- *Corresponding Author:
- L. Kumar
Departments of Pharmaceutics and Pharmaceutical Chemistry, Manipal College of Pharmaceutical Sciences, Manipal University, Manipal-576 104, India
E‑mail: lalitkumar_pharma@yahoo.co.in
| Date of Submission | 19 March 2013 |
| Date of Revision | 15 July 2013 |
| Date of Acceptance | 21 July 2013 |
| Indian J Pharm Sci 2013;75(5):585-590 |
Abstract
Objective of the present study was to develop and evaluate vaginal films with essential in vitro studies. Films were developed using hydroxypropyl methylcellulose as a polymer and formulations were coded. The developed films were evaluated with Fourier transform infrared spectroscopy, drug content, viscosity, surface pH, thickness, mechanical characterisation and in vitro drug release study. Fourier transform infrared spectroscopy results confirmed that there is no chemical interaction between drug and stabilisers/excipients. The batch variation was not more than 5% for average thickness and weight of the films. The drug content for the prepared formulation was in the range of 72.32±0.18% to 94.48±0.54%. Viscosity of the formulations increased with the increase in concentration of polymer. Mechanical characterisation revealed that tensile strength and percentage elongation of the films improved as there is increase in degree of substitution of the polymer, but the values of modulus decreased which confirmed that all the prepared films are soft in nature. The in vitro study indicated that 1 and 2% concentrations of polymer are the least concentrations to control the release of drug whereas the 4% concentration of polymer is a good and more effective concentration to control the release. Only one prepared formulation released the drug by following anomalous transport whereas other film formulations released the fluconazole by following Fickian diffusion mechanism. Prepared vaginal films may be an important alternative for the treatment of vaginal candidiasis, because these prepared films suggest the benefits of controlled release of fluconazole at the site of absorption.
Keywords
Fluconazole, vaginal films, vaginal candidiasis, antimicrobial agent
Vaginal fungal infections are common in almost all women of all age groups due to certain factors. These may be related to menstrual cycles, sexual intercourses, birth control methods, vaginal infections, aging, medicines, or hormonal changes after (or) at the time of pregnancy [1]. Vaginal infection is the major problem for all above given problems. It is caused by the presence or excessive growth of yeast cells, bacteria or viruses. Sometimes the infection occurs due to the imbalance or changes in vaginal conditions like the vaginal pH. In normal adult women the vaginal pH is 3.5 to 5.0 and is naturally maintained by the production of lactic acid by the vaginal microflora, but in the presence of semen in vagina it changes to slight alkaline i. e., pH 7.0 to 8.0. This alkaline or slight alkaline condition is more favorable condition for vaginal infection [2,3].
Vaginal candidiasis is a major kind of vaginal infection which occurs mainly due to over growth of yeast or fungus in vagina. As per the literature [4‑6], most women have a vaginal yeast infection at least once during their lifetime. Actually, candida and some other types of germs lives in vagina which keep each other in balance. But, sometimes due to some changes like wetting or discharge in vagina or due to the use of antibiotic or other factors which affects the normal balance of bacteria in vagina, the number of Candida albicans increases which leads to yeast infection.
In recent years [4,5], the medications most commonly used for the treatment of vaginal yeast infections are imidazole derivatives. Fluconazole is a drug which also comes under imidazole antifungal category. Fluconazole is available in various dosages forms in market like powder for oral suspension, oral tablets and others. But oral formulations cause more gastrointestinal side effects (like nausea, vomiting, diarrhea and abdominal discomfort) in comparison of vaginal products [7]. On the other hand, conventional vaginal dosage forms of antifungal drugs has limitations, which are poor retention, leakage and messiness causing inconvenience to users, leading to poor patient compliance, loss of therapeutic efficacy [8‑10]. However, a clear need is required to increase the patient compliance and therapeutic activity of the drug. In the present study, vaginal films were prepared to increase the therapeutic activity of drug and to overcome the problems due to conventional dosage forms.
Materials and Methods
Fluconazole was obtained as gift sample from Ipca Laboratories Ltd, Mumbai, India. Hydroxypropyl methylcellulose CP 50 was purchased from Loba Chemie Pvt. Ltd., Mumbai, India; Polyethylene glycol (PEG 400) and glacial acetic acid were purchased from Merck Ltd., Mumbai, India. Sodium acetate, dichloromethane and methanol were purchased from SD Fine Chem Ltd., Mumbai, India.
Drug‑polymer compatibility studies
Before preparation of vaginal films, compatibility of drug with polymer present in film formulation was studied using FTIR spectrophotometer (Shimadzu‑8400 S, Japan) by KBr pellet method in the range of 4000-400 cm‑1. The spectra of pure drug was compared with the spectra of drug and polymer mixture [8,11].
Preparation of films
Hydroxypropyl methylcellulose films were prepared using solvent evaporation technique. Solutions containing different ratios of polymers were prepared in dichloromethane:methanol (8:2) and coded as FLZF1, FLZF2, FLZF3 and FLZF4. In one beaker calculated amount of drug was dissolved in methanol and in another beaker HPMC polymer was dissolved in dichloromethane portion. Then first beaker solution was added into the second beaker solution followed by stirring. In required quantity PEG 400 as a plasticiser and permeation enhancer was added in to the mixed solution with continuous stirring at 800 rpm for 2 h. Then 15 gm of polymer solution was poured on a glass film mould of defined area after putting on a horizontal flat surface followed by drying at a room temperature for 48 h. Dried films were removed from the glass moulds and kept at a butter paper. All the films were cut into pieces of defined size (2×2 cm2). The films were wrapped in big size butter paper followed by wrapping with laminated aluminium foil. The wrapped films were stored in a desiccator for further studies. Compositions of different formulations are given in Table 1 [8,11‑14].
| Ingredients | Formulation code | |||
|---|---|---|---|---|
| FLZF1 | FLZF2 | FLZF3 | FLZF4 | |
| Drug (mg) | 275 | 275 | 275 | 275 |
| HPMC (%) | 1.0 | 2.0 | 3.0 | 4.0 |
| Polyethylene glycol 400 (%) | 0.5 | 0.5 | 0.5 | 0.5 |
| Dichloromethane:methanolq.s.(g) | 15 | 15 | 15 | 15 |
FLZF=Fluconazole film formulation, HPMC=Hydroxypropyl methylcellulose
Table 1: Formulation composition of vaginal
Fourier transform infrared spectroscopy of vaginal films
The stability of the drug (drug interaction with other excipients and solvents) in the prepared films was analysed using FTIR spectra of prepared vaginal films. The FTIR spectra of prepared films were studied as mentioned earlier. The spectra of pure drug is compared with films spectra [8,11].
Physical characterisation of films
All prepared vaginal film formulations were characterised for various aesthetic (visual) and physical parameters such as colour, transparency, nature, thickness and weight [11,13]. The thickness of all prepared film formulations were measured using a screw gauge.
Determination of drug content
The films of defined size (2×2 cm2) were taken into 10 ml volumetric flask and dissolved in acetate buffer pH 4.6. All the samples were diluted appropriately and the absorbance was examined at 261 nm using spectrophotometer (Shimadzu UV‑2450, Japan) [8,13].
Determination of surface pH
The surface pH of the prepared vaginal films was determined to evaluate the possible irritation effects of the mucosa. The films were left to swell in 5 ml of acetate buffer pH 4.6 in petri‑dish for 2‑3 min. Films were removed from the Petri dish and surface pH was analysed with the help of pH paper [8].
Measurement of mechanical characteristics of films
Mechanical characteristics of the prepared films were measured as tensile strength, percentage elongation and modulus. Mechanical properties of films were measured using an Instron universal testing machine, UK (Model 3366, Instron). It consisted of two loaded grips. The upper one was movable and the lower one was fixed. The test film specimen of definite size (2×2 cm2) was attached between these load grips and force was gradually applied till the film broke. The stress‑strain curves were recorded for each sample and the tensile strength at break, elongation and modulus were taken directly from the software of Instron universal testing machine [8,15,16].
The Young’s modulus of elasticity was determined for the prepared films. As per Wu and McGinity [15], Young’s modulus provides the information about the hardness, flexibility and stiffness of a polymer. Stiffness defines the capacity of the films to resist deformation in the elastic range. Higher value of Young’s modulus resembles the greater stiffness of the films whereas the lower value of modulus resembles the softness of films.
Viscosity measurement
A Brookfield DV III Ultra Programmable Rheometer (LV) was used to measure the viscosity (in cps) of the formulated gels/solutions of different polymeric films [17].
In vitro drug release studies
In vitro drug diffusion studies were carried out by using modified Keshery‑Chien (KC) diffusion cell with cellophane membrane. Cellophane membrane was soaked in acetate buffer pH 4.6 for 24 h. Cellophane membrane was fixed to one end of the cylindrical donor compartment such that lower end just touched the surface of medium of receptor compartment. For the present study acetate buffer pH 4.6 was taken as medium in the receptor compartment. Specific area of film was cut (2×2 cm2) and placed on the surface of the processed cellophane membrane in the donor compartment. Acetate buffer (0.5 ml) was also placed in the donor compartment and then its top end was covered with a parafilm to avoid the evaporation of solvent. The receptor medium was allowed to stir continuously by means of magnetic stirrer at 75±5 rpm and the temperature was maintained at 37±2°. Five millilitre of receptor samples were withdrawn through the sampling port of the receptor compartment at predetermined time intervals to study the release of drug. Sample quantity (5.0 ml) of fresh acetate buffer pH 4.6 was refilled in to the receptor compartment to maintain the sink condition as well as its attachment with the donor compartment. Then the drug was estimated in the collected receptor samples by using UV/Vis spectrophotometer at 261 nm (λmax) [8,18,19].
In vitro drug release kinetics
In order to investigate the kinetics and mechanism of drug release from prepared films of different drug and polymers ratios, the release data were examined using Zero‑order kinetic, First order kinetic, Higuchi kinetic and Korsmeyer‑Peppas model [18].
For the zero‑order kinetic, data obtained from in vitro drug release studies were plotted as cumulative amount of drug released versus time whereas for the first‑order kinetic, the obtained data were plotted as log cumulative percentage of drug remaining versus time. For Higuchi kinetic, the obtained data of in vitro drug release were plotted as cumulative percentage drug release versus square root of time [20].
Furthermore, to find the mechanism of drug release, the first 60% of cumulative drug release data were fitted in Korsmeyer‑Peppas model, because Korsmeyer‑Peppas model is valid up to initial 60% cumulative drug release. The obtained in vitro drug release data were plotted as log cumulative percentage drug release versus log time to find the drug release mechanism using Korsmeyer‑Peppas model [18,20].
Results and Discussion
In the present study, fluconazole‑HPMC films were prepared in various compositions followed by characterisation. HPMC was chosen as film forming polymer in this study because as per literature review it was reported that it is nontoxic, nonirritant, stable at vaginal pH, imparts the viscosity of the solution, has good wetting properties and maintains prolonged contact with mucous membrane [8,11,21‑24].
Different vaginal films were prepared with solvent evaporation method and coded as FLZF1 to FLZF4 (Table 1). FTIR spectroscopy was carried out for drug‑polymer interaction and to confirm the stability of drug in the prepared films with other solvents and excipients. Spectra of drug‑polymer mixture and prepared films were compared with pure drug and the data is given in the Table 2.
| Vibration mode | Frequency (cm-1) | |||||
|---|---|---|---|---|---|---|
| Drug | Drug+ Polymer | FLZF1 | FLZF2 | FLZF3 | FLZF4 | |
| C‑H Str | 2966.52 | 2956.87 | 2956.87 | 2966.52 | 2966.52 | 2966.52 |
| O‑H Str | 3176.76 | 3192.19 | 3176.76 | 3242.34 | 3178.69 | 3176.76 |
| O‑H def | 1390.68 | 1383.43 | 1384.89 | 1383.43 | 1384.89 | 1384.89 |
| C‑F Str | 1139.93 | 1139.93 | 1139.93 | 1139.93 | 1139.93 | 1139.93 |
| C=C and C=N Str | 1560.41 | 1562.34 | 1562.34 | 1560.41 | 1562.34 | 1562.34 |
| C=C | 1620.21 | 1620.21 | 1614.42 | 1624.06 | 1616.35 | 1624.06 |
FLZF=Fluconazole film formulation
Table 2: Fourier transform infrared spectroscopy
Vaginal films were prepared in different drug: polymer ratios and their physical properties were examined (Table 3). All the formulations were found to be homogeneous, colorless, transparent and flexible except film FLZF1. This is indicating that the quantity of polymer in FLZF1 is not sufficient to produce a good film. All the films were found to be easily peeled off from the moulds. This study suggests that the 0.5% w/v concentration of plasticiser PEG 400 for vaginal films preparation is sufficient. The batch variation was not found more than 5% for average thickness and weight of the films. This is indicating the consistency in the preparation of vaginal films.
| Formulation code | Physical characteristics of film | ||||
|---|---|---|---|---|---|
| Color | Transparency | Nature | Thickness* (mm) |
Weight* (mg) |
|
| FLZF1 | White | Cloudy | Flexible | 0.08±0.00 | 49.62±3.10 |
| FLZF2 | Colorless | Transparent | Flexible | 0.08±0.01 | 50.06±1.57 |
| FLZF3 | Colorless | Transparent | Flexible | 0.10±0.01 | 53.72±2.27 |
| FLZF4 | Colorless | Transparent | Flexible | 0.07±0.00 | 46.75±4.61 |
FLZF=Fluconazole film formulation, *=Reflecting data are Mean±Standard error of mean (n=3)
Table 3: Physical characteristics of vaginal films
The drug content study shows that the low polymer concentration (1%) and high polymer concentration (4%) affects the uniform drug distribution into the prepared films. The improper distribution of drug in films with low polymer concentration may be affected due to low viscosity and high solubility of drug which may accumulate the drug at one place. Similarly, the distribution of drug in the films with high polymer concentration may be affected due to the high viscosity which may affect for the proper distribution of drug in films. Hence, the 2% and 3% concentration of polymer is good, as it does not affect the distribution of drug. The pH of all the films was found to be almost weakly acidic. Drug content and surface pH of all the formulations is given in the Table 4.
| Formulation code |
Drug content* (%) |
pH | Viscosity of formulation dispersions (cps) |
|---|---|---|---|
| FLZF1 | 94.48±0.54 | 5.8 | 1.92 |
| FLZF2 | 82.35±0.19 | 5.9 | 2.02 |
| FLZF3 | 83.10±0.14 | 5.9 | 2.21 |
| FLZF4 | 72.32±0.18 | 6.1 | 2.99 |
FLZF=Fluconazole film formulation, cps=Centipoise, *=Reflecting data are Mean±Standard error of mean (n=3)
Table 4: Drug content and ph and viscosity (of formulation dispersions) of vaginal films
The mechanical characteristics of the prepared films were determined as the tensile strength, percent elongation and modulus. Increasing the amount of polymers in the prepared films leads to an increase of the maximum force due to degree of HPMC polymer substitution. All drug‑polymer formulations showed a significant flexibility of the prepared polymeric films [15,16].
As seen in the Table 5, the tensile strength and percent elongation increased as the concentration of the polymer is increased in the films due to increase in the molecular weight. The Young’s modulus decreased as the concentration of the polymer is increased in the films (Table 5). Hence, the study shows that the increase in molecular weights contribute to an improvement in tensile strength and percentage elongation, but the value of modulus decreases which confirms that all the prepared films are soft in nature.
| Formulation code |
Tensile extension at break*(mm) |
Elongation* (%) |
Modulus* (MPa) |
|---|---|---|---|
| FLZF1 | 0.77±0.11 | 30.11±14.99 | 3.15±1.73 |
| FLZF2 | 1.46±1.04 | 35.27±7.29 | 1.94±0.52 |
| FLZF3 | 1.69±0.70 | 38.01±10.65 | 1.64±0.18 |
| FLZF4 | 1.87±0.15 | 44.19±4.44 | 1.58±0.05 |
FLZF=Fluconazole film formulation, MPa=Megapascal, mm=Millimetre, *=Reflecting data are Mean±Standard error of mean (n=3)
Table 5: Mechanical properties of vaginal films
Viscosity data of prepared films (Table 4) exhibits that as the concentration of polymer is increases the viscosity of prepared films increases. This may be due to increase in degree of substitution of HPMC in prepared formulations. The study is indicating that the increase in polymer concentration based viscosity may be helpful to control the release of fluconazole from film formulations.
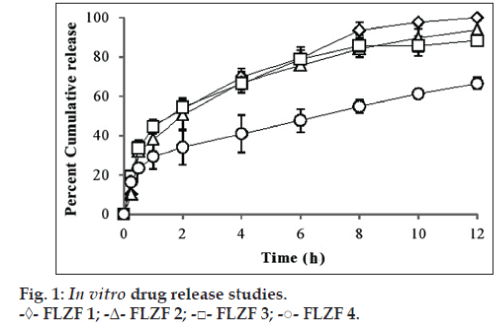
Fig. 1 shows that in vitro drug release behaviour of drug from the prepared films in acetate buffer pH 4.6 to mimic the physiological conditions of vagina in the study. In vitro drug release study shows that as the concentration of HPMC is increasing, the drug release from the formulations is decreasing. The films containing 1, 2, 3 and 4% of polymer released 99.94, 93.87, 88.31 and 66.50%, respectively, over a period of 24 h. The fastest release of drug was observed from FLZF1 which is having 1% concentration of HPMC while slower release was found from FLZF4 which is having 4% polymer concentration. This slow release of the drug from FLZF4 may be due to high molecular weight or due to high viscosity [15,23]. The study is indicating that the 1% and 2% concentrations of HPMC are the least concentrations to control the release of drug from the films whereas the 4% concentration of HPMC is a good and more effective concentration to control the release.
The correlation coefficient values were calculated from the in vitro drug release data for zero‑order, first‑order, Higuchi and Korsmeyer‑Peppas models to find out the best fit model. Almost all the formulations releases the fluconazole from the films by following Higuchi and Korsmeyer‑Peppas model except FLZF1 and FLZF2 and those formulations even also follows first‑order with Higuchi model. The present study indicates that the 1 and 2% concentrations of polymer is able to control the release of drug from the formulations but that is less effective to control the release in comparison of 3% and 4% concentrations of polymer.
The drug release mechanism was confirmed by using Korsmeyer‑Peppas model. As a result, the drug release mechanism of the films changes from anomalous (non‑Fickian) transport (superposition of both phenomena) to Fickian diffusion mechanism (indicating diffusion‑controlled drug release) with a decrease in n‑value. FLZF1 releases the drug by following anomalous transport whereas other film formulations release the fluconazole by following Fickian diffusion mechanism indicating diffusion-controlled drug release [18,23,25‑30]. As per Fickian diffusion mechanism, the release of fluconazole depends upon the square root of time [30]. Table 6 shows the data for correlation coefficient values and drug‑release mechanisms.
| Formulation code |
Correlation coefficient values (r2)* | Mechanism* n‑value |
|||
|---|---|---|---|---|---|
| Zero– order |
First– order |
Higuchi | Korsmeyer– peppas |
||
| FLZF1 | 0.83±0.04 | 0.92±0.01 | 0.94±0.02 | 0.89±0.05 | 0.53±0.08 |
| FLZF2 | 0.80±0.04 | 0.97±0.01 | 0.92±0.02 | 0.72±0.03 | 0.45±0.02 |
| FLZF3 | 0.83±0.03 | 0.90±0.05 | 0.95±0.02 | 0.93±0.03 | 0.37±0.01 |
| FLZF4 | 0.84±0.09 | 0.91±0.06 | 0.96±0.04 | 0.94±0.04 | 0.34±0.03 |
FLZF=Fluconazole film formulation, *=Reflecting data are Mean±Standard error of mean (n=3), n-value=Reflecting release exponent characterising the diffusion mechanism
Table 6: In vitro drug release kinetics
The vaginal films were successfully prepared with solvent evaporation method for controlled release of drug. The developed films were evaluated by FTIR and for parameters like drug content, viscosity, surface pH, thickness, mechanical characterisation, in vitro drug release study and other. FTIR results confirm that there is no chemical interaction between drug and stabilisers/excipients. The batch variation is not more than 5% for average thickness and weight of the films. The surface pH studies of the formulations conclude that all the films are almost weak acidic in nature. FLZF1 showed high drug content (94.48±0.54) whereas FLZF4 showed low drug content (72.32±0.18). Viscosity of the formulations increased with the increase in concentration of polymer. Mechanical characterisation shows that tensile strength and percentage elongation of the films improves as degree of substitution of the polymer increases, but the values of modulus decreases which confirms that all the prepared films are soft in nature. The in vitro study is indicating that the 1% and 2% concentrations of HPMC are the least concentrations to control the release of drug whereas the 4% concentration of HPMC is a good and more effective concentration to control the release. FLZF1 releases the drug by following anomalous transport whereas other film formulations release the fluconazole by following Fickian diffusion mechanism. Prepared vaginal films may be an important alternative for the treatment of vaginal candidiasis, because these prepared films suggest the benefits of controlled release of fluconazole at the site of absorption.
Acknowledgements
Authors thank to Ipca Laboratories Ltd, Mumbai, India for the gift sample of drug. The authors thank the Manipal Dental Material Department, Manipal University, Manipal, India for help in performing the analysis of mechanical characteristics. Authors are also thankful to Manipal College of Pharmaceutical Sciences, Manipal University, Manipal, India for providing research facility to do this work in the campus.
References
- Available from: http://www.femalenetwork.com/girltalk/index. php?topic=242138.0. [Last accessed on 2012 Jul 15].
- Barnhart K, Shalaby W. In: Rencher WF, editor. Vaginal microbicide formulations workshop. Philadelphia: Lippincott‑Raven Publishers; 1998. p. 1‑15. Available from: http://pdf.usaid.gov/pdfdocs/PNACK016. pdf. [Last accessed on 2012 Dec 20].
- Alexander NJ, Baker E, Kaptein M, Karck U, Miller L, Zampaglione E. Why consider vaginal drug administration? FertilSteril 2004;82:1‑12.
- Available from: http://www.nlm.nih.gov/medlineplus/ency/article/001511. htm. [Last accessed 2012 Jul 16].
- Bachhav YG, Patravale VB. Microemulsion based vaginal gel of fuconazole: Formulation, in vitro and in vivo evaluation. Int J Pharm 2009;365:175‑9.
- Albertini B, Passerini N, Sabatino MD, Vitali B, Brigidi P, Rodriguez L. Polymer–lipid based mucoadhesive microspheres prepared by spray‑congealing for the vaginal delivery of econazole nitrate. Eur J Pharm Sci 2009;36:591‑601.
- Available from: http://dailymed.nlm.nih.gov/dailymed/archives/ fdaDrugInfo.cfm?archiveid=18356. [Last accessed 2012 Dec 20].
- Sudeendra BR, Umme H, Gupta RK, Shivakumar HG. Development and characterization of bioadhesive vaginal films of clotrimazole for vaginal candidiasis.Acta Pharm Sci 2010;52:417‑26.
- Vermani K, Garg S. The scope and potential of vaginal drug delivery. Pharm SciTechnol Today 2000;3:359‑64.
- Robinson JR, Bologna WJ. Vaginal and reproductive system treatments using a bioadhesive polymer. J Control Release 1994;28:87‑94.
- Garg S, Vermani K, Garg A, Anderson RA, Rencher WB, Zaneveld LJ. Development and characterization of bioadhesive vaginal films of sodium polystyrene sulfonate (PSS), a novel contraceptive antimicrobial agent. Pharm Res 2005;22:584‑95.
- Krishna Murthy TE, Kishore VS. Effect of casting solvent on permeability of antihypertensive drugs through ethyl cellulose films. J SciInd Res 2008;67:147‑50.
- Yoo JW, Dharmala K, Lee CH. The physicodynamic properties of mucoadhesive polymeric films developed as female controlled drug delivery system. Int J Pharm 2006;309:139‑45.
- Dobaria NB, Badhan AC, Mashru RC. A novel itraconazolebioadhesive film for vaginal delivery: Design, optimization and physicodynamic characterization. AAPS PharmSciTech 2009;10:951‑9.
- Wu C, McGinity JW. Non‑traditional plasticization of polymeric films.Int J Pharm 1999;177:15‑27.
- IPC‑TM‑650 Test methods manual. Available from: http://www.ipc. org/4.0_Knowledge/4.1_Standards/test/2.4.18.3.pdf. [Last accessed 2012 Dec 11].
- Sandri G, Rossi S, Ferrari F, Bonferoni MC, Muzzarelli C, Caramella C. Assessment of chitosan derivatives as buccal and vaginal penetration enhancers. Eur J Pharm Sci 2004;21:351‑9.
- Chatterjee A, Kumar L, Bhowmik BB, Gupta A. Micoparticulated anti‑HIV vaginal gel: In vitro‑in vivo drug release and vaginal irritation study. Pharm DevTechnol 2011;16:466‑73.
- Hajare A. In: Physical Pharmacy. Kolkata: New Central Book Agency (P) Ltd; 2012. p. 364‑406.
- Dash S, Murthy PN, Nath LK, Chowdhury P. Kinetic modelling on drug release from controlled drug delivery systems. Acta Pol Pharm 2010;67:217‑23.
- Toda I, Shinozaki N, Tsubota K. Hydroxypropyl methylcellulose for the treatment of severe dry eye associated with Sjogren’s syndrome. Cornea 1996;15:120‑8.
- Ludwig A. The use of mucoadhesive polymers in ocular drug delivery.Adv Drug Deliv Rev 2005;57:1595‑639.
- El‑Sousi S, Nacher A, Mura C, Catalan‑Latorre A, MerinoV, Merino‑Sanjuan, et al. Hydroxypropyl methylcellulose films for the ophthalmic delivery of diclofenac sodium. J Pharm Phamacol 2013;65:193‑200.
- Yamsani VV, Gannu R, Kolli C, Rao ME, Yamsani MR. Development and in vitro evaluation of buccoadhesivecarvedilol tablets. Acta Pharm 2007;57:185‑97.
- Chandak AR, Verma PR. Design and development of hydroxypropyl methylcellulose (HPMC) based polymeric films of Methotrexate: Physicochemical and pharmacokinetic evaluations. YakugakuZasshi 2008;128:1057‑66.
- Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC).Adv Drug Deliv Rev 2001;48:139‑57.
- Kulkarni RV, Mutalik S, Mangond BS, Nayak UY. Novel interpenetrated polymer network microbeads of natural polysaccharides for modified release of water soluble drug: In vitro and in vivo evaluation. J Pharm Phamacol 2012;64:530‑40.
- Ritger PL, Peppas NA. A simple equation for description of solute release I. Fickian and non‑Fickian release from non‑swellable devices in the form of slabs, spheres, cylinders or discs. J Control Release 1987;5:23‑36.
- Ritger PL, Peppas NA. A simple equation for description of solute release II. Fickian and anomalus release from swellable devices. J Control Release 1987;5:37‑42.
- Xu G, Sunada H. Influence of formulation change on drug release kinetics from hydroxypropyl methylcellulose matrix tablets. Chem Pharm Bull 1995;43:483‑7.