- *Corresponding Author:
- Yan Wang
College of Pharmacy, Dali University, Dali, Yunnan Province 671000, China
E-mail: jessica9428@sina.com
| Date of Received | 16 August 2023 |
| Date of Revision | 19 March 2024 |
| Date of Acceptance | 05 August 2024 |
| Indian J Pharm Sci 2024;86(4):1347-1354 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
We adopted a network pharmacology approach, including the collection of active ingredients through literature retrieval, drug target prediction, gene ontology enrichment and Kyoto encyclopedia of genes and genomes enrichment, to systematically explore the underlying mechanisms of Periplaneta americana (Linnaeus) extracts in the treatment of immunocompromised diseases. A total of 53 chemical components of Periplaneta americana (Linnaeus) extracts were identified through literature retrieval, and 1267 possible therapeutic targets that may improve immune function were predicted by the database. Gene ontology analysis results illustrated that the targets of Periplaneta americana (Linnaeus) extracts, which enhance immunomodulatory function, were primarily concentrated in biological processes such as cellular response to chemical stress, leukocyte cell-cell adhesion, and leukocyte migration. Kyoto encyclopedia of genes and genomes analysis demonstrated that the target genes were mainly involved in programmed cell death-ligand 1 expression and the programmed cell death-1 checkpoint pathway in cancer, T helper 17 cell differentiation, and the nuclear factor kappa B signaling pathway. According to protein-protein interaction network analysis, Periplaneta americana (Linnaeus) extracts attenuated immunodeficiency effects which may be related to the key therapeutic proteins such as epidermal growth factor receptor, heat shock protein 90 alpha family class A member 1, RELA, signal transducer and activator of transcription 3, estrogen receptor alpha, protein kinase B alpha, inhibitor of kappa light polypeptide gene enhancer in b-cells kinase gamma, Erb-B2 receptor tyrosine kinase 2, TANK-binding kinase 1, and mechanistic target of rapamycin. This study revealed that the therapeutic effects of Periplaneta americana (Linnaeus) extracts on immunocompromised diseases were regulated by interactions among multiple components, targets, and pathways.
Keywords
Periplaneta americana (Linnaeus), network pharmacology, immunocompromised diseases, protein-protein interaction
Modern pharmacological studies have demonstrated that immunosuppression is the root cause of many diseases, such as type 2 diabetes, nonalcoholic fatty liver disease, upper respiratory tract infection, urinary tract infection and irritable bowel syndrome. Around the world, due to various causes of congenital deficiency or acquired malnutrition, the immune function of people against various chronic diseases is constantly weakened. Therefore, maintaining the immune system in a normal state is important for preventing the occurrence of various diseases. This version provides a smoother transition and maintains clarity.
Traditional Chinese Medicines (TCMs) have been used in China for the treatment of various diseases for millennia. In recent years, medicinal insects have been shown to modulate immune responses by acting directly on immune cells. However, the mechanisms of immune-enhancing activity of Periplaneta americana (Linnaeus) (Periplaneta americana L.) remain unclear due to the complexity of its ingredients. Hence, we systematically explored the underlying mechanisms of Periplaneta americana L. extracts in the treatment of immunocompromised diseases based on network pharmacology. This study provided an important scientific and theoretical basis for the promotion, development and application of Periplaneta americana L.
Materials and Methods
Chemical components collection:
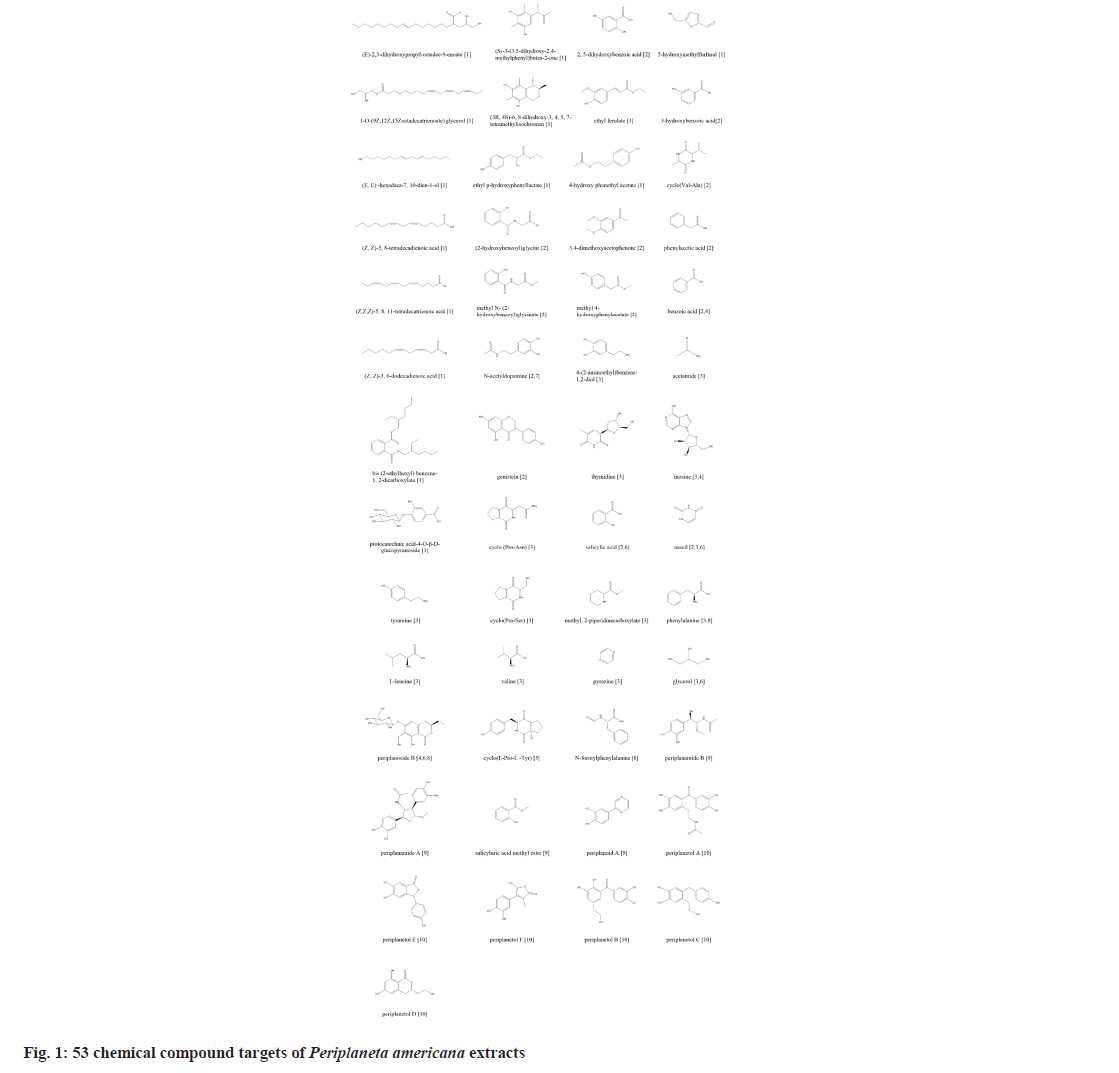
There are no relevant chemical constituents of Periplaneta americana L. included in the TCMSP database. We use “American cockroach” and “Periplaneta americana L.” as the keyword to retrieve in all databases. 53 compounds were collected through literature researchs[1-10].
Immunocompromised targets for chemical components collection:
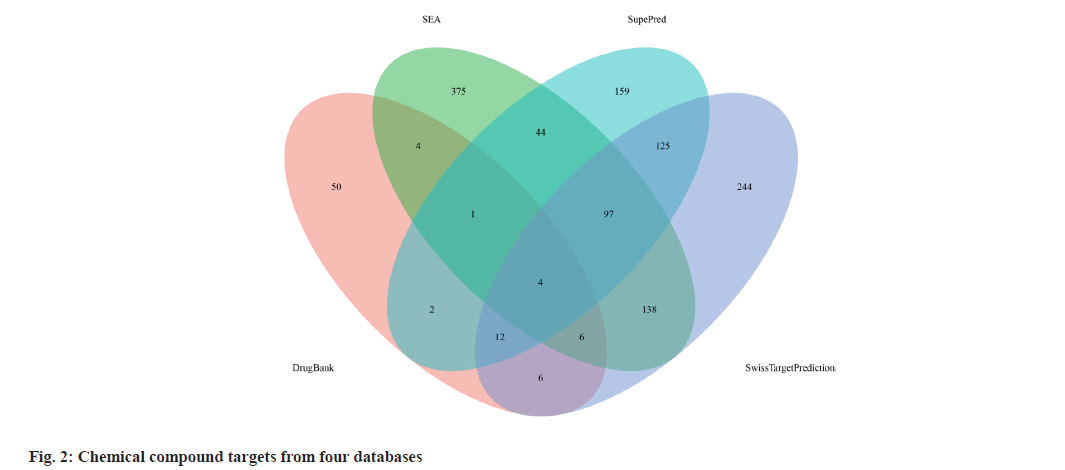
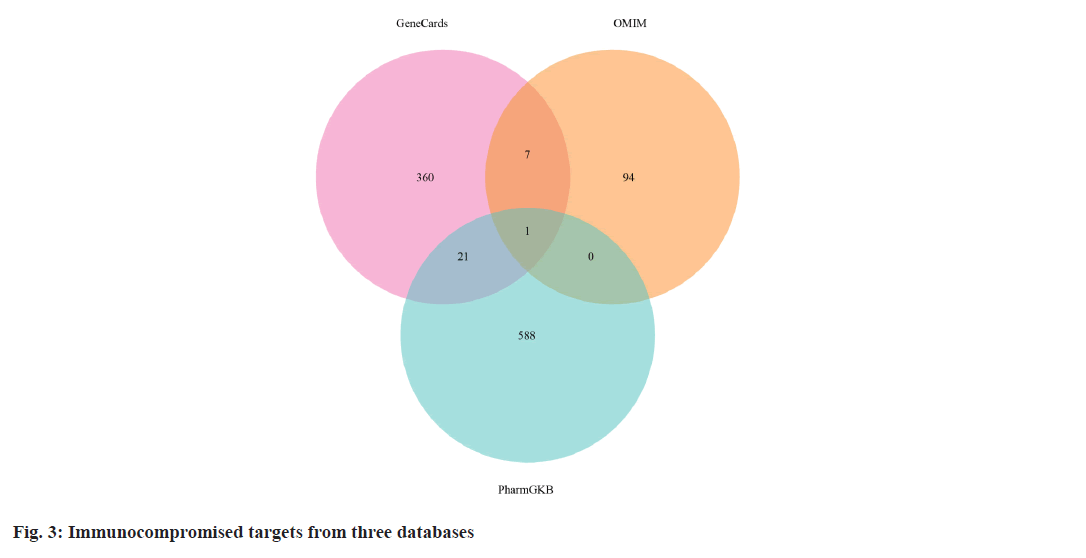
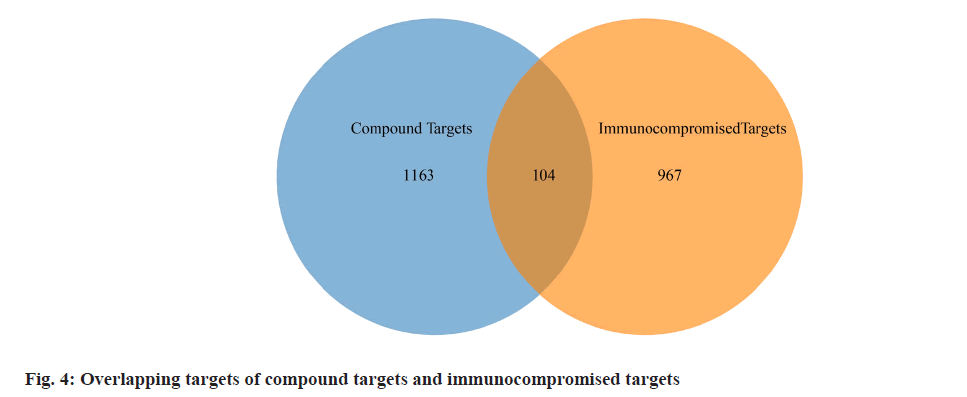
The targets of the 53 chemical compounds were obtained by consolidating data from databases including PharmMapper, SEA, SupePred, and SwissTargetPrediction. Subsequently, the quest to identify targets associated with the immunocompromised properties of active compounds encompassed an exploration of databases like GeneCards, PharmGKB, and Online Mendelian Inheritance in Man (OMIM®). To determine potential targets responsible for the immunocompromised properties of Periplaneta americana L. extracts, the intersection between compound targets and immunocompromised targets was considered.
Establishing the Protein-Protein Interaction (PPI) network and uncovering key genes?
A PPI network was constructed using the STRING platform. To ensure network reliability, overlapping genes were uploaded to STRING with 'experiment' as the sole active interaction source. A minimum interaction score of 0.4 was specified, Homo sapiens was selected as the organism, and disconnected nodes were hidden to prioritize meaningful interactions. The resulting PPI network was visualized using the R language. Identification of key genes was achieved through the utilization of the Cytohubba plugin for Cytoscape 3.9.1, with the top 10 core genes ranked according to their degree values.
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses:
Employing the "clusterProfiler" R package, the analysis encompassed both GO Enrichment Analysis and KEGG pathway analysis. This involved investigating the three classic GO subschemas: Biological Process (BP), Cellular Component (CC), and Molecular Function (MF). Significance thresholds for results were determined at p-values <0.05 and Q-values <0.05.
Building the interaction network for compounds, targets, and pathways:
To construct the interaction network for compounds, targets, and pathways, we utilized relevant compounds, overlapping targets, and KEGG pathways integrated within Cytoscape 3.9.1.
Results and Discussion
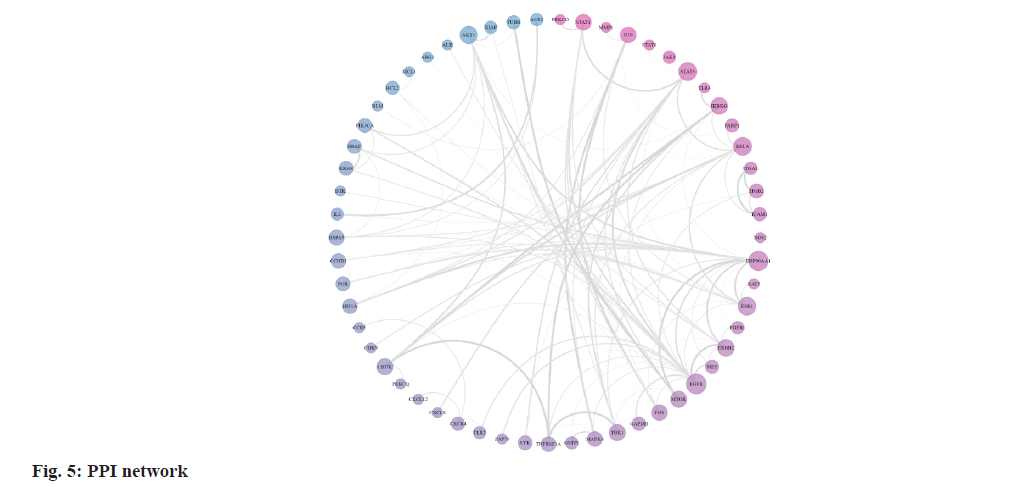
We retrieved 53 results relevant to Periplaneta americana L. by conducting keyword searches, specifically "American cockroach and Periplaneta americana L. ". The chemical structures of these compounds, along with their corresponding references are displayed in (fig. 1). The total of 1267 chemical compound targets is presented in fig. 2, while fig. 3, highlights the 1071 immunocompromised targets. The intersection of these targets, showcased in fig. 4, reveals 173 potential targets associated with immunocompromised effects.
The STRING database provided data for 104 targets, resulting in a PPI network of 57 nodes and 254 edges after the removal of disconnected nodes. The PPI network visualization in fig. 5, displays genes with higher degree values in dark pink and those with lower degree values in dark blue. The size of each node corresponds to its degree value. The top ten key genes, including Epidermal Growth Factor Receptor (EGFR), Heat Shock Protein 90 Alpha family class A member 1 (HSP90AA1), Nuclear Factor kappa B (NF-κB) subunit 3 (RELA, a subunit of NF-κB), Signal Transducer and Activator of Transcription 3 (STAT3), Estrogen Receptor 1 (ESR1), protein kinase B (AKT) serine/threonine kinase 1 (AKT1), Inhibitor of kappa light polypeptide gene enhancer in B-cells Kinase Gamma (IKBKG), Erb-B2 receptor tyrosine Kinase 2 (ERBB2), TANK-Binding Kinase 1 (TBK1), and Mechanistic Target of Rapamycin (MTOR), were identified using the cytoHubba analysis tool according to their degree values.
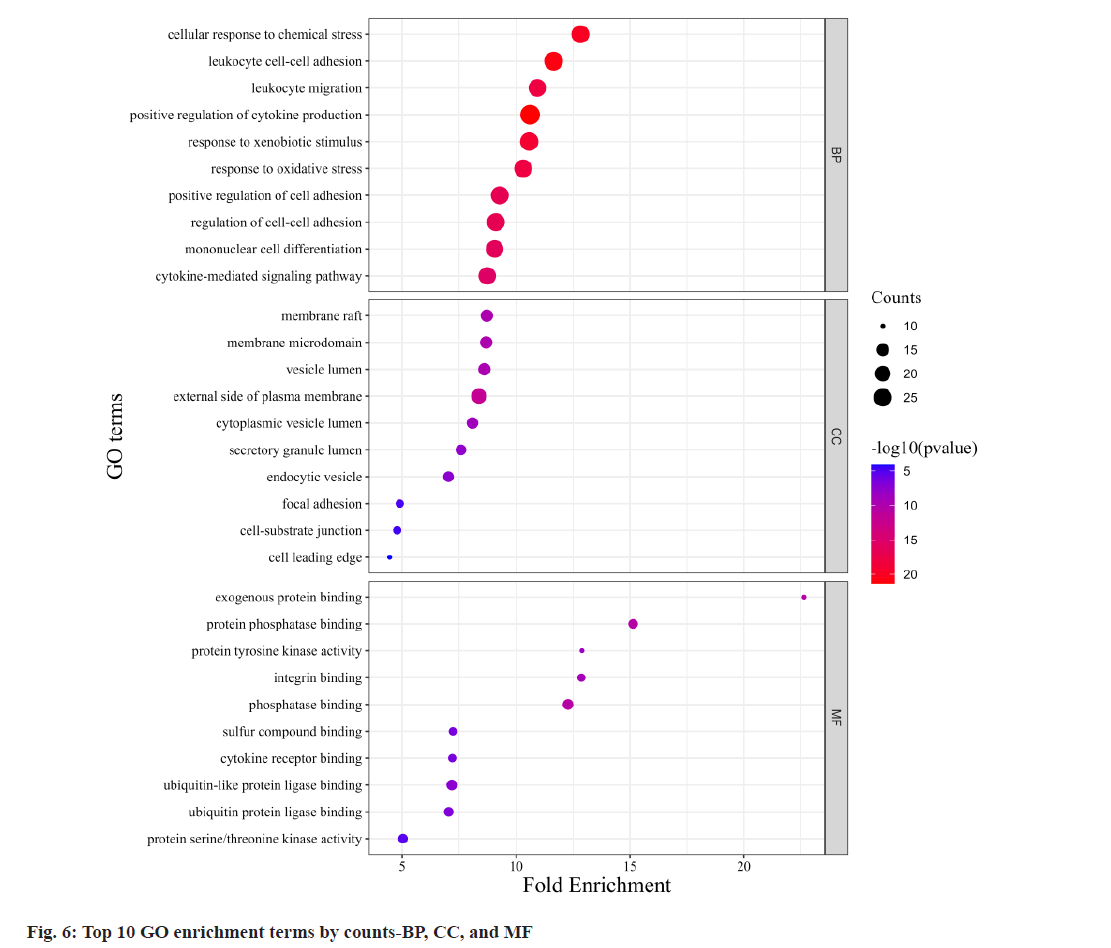
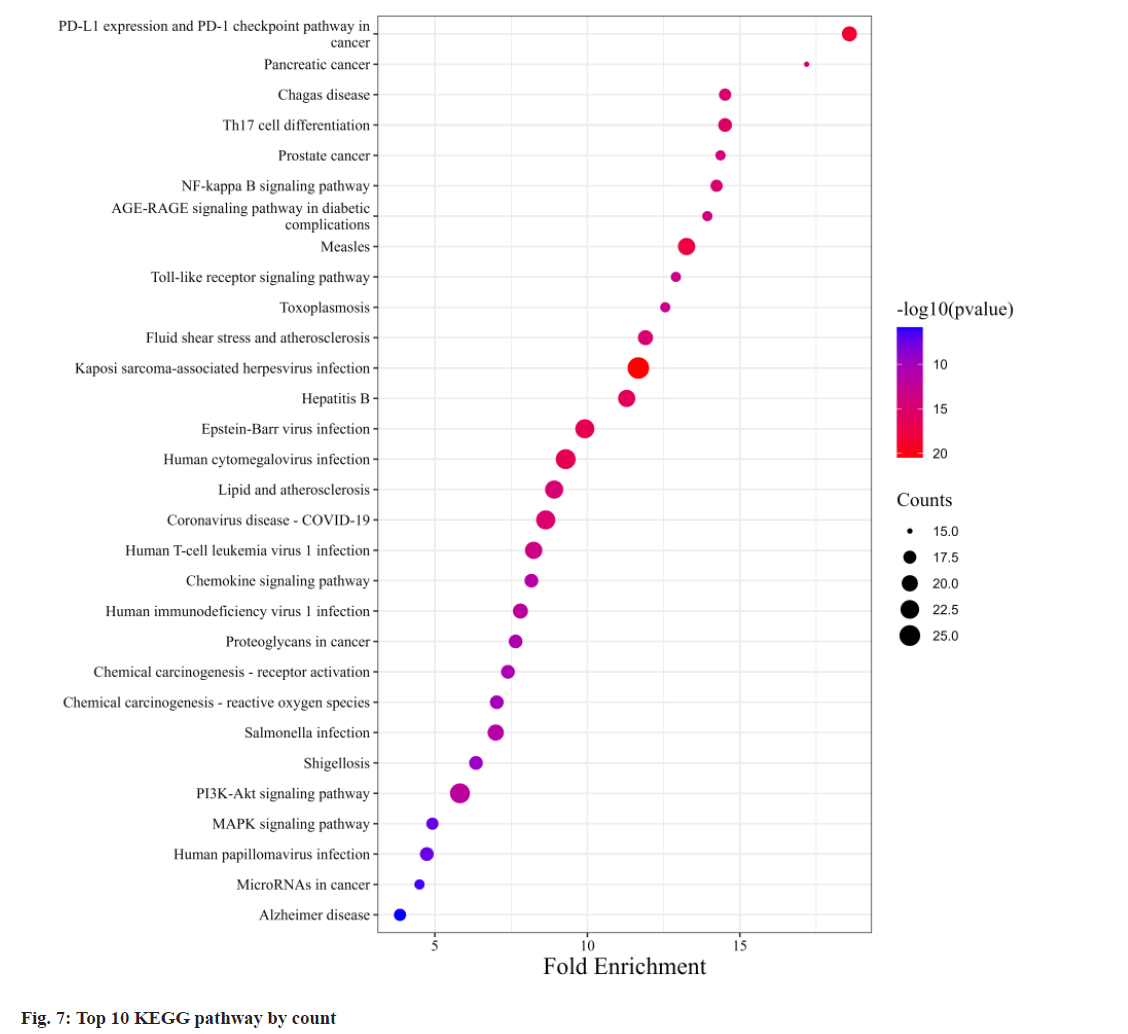
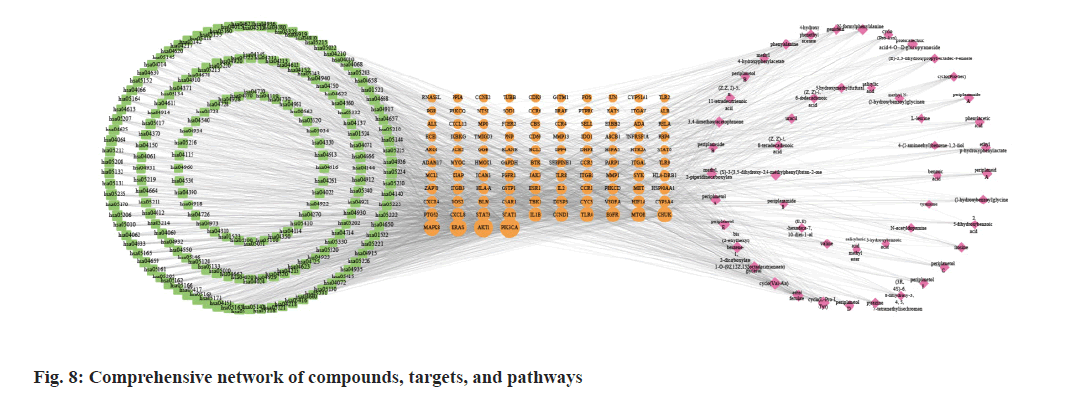
Fig. 6, illustrates GO annotations for the overlapping targets, encompassing BP, CC, and MFs. Notable findings from the BP analysis include the dominance of cellular response to chemical stress, leukocyte cell-cell adhesion, and leukocyte migration. In the CC analysis, membrane raft, membrane microdomain, and vesicle lumen received the highest rankings. The MF analysis highlighted exogenous protein binding, protein phosphatase binding, and protein tyrosine kinase activity. Regarding the KEGG annotation presented in fig. 7, these extracts were found to exert immunomodulatory functions associated with Programmed cell Death-Ligand 1 (PD-L1) expression and the Programmed cell Death-1 (PD-1) checkpoint pathway in cancer, T helper 17 (Th17) cell differentiation, and the NF-κB signaling pathway. Illustrated in fig. 8, the comprehensive network visually portrays the intricate connections between compounds, targets, and pathways. Within this network, ingredients are symbolized by fuchsia diamonds, overlapping targets are represented by orange ellipses, pathway codes are denoted by green rounded rectangles, and larger shapes correspond to larger degree values.
The key genes identified in this study play crucial roles in shaping immune responses within their respective environments. Understanding their mechanisms offers innovative therapeutic possibilities. Notably, EGFR takes the top position among these key genes, signifying its potential for a significant impact on immune responses and modulation. Stressexposed EGFRs have been linked to innate immune responses to viral infections, a finding that highlights their crucial role[11]. These stress-exposed EGFRs undergo internalization through clathrin-mediated endocytosis, a process intricately tied to EGFR phosphorylation[12]. Furthermore, several studies have reported that EGFR inhibition enhances the expression of Major Histocompatibility Complex class-I (MHC-I), MHC-II, and Class II major histocompatibility complex Transactivator (CIITA) complexes and suggests that self-reactive T cells may be involved and associated with skin rashes[13,14]. Additionally, the inhibition of EGFR shows promise in boosting antigen-specific T cell-mediated tumor killing, offering potential benefits for cancer immunotherapy[14]. ERBB2, like EGFR, belongs to the ErbB receptor family and exhibits a Gainof- Function (GoF) in primary tumors, undergoing GoF alterations in primary tumors. The heightened immune cell infiltration, boosted adaptive immunity, and reduced T regulatory cells, driven by ERBB2 GoF, adversely impact the tumor microenvironment and patient survival, underscoring the significance of ERBB2 gene demethylation in modulating immune responses in cancer[15].
The KEGG annotation emphasizes the immunomodulatory potential of these extracts, particularly their impact on key pathways in cancer immunotherapy and immune responses. Specifically, the PD-1/PD-L1 immune checkpoint pathway plays a pivotal role in tumor immunotherapy. PD- 1, primarily found on activated T cells, B cells, macrophages, and bone marrow cells, interacts with PD-L1 on the surface of tumor cells, hampering T lymphocyte activation and triggering T cell apoptosis via signaling pathway inhibition[16,17]. Concurrently, increased PD-L1 on dendritic cells inhibits cytokine production by T lymphocytes in the tumor microenvironment, diminishing the immune surveillance function of T cells and allowing tumor cells to evade the immune system[18-20]. PD-1 and PD-L1 not only suppress tumor-specific T cells but also promote regulatory T cell development[21]. Furthermore, exhausted CD8+ T cells, characterized by elevated PD-1 expression leading to reduced responsiveness to tumor cells, share this exhaustion pattern with CD4+ T cells and tumor-associated macrophages, all displaying heightened PD-1 expression[22-24]. Recent findings suggest that PD-1 therapy can enhance antitumor immunity through diverse cell-dependent mechanisms, particularly by reinvigorating exhausted T cells and reducing PD- 1-related inhibition, thereby improving the body's response to tumors[24-26]. NF-κB plays a key role in immune responses, activating genes responsible for immune functions such as cytokines, chemokines, and adhesion molecules[27]. This activation is especially vital for expressing cytokine genes in Th17 cells, which are instrumental in defending the body against extracellular pathogens and fungal infections[28].
In summary, EGFR and ERBB2 may be important target proteins in the treatment of immunocompromised diseases. Moreover, these pathways may be involved in regulating immune activity through PD-L1 expression and the PD-1 checkpoint pathway in cancer, Th17 cell differentiation, and the NF-κB signaling pathway. This study elucidated the relationship between the main components, targets, pathways, and diseases of Periplaneta americana extracts by means of network pharmacology. It clarified that the key constituents of Periplaneta americana extracts involved in the treatment of immunocompromised diseases were regulated by a multi-target and multi-pathway interaction network. The above findings revealed the scientific connotation of medicinal insect usage, and provided an important theoretical basis for further validation research.
Acknowledgments:
This research work was funded by the Special Basic Cooperative Research Programs of Yunnan Provincial Undergraduate Universities Association (202101BA070001-217 and 202101AO070268) and research program of Yunnan Provincial Key Laboratory of Entomological Biopharmaceutical R&D (AS2022001).
Conflict of interests:
The authors declared no conflict of interests.
References
- Guo JC, Luo Y, Niu H, Zhang D, Lei H. Chemical constituents from Periplaneta americana and their antioxidant activities. Chin Tradit Pat Med 2021;43:3355-9.
- Si JG, Zhang T, Li LY, W MH, Zou ZM. Chemical constituents from Periplaneta americana. Chin Pharm J 2018;53(3):178-81.
[Crossref]
- Gao Y, Liang LC, Wang R, Cheng L, Guo FJ, Li YM. Chemical constituents from Periplaneta americana. Chin Tradit Pat Med 2018;40:375-8.
- Xu J, Che Y, Liu X, Liu C, Meng D, Pang X, et al. The regulating effect of CII-3 and its active components from Periplaneta americana on M1/M2 macrophage polarization. Molecules 2022;27(14):4416.
- Li Y, Wang F, Zhang PZ, Yang M. Chemical constituents from Periplaneta americana. Zhong Yao Cai 2015;38(10):2038-41. [Google Scholar]
[PubMed]
- Yang YX, Luo Q, Hou B, Yan YM, Wang YH, Tang JJ, et al. Periplanosides A-C: New insect-derived dihydroisocoumarin glucosides from Periplaneta americana stimulating collagen production in human dermal fibroblasts. J Asian Nat Prod Res 2015;17(10):988-95.
[Crossref] [Google Scholar] [PubMed]
- Wang XL, Xiang B, Li YM, Qin FY, Geng FN, Yan YM et al. Nitrogen containing chemical constituents from Periplaneta americana and their wound-healing promoting activities. Mod Chin Med 2018;20(1):20-5.
- Zhang HS, Yan YM, Wang DW, Lv Q, Cheng YX, Wang SM. Small molecule constituents of Periplaneta americana and Their IL-6 inhibitory activities. Nat Prod Commun 2021;16(9):1934578X211033180.
- Yan YM, Xiang B, Zhu HJ, Qi JJ, Hou B, Geng FN, et al. N-containing compounds from Periplaneta americana and their activities against wound healing. J Asian Nat Prod Res 2019;21(2):93-102.
[Crossref] [Google Scholar] [PubMed]
- Bai HF, Li YP, Qin FY, Yan YM, Wang SM, Zhang HX, et al. Periplanetols A-F, phenolic compounds from Periplaneta americana with potent COX-2 inhibitory activity. Fitoterapia 2020;143:104589.
[Crossref] [Google Scholar] [PubMed]
- Zeng X, Carlin CR. Adenovirus early region 3 RIDa protein limits NF?B signaling through stress-activated EGF receptors. PLoS Pathog 2019;15(8):e1008017.
[Crossref] [Google Scholar] [PubMed]
- Grandal MV, Grovdal LM, Henriksen L, Andersen MH, Holst MR, Madshus IH, et al. Differential Roles of Grb 2 and AP-2 in p38 MAPK-and EGF-Induced EGFR Internalization. Traffic. 2012;13(4):576-85.
[Crossref] [Google Scholar] [PubMed]
- Pollack BP, Sapkota B, Cartee TV. Epidermal growth factor receptor inhibition augments the expression of MHC class I and II genes. Clin Cancer Res 2011;17(13):4400-13.
[Crossref] [Google Scholar] [PubMed]
- Im JS, Herrmann AC, Bernatchez C, Haymaker C, Molldrem JJ, Hong WK, et al. Immune-modulation by epidermal growth factor receptor inhibitors: Implication on anti-tumor immunity in lung cancer. PLoS One 2016;11(7):e0160004.
[Crossref] [Google Scholar] [PubMed]
- Wang H, Jiang Y, Jin H, Wang C. ERBB2 promoter demethylation and immune cell infiltration promote a poor prognosis for cancer patients. Front Oncol 2022;12:1012138.
[Crossref] [Google Scholar] [PubMed]
- Chocarro de Erauso L, Zuazo M, Arasanz H, Bocanegra A, Hernandez C, Fernandez G, et al. Resistance to PD-L1/PD-1 blockade immunotherapy. A tumor-intrinsic or tumor-extrinsic phenomenon? Front Pharmacol 2020;11:441.
[Crossref] [Google Scholar] [PubMed]
- Tran L, Theodorescu D. Determinants of resistance to checkpoint inhibitors. Int J Mol Sci 2020;21(5):1594.
[Crossref] [Google Scholar] [PubMed]
- Udall M, Rizzo M, Kenny J, Doherty J, Dahm S, Robbins P, Faulkner E. PD-L1 diagnostic tests: A systematic literature review of scoring algorithms and test-validation metrics. Diagn Pathol 2018;13:1-1.
[Crossref] [Google Scholar] [PubMed]
- Upadhaya S, Neftelino ST, Hodge JP, Oliva C, Campbell JR, Yu JX. Combinations take centre stage in PD1/PDL1 inhibitor clinical trials. Nat Rev Drug Discov 2021;20(3):168-70.
[Crossref] [Google Scholar] [PubMed]
- Martorana F, Colombo I, Treglia G, Gillessen S, Stathis A. A systematic review of phase II trials exploring anti-PD-1/PD-L1 combinations in patients with solid tumors. Cancer Treat Rev 2021;101:102300.
[Crossref] [Google Scholar] [PubMed]
- Cui Y, Shi J, Cui Y, Zhu Z, Zhu W. The relationship between autophagy and PD-L1 and their role in antitumor therapy. Front Immunol 2023;14:1093558.
[Crossref] [Google Scholar] [PubMed]
- Zha H, Jiang Y, Wang X, Shang J, Wang N, Yu L, et al. Non-canonical PD-1 signaling in cancer and its potential implications in clinic. J Immunother Cancer 2021;9(2).
[Crossref] [Google Scholar] [PubMed]
- Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 2015;15(8):486-99.
[Crossref] [Google Scholar] [PubMed]
- Kim MJ, Ha SJ. Differential role of PD-1 expressed by various immune and tumor cells in the tumor immune microenvironment: Expression, function, therapeutic efficacy, and resistance to cancer immunotherapy. Front Cell Dev Biol 2021;9:767466.
[Crossref] [Google Scholar] [PubMed]
- Hudson WH, Gensheimer J, Hashimoto M, Wieland A, Valanparambil RM, Li P, et al. Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1+ stem-like CD8+ T cells during chronic infection. Immunity 2019;51(6):1043-58.
[Crossref] [Google Scholar] [PubMed]
- Nicodemus CF. Antibody-based immunotherapy of solid cancers: Progress and possibilities. Immunotherapy 2015;7(8):923-39.
[Crossref] [Google Scholar] [PubMed]
- Hayden MS, Ghosh S. NF-?B in immunobiology. Cell Res 2011;21(2):223-44.
[Crossref] [Google Scholar] [PubMed]
- Park SH, Cho G, Park SG. NF-?B activation in T helper 17 cell differentiation. Immune Netw 2014;14(1):14-20.
[Crossref] [Google Scholar] [PubMed]