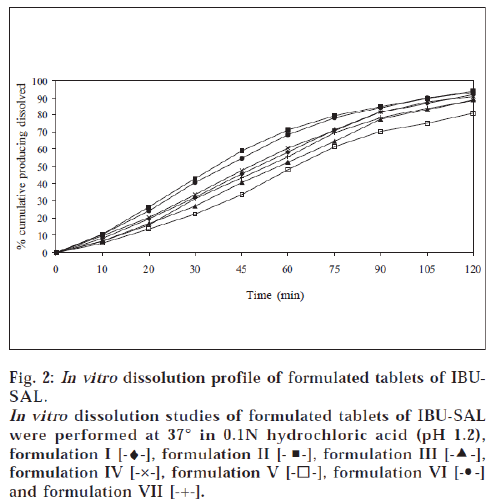
All the tablets prepared by direct compression method were subjected to quality control tests for tablets – like thickness, content of uniformity, weight variation, disintegration, hardness and friability [17-18]. Results are shown in Tables 4 and 5. The dissolution rate of tablets of prodrugs was studied in 0.1N hydrochloric acid (pH 1.2) using USP XXXII dissolution apparatus I [19]. The samples were withdrawn at specific time intervals and the percent of prodrugs (IBU-PA and IBU-SAL) was estimated by HPLC method as above. The percent cumulative prodrug dissolved is illustrated in figs. 1 and 2.
| Quality control test | Formulations | ||||||
|---|---|---|---|---|---|---|---|
| I | II | III | I V | V | V I | VII | |
| *Thickness of tablet (mm) | 3.28 ± 0.20 | 3.25 ± 0.18 | 3.60 ± 0.12 | 3.50 ± 0.15 | 3.62 ± 0.14 | 3.65 ± 0.18 | 3.62 ± 0.15 |
| **Content of uniformity (%) | 98.8 ± 0.8 | 101.1 ± 0.9 | 98.5 ± 0.4 | 99.2 ± 0.7 | 101.4 ± 0.4 | 99.9 ± 0.4 | 97.5 ± 1.2 |
| Average weight of tablet (mg) | 199.19 | 203.03 | 198.89 | 198.14 | 201.44 | 199.98 | 202.24 |
| #Disintegration time (min) | 6.25 ± 0.23 | 4.30 ± 0.24 | 7.15 ± 0.20 | 8.10 ± 0.21 | 9.25 ± 0.23 | 4.76 ± 0.26 | 9.54 ± 0.18 |
| #Hardness (Kg/ cm2) | 4.71 ± 0.32 | 5.53 ± 0.23 | 4.54 ± 0.36 | 5.92 ± 0.45 | 4.95 ± 0.44 | 4.67 ± 0.37 | 5.78 ± 0.45 |
| **Friability (%) | 0.39 ± 0.03 | 0.74 ± 0.05 | 0.89 ± 0.04 | 0.54 ± 0.06 | 0.26 ± 0.07 | 0.29 ± 0.03 | 0.34 ± 0.06 |
Table 4: Quality Control Tests Of Formulated Tablets Of Ibu-Pa
| Quality control test | Formulations | ||||||
|---|---|---|---|---|---|---|---|
| I | II | III | I V | V | V I | VII | |
| *Thickness of Tablet (mm) | 3.68 ± 0.18 | 3.40 ± 0.20 | 3.13 ± 0.52 | 3.50 ± 0.48 | 3.65 ± 0.39 | 3.32 ± 0.21 | 3.15 ± 0.37 |
| **Content of uniformity (%) | 97.9 ± 0.9 | 100.2 ± 0.6 | 98.2 ± 0.9 | 100.4 ± 0.1 | 99.6 ± 0.9 | 99.3 ± 0.4 | 99.4 ± 0.4 |
| Average weight of tablet (mg) | 201.53 | 199.47 | 197.47 | 204.25 | 203.06 | 198.41 | 201.06 |
| #Disintegration time (min) | 7.07 ± 0.31 | 3.39 ± 0.12 | 9.45 ± 0.40 | 8.12 ± 0.17 | 7.18 ± 0.25 | 5.15 ± 0.22 | 7.38 ± 0.20 |
| #Hardness (Kg/ cm2) | 4.83 ± 0.54 | 4.96 ± 0.35 | 4.64 ± 0.38 | 5.35 ± 0.43 | 4.92 ± 0.24 | 5.14 ± 0.21 | 5.31 ± 0.27 |
| **Friability (%) | 0.30 ± 0.06 | 0.27 ± 0.03 | 0.64 ± 0.02 | 0.77 ± 0.03 | 0.85 ± 0.05 | 0.92 ± 0.04 | 0.62 ± 0.06 |
Table 5: Quality Control Tests Of Formulated Tablets Of Ibu-Sal
In vivo evaluation of tablets of mutual prodrugs
The tablet formulation II of IBU-PA and IBU-SAL was selected for in vivo evaluation studies in rats on the basis of dissolution rate. The protocol of all experiments was approved by the Institutional Animal Ethical Committee. Healthy Wistar rats of either sex (each weighing 150 to 175 g) were used during this study. The animals were fasted overnight (water ad libitum) prior to product administration. The selected animals were divided into three groups of six animals each. Two groups of animals were used for tablet formulation II of IBU-PA and IBU-SAL and one group was used for ibuprofen. The doses of prodrugs were calculated on equimolar basis of ibuprofen (11.43 mg/kg), i.e., IBU-PA: 18.80 mg/kg; and IBU-SAL: 18.05 mg/kg. The parent drug (ibuprofen) was administered in tablets which were prepared using same excipients as used in the formulation of prodrugs. The tablets of prodrugs and drugs were broken into small pieces. The animals then received doses equivalent to equimolar weight of powder with water.
Blood samples (0.5 ml) were withdrawn from retro orbital plexus with the help of capillary under ether anaesthesia at intervals of 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12 and 24 h. The blood samples so collected were added to a series of graduated centrifuge tubes containing 1.0 ml of sodium citrate solution (4.0% w/v). The samples were centrifuged at 2500 rpm for 10 min to separate the plasma. The 1.0N hydrochloric acid (2 ml) was added to test tubes containing plasma and these samples were centrifuged at 2500 rpm for 5 min. The supernatant of each sample was then pipetted into a series of separating funnels of 25 ml capacity each and extracted three times with chloroform (5 ml) in case of ibuprofen and IBU-PA tablet formulations and ether in case of IBU-SAL (5 ml) tablet formulation and washed several times with distilled water. Organic phase was evaporated to dryness after separation from aqueous phase. The residue was dissolved in acetonitrile and diluted suitably to estimate the parent drug, i.e., ibuprofen, by HPLC method as above.
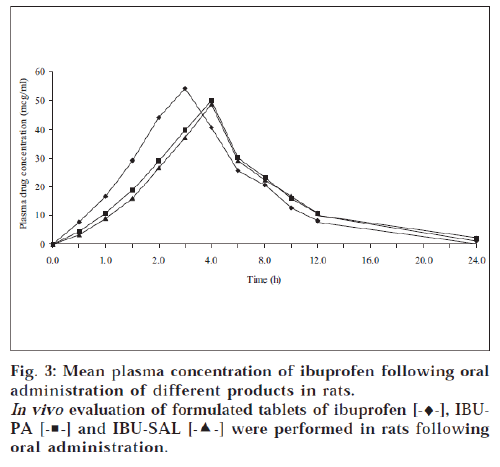
The mean plasma concentration obtained after oral administration of tablets is illustrated in fig. 3. The Cmax and tmax were obtained from the plot of plasma drug concentration versus time and AUC0-24 was calculated by trapezoid method20. Absorption rate constant (Ka) and elimination rate constant (KE) were obtained by the method of residual [21,22]. A plot of log C versus t yields a biexponential curve with a terminal linear phase having slope equal to - KE/ 2.303. A plot of log Cr (residual drug concentration) versus t yields a straight line with slope equal to - Ka/ 2.303. The relative bioavailability of products was then calculated [23,24]. The absorption rate constant (Ka), elimination rate constant (KE), elimination half life, lag time and relative bioavailability are reported in Table 6.
| Pharmacokinetic parameter | Ibuprofen | IBU-PA | IBU-SAL |
|---|---|---|---|
| *Cmax (µg/ ml) | 54.2 ± 3.38 | 49.9 ± 2.64 | 48.8 ± 3.71 |
| tmax (h) | 3.00 | 4.00 | 4.00 |
| AUC0–24 (µg-h/ ml) | 356 | 376 | 368 |
| Absorption rate constant (Ka ) (h-1) |
7.40 | 7.08 | 7.07 |
| Elimination rate constant (KE ) (h-1) |
1.66 | 1.40 | 1.34 |
| Elimination half life (h) | 2.80 | 3.10 | 3.30 |
| Lag time (min) | 13.8 | 22.1 | 23.8 |
| Relative bioavailability (%) | - | 64.17 | 65.49 |
Table 6: Pharmacokinetic Parameters Of Ibuprofen, Ibu-Pa And Ibu-Sal After Oral Administration Of Tablet Formulations In Rats
Results and Discussion
Preformulation studies were performed in order to optimize the solid (tablet) formulations of prodrugs (IBUPA and IBU-SAL). Flow properties of prodrugs were studied by determining angle of repose and Carr’s compressibility index. The angle of repose of IBU-PA and IBU-SAL was found to be 27.35° and 26.40° respectively. Carr’s compressibility index of IBU-PA and IBU-SAL was found to be 14.66% and 12.37% respectively. These results showed their good flow properties. Therefore, these prodrugs are good candidates for tablet formulation, especially for direct composition.
The result of solid state stability studies revealed that the prodrugs were very slowly degraded at elevated temperatures (40°, 50° and 60°) and in 90 d, 1-3% of prodrug was degraded. The prodrugs were found to be physically stable. There was no discolouration, no caking, no liquefaction and no change in odour at elevated temperatures. The shelf life of prodrugs was found to be 877 d for IBU-PA and 863 d for IBU-SAL at 25°. A perusal of Table 1 indicated that these prodrugs have good solid state stability. Hence these are good candidates for solid dosage forms.
Solid state stability studies of prodrugs at different humidity conditions (31% RH, 52% RH, 79.3% RH and 87% RH) were performed. The shelf life of IBU-PA was found to be 175 d at 31% RH, 80 d at 52% RH, 70 d at 79.3% RH and 48 d at 87% RH. The shelf life of IBUSAL was found to be 93 d at 31% RH, 58 d at 52% RH, 31 d at 79.3% RH and 26 d at 87% RH. This study indicates that the prodrugs are unstable under different humidity conditions. This may be attributed to hydrolysis of prodrugs. Therefore it was recommended that prodrugs should be stored in airtight containers or protected from humidity. The prodrugs were exposed to light intensity of 600 fc for 4 w and were found to have no change in their appearance or colour. These observations revealed that prodrugs are quite stable to light; hence these are nonphotolytic.
Compatibility studies of prodrugs with tablet excipients were performed at 55° (except for dicalcium phosphate, at 45°) and showed no change in their appearance, colour and liquefaction and other physical properties. The samples were then examined for interaction by TLC using benzene:methanol (4:1) mobile phase system. This study showed a single spot and no change in Rf value of prodrugs. A perusal of Table 2 indicated that there was no incompatibility or interaction between prodrugs and the excipients that were tried. Therefore, these excipients were used in tablet formulations of prodrugs.
The tablets of each formulation showed uniform thickness. This indicates that the materials behaved uniformly throughout the compression process. The content of prodrug in the tablets of each product was found to be in the range of 97.50-101.37%. This confirms an excellent content uniformity of prodrug in each product, which is also in conformity with the pharmacopoeial limit of any product. A perusal of Tables 4 and 5 indicated that all the formulated products confirmed to the general pharmacopoeial requirement of weight variation of 200 mg tablet, i.e., not more than ±7.5% deviation [18]. This result indicated adequate lubrication and free-flowing nature of materials.
The disintegration time of formulated tablets of prodrugs in distilled water at 37 ± 2° was found to be in the range of 3.39-9.54 min, which is also within the pharmacopoeial limits. The formulation II of all prodrugs showed faster disintegration of tablet as compared to other formulations (disintegration time – 4.30 min for BU-PA and 3.39 min for IBU-SAL). The formulation II of all prodrugs was formulated with starch as a diluent and disintegrant. This study showed that starch is not only an excellent diluent but also a superior disintegrant due to its hydrophilicity and swelling property.
A tablet should have sufficient hardness to withstand handling during packaging and transportation. All the products showed good hardness and it was found to be in the range of 4.54-5.92 kg/cm2. All the products were seen to have friability values less than 1%, i.e., within the reported range [17]. This property shows that tablets will resist chipping, abrasion or breakage under conditions of storage, transportation and handling. In vitro dissolution studies of tablet formulations of prodrugs were performed in 0.1N hydrochloric acid (pH 1.2) for 2 h. This study showed that 77.9-94.5% of IBU-PA and 80.9-94.3% of IBU-SAL was dissolved in 2 h. A perusal of figs. 1 and 2 indicated that all formulations followed pseudo zero-order release kinetics. The t70% of formulation II was found to be 63.2 min for IBU-PA formulation and 58.8 min for IBU-SAL formulation. It was also indicated that formulation II showed faster dissolution as compared to other formulations.
The formulations II contained starch 1500, which showed the fastest dissolution rate among all products. This may be attributed to better and more thorough disintegration due to hydrophilicity and swelling property of starch. The formulations II contained a minimum percentage of magnesium stearate as a lubricant. Magnesium stearate forms a thin hydrophobic film around the tablet excipients, thereby inhibiting the penetration of water into the tablet pores and delaying disintegration and dissolution. But formulation II was designed with minimum percentage of magnesium stearate (0.5%) as compared to other formulations. Therefore, the minimum concentration of lubricant may have less effect or no inhibiting effect on penetration of water into tablet pores and hence faster disintegration and dissolution of product was observed.
The formulated products of prodrugs were evaluated for their bioavailability in order to ascertain in vivo performance. A perusal of Table 6 indicated that the product of prodrugs (i.e., IBU-PA and IBU-SAL) showed delayed tmax (4 h) as compared to ibuprofen (3 h). This may be attributed to the time taken for the hydrolysis of prodrugs in the body because ibuprofen was estimated in blood and it was available only after hydrolysis of prodrugs. The hydrolysis of the prodrug might have taken place mainly after absorption of prodrugs in blood and passage through the liver.
The extent of bioavailability (indicated by AUC0-24) of drugs from tablet formulation was found to be 376.4 μg-h/ ml for IBU-PA and 368.9 μg-h/ ml for IBU-SAL against 356.6 μg-h/ ml for ibuprofen. The better bioavailability of ibuprofen from formulations of prodrugs may be attributed to faster absorption of prodrugs (an ester form of parent drug), even before it was hydrolysed in GIT. It may be attributed to its greater lipophilicity compared to the parent drug [4]. Lag time was delayed in case of prodrug formulations (22.15 min for IBU-PA and 23.07 min for IBU-SAL) as compared to ibuprofen formulation (13.84 min), which may be accounted for by the time taken for hydrolysis of prodrugs. Since the semilogarithmic plot of the residual value against time yielded a straight line of slope - Ka/ 2.303, the absorption of drug, i.e., ibuprofen, followed first-order kinetics. The absorption rate constant (Ka) of drugs from tablet formulations of prodrugs (7.037 h-1 for IBU-PA and 7.066 h-1 for IBU-SAL) was found less than that of parent drug (7.397 h-1 for ibuprofen), once again suggesting that prodrug has taken some time to produce parent drug, i.e., ibuprofen. This may be application of prodrugs for sustaining the action of parent drug.
Similarly, the elimination rate constant (KE) of ibuprofen from tablet formulations of prodrugs (1.439 h-1 for IBU-PA and 1.343 h-1 for IBU-SAL) was found to be less than parent drug (1.658 h-1 for ibuprofen). The elimination half lives of drugs from prodrug formulations (3.1 h for IBU-PA and 3.3 h for IBU-SAL) were found to be more than the parent drug formulations (2.8 h), which is indicating slower elimination of drug from prodrug formulations as compared to parent drug. The extended time for elimination may also be accounted for by the time taken for the hydrolysis of prodrugs in the body. The relative bioavailability of tablet formulation of prodrugs was found to be 64.17% for IBUPA and 65.49% for IBU-SAL. The in vivo study of tablet formulations of prodrugs confirmed that they possessed the ability of parent drug, i.e., ibuprofen.
Acknowledgements
The authors wish to thank the Head of the Department of Pharmaceutical Sciences, Dr. H. S. Gour Vishwavidyalaya, Sagar, for the facilities provided.
References
- Bundgaard, H., In; Bundgaard, H., Eds., Design of Prodrugs, Elsevier, Amsterdam, 1985, 1.
- Singh, G. and Sharma, P., Indian J. Pharm. Sci., 1994, 56, 69.
- Bahekar, H.R. and Gaikwad, H.J., Indian Drugs, 1998, 35, 648.
- Bhosale, A.V., Agrawal, G.P. and Mishra, P., Indian J. Pharm.Sci., 2004, 66, 158
- Rudnic, E. and Schwartz, J.B., In; Gennaro, A.R., Eds., Remington’s Pharmaceutical Sciences, 18th Edn., Mack Publishing Company, Easton, Pennsylvania, 1990, 1633.
- Marshall, K. and Rudnic, E.M., In; Banker, G.S. and Rhodes, C.T.,Eds., Modern Pharmaceutics, 2nd Edn., Marcel Dekker, Inc., NewYork, 1990, 355.
- Peck, G.E., Baley, G.J., McCurdy, V.E. and Banker, G.S., In;Lieberaman, H.A., Lachman, L. and Schwartz, J.B., Eds., Pharmaceutical Dosage Forms: Tablets Vol. I, 2nd Edn.,Marcel Dekker Inc., New York, 1989, 78.
- Pilpel, N., Chem. Process Eng., 1965, 46, 467.
- Carr, R.L., Chem. Eng., 1965, 72, 163.
- Radebaugh, G.W., In; Gennaro, A.R., Eds., Remington’s Pharmaceutical Sciences, 18th Edn., Mack Publishing Co., Easton,PA, 1990, 147.
- Carstensen, J.T., J. Pharm. Sci., 1974, 63, 1.
- Walter, L., Eds., In; The Pharmaceutical Codex, 12th Edn.,ThePharmaceutical Press, London, 1994, 286.
- Moyers, C.G. and Baldwin, G.W., In; Green, D.W. and Maloney, J.O.,Eds., Perry’s Chemical Engineer’s Handbook, 7th Edn., McGraw Hill, New York, 1997, 12.
- Wells, J.I., In; Pharmaceutical Preformulation: The Physicochemical Properties of Drug Substances, 1st Edn., Ellis Horwood Ltd.,Chichestar, England, 1988, 173.
- Carstensen, J.T., In; Banker, G.S. and Rhodes, C.T., Eds., Modern Pharmaceutics, 2nd Edn., Marcel Dekker, Inc., New York, 1990, 239.
- Zweig, G. and Sharma, J., In; Handbook of Chromatography, Vol. 1,CRC Press, Ohio, 1976, 462.
- Banker, G.S. and Anderson, N.R., In; Lachman, L., Lieberman, H.A.,and Kanig, J.L., Eds., The Theory and Practice of Industrial Pharmacy,3rd Edn., Varghese Publishing House, Mumbai, 1990, 296.
- The Indian Pharmacopoeia, 1996, Vol. II, Govt. of India, Ministry ofHealth and Family Welfare, The Controller of Publications, New Delhi, 735
- The United States Pharmacopeia, 23rd Edn., The National Formulary,18th Edn., United State Pharmacopeial Convention, INC., Rockville, M.D., 1995, 1791.
- Rowland, M. and Tozer, T.N., In; Clinical Pharmacokinetics: Conceptsand Applications, 3rd Edn., B.I. Waverly Pvt. Ltd., New Delhi,1996, 120.
- Gibaldi, M. and Perri, D., In; Pharmacokinetics, 2nd Edn.,Marcel Dekker, Inc., New York, 1982, 445.
- Brahmankar, D.M. and Jaiswal, S.B., In; BiopharmaceuticsandPharmacokinetics A Teratise, 1st Edn.,Vallabh Prakashan, New Delhi, 1999, 225.
- Abdou, H.M., In; Dissolution, Bioavailability and Bioequivalence, Mack Publishing Co., Easton, Pennsylvania, 1989, 404.
- Shargel, L. and Andrew, Y., In; Applied Biopharmaceutics and Pharmacokinetics, 4th Edn., Prentice-Hall International (UK) Inc.,London, 1999, 234.