- *Corresponding Author:
- Ö. Bahadir Acikara
Ankara University, Faculty of Pharmacy, Department of Pharmacognosy, Ankara, Turkey
E-mail: bahadir-ozlem@hotmail.com
| Date of Submission | 04 November 2016 |
| Date of Revision | 04 February 2017 |
| Date of Acceptance | 14 July 2017 |
| Indian J Pharm Sci 2017;79(5): 715-723 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Evaluation of hepatoprotective activities of Scorzonera roots and their major compounds, was aimed in current study. Scorzonera latifolia, S. tomentosa, S. mollis ssp. szowitsii, S. parviflora and S. cana var. jacquiniana roots, methanol-water (80:20) extracts together with chlorogenic acid, scorzotomentosin-4'-O-β-glucoside, hydrangenol-8-O-β-glucoside as major compounds isolated from S. latifolia roots were tested for their hepatoprotective activities. Sprague Dawley rats were used for experiment and hepatotoxicity was induced by carbon tetrachloride. Aspartate aminotransferase and alanine aminotransferase levels were measured and all results were confirmed by histopathological examination. Plasma aspartate aminotransferase and alanine aminotransferase levels of examined groups were not significant when compared to carbon tetrachloride-treated groups. However histopathological results have revealed that all tested groups have less damage when compared to carbon tetrachloride group except scorzotomentosin-4'-O-β-glucoside and hydrangenol-8-O-β-glucoside groups. Scorzonera species displayed moderate hepatoprotective activities against carbon tetrachloride induced acute toxicity. Chlorogenic acid, among tested compounds exhibited higher activity than all tested Scorzonera species as well as other isolated compounds. Therefore chlorogenic acid could be suggested as responsible compounds.
Keywords
Scorzonera sp., hepatoprotective effect, carbon tetrachloride, chlorogenic acid
In Turkish folk medicine, the roots of Scorzonera latifolia and similar species are mainly used as analgesic, antihelmintic, wound healer, and for treatment of women infertility [1,2]. Additionally, the usage of this genus plants against hypertension, kidney diseases, diabetes mellitus, arteriosclerosis, and rheumatism have been recorded [1]. Analgesic activities of the S. latifolia, S. tomentosa, S. mollis ssp. szowitsii, S. suberosa ssp. suberosa were proven scientifically by previous studies and triterpenoids were isolated as responsible compounds [3-5]. Antiinflammatory, antioxidant, and wound healing activities have also been reported [6-9]. Phytochemical analyses revealed that S. latifolia roots contained taraxasteryl myristate, taraxasteryl acetate, motiol, 3-β-hydroxy fern-8 en-7- one acetate, urs-12-en-11-one-3-acetyl, 3-β-hydroxyfern- 7-en-6-one-acetate, olean-12-en-11-one-3- acetyl, fern-7-en-3-β-one, leucodin, β-sitosterol [3,5,10], chlorogenic acid, chlorogenic acid methyl ester, 1,5-dicaffeoyl quinic acid, 3,5-dicaffeoyl quinic acid, methylester of 3,5-dicaffeoyl quinic acid, hydrangenol- 8-O-β-glucoside, hydrangenol-4'-O-β-glucoside, scorzotomentosin-4'-O-β-glucoside and a new isocoumarine derivative [11] as well as scorzoveratrin 4'-O-β-glucoside, scorzoveratrin, scorzoveratrozit, 4,5-dicaffeoylquinic acid, 4,5-dicaffeoylquinic acid methyl ester and caffeic acid [12]. Scorzotomentosin, scorzotomentosin-4'-O-β-glucoside, scorzophtalide, scorzoerzincanin, hydrangenol, hydrangenol-4'-O-β- glucoside, hydramacrophyllol A and B have been isolated from S. tomentosa roots previously [13]. Chlorogenic acid has been determined in roots of Scorzonera sp., including S. latifolia, S. tomentosa, S. mollis ssp. szowitsii, S. parviflora and S. cana var. jacquiniana as one of the major compound by high-performance liquid chromatography (HPLC) [6,14]. Chlorogenic acid has potent antioxidant and hepatoprotective activities. Liver damage and symptoms of liver fibrosis were reduced significantly by chlorogenic acid as well as serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT), alkaline phosphatase (ALP) and total biluribin levels were lowered [15,16]. In current study the roots of S. latifolia, S. tomentosa, S. mollis ssp. szowitsii, S. parviflora and S. cana var. jacquiniana were investigated for their potential hepatoprotective activities due to their high content of chlorogenic acid as well as caffeoylquinic acid derivatives. Chlorogenic acid and isocoumarine derivatives; hydrangenol-8- O-glucoside and scorzotomentosin-4'-O-β-glucoside obtained from S. latifolia roots as major components were also evaluated for their activities.
Materials and Methods
Scorzonera species were collected from different parts of Turkey. The taxonomic identification of the plants was confirmed in the Department of Biological Sciences, Faculty of Art and Sciences, Gazi University. Voucher specimens are kept in the herbarium of Ankara University, Faculty of Pharmacy (Table 1). Carbon tetrachloride (CCl4) was obtained from Merck (Darmstadt, Germany), and olive oil was obtained from Fluka (Steinheim-Germany). CCl4 dissolved in olive oil (v/v, 1:1) and Scorzonera extracts were prepared using water (w/v).
| Species | Place | Herbarium number |
|---|---|---|
| S. cana(C.A. Meyer) Hoffm. var. jacquiniana (W. Koch) Chamberlain | Camlidere, Ankara, 2013 | AEF 23834 |
| S. latifolia(Fisch. &Mey.) DC. | Kop passage, 2010 | AEF 23830 |
| S. mollisBieb. subsp. szowitsii (DC.) Chamberlain | Kizilcahamam, Ankara, 2006 | AEF 23844 |
| S. parvifloraJacq. | Gölbasi, Ankara, 2012 | AEF 25894 |
| S. tomentosaL. | Akdagmadeni,Yozgat, 2013 | AEF 23841 |
Table 1: Voucher Specımens in the Herbarıum
Extraction of plant material
Dried and powdered S. latifolia roots (700 g) were extracted with methanol-water (80:20) at room temperature for 24 h×3 times by continuous stirring. Methanol-water extract was filtered and concentrated to dryness under reduced pressure and low temperature (40-50°) on a rotary evaporator to give crude extract (182.46 g).
Isolation and identification of the compounds
Solvent-solvent partition was performed with petroleum ether, chloroform, ethylacetate, respectively, and with the remain methanolic part four different extract were obtained. Ethylacetate part (11.02 g) was subjected to column chromatography on silica gel column eluting with ethylacetate:methanol:water (100:13.5:10) to yield 92 fraction. Fraction 24-26 and fraction 38-42 gave hydrangenol-8-O-β-glucoside and scorzotomentosin-4'-O-β-glucoside, respectively. Chlorogenic acid was obtained from 89-92 fractions as a dirty white precipitate. Structures of the compounds were established using MS (Waters 2695 Alliance Micromass ZQ, LC/MS), 1H and 13C NMR (Varian Mercury 400, 400 MHz High Performance Digital FTNMR Spectrometer) techniques.
Experimental animals
The study protocol (01-06-2015/41) was approved by the Ethical Committee of, İstanbul Medipol University. Male and female Sprague-Dawley rats (170-240 g) were used in this experiment. The animals were housed in standard cages (48×35×22 cm) at room temperature (22±2°), with artificial light from 7.00 am to 7.00 pm, and provided with pelleted food and water ad libitum.
Hepatoprotective activity assay
Hepatotoxicity test model induced by CCl4 was used with slight modification [16,17]. Liver toxicity was induced by i.p. (intraperitoneal) administration of CCl4 (0.8 ml/kg) diluted in olive oil (1:1 v/v) for two days. Animal groups were designed as follow (n=5): control group 1 received isotonic saline solution (ISS) 0.1 ml, group 2 received CCl4 0.8 ml/kg i.p. Group 3 received S. cana var. jacquiniana root extract (100 mg/kg)+CCl4 (0.8 ml/kg), group 4 received S. latifolia root extract (100 mg/kg)+CCl4 (0.8 ml/kg), group 5 received S. mollis ssp. mollis root extract (100 mg/kg)+ CCl4 (0.8 ml/kg), group 6 received S. parviflora root extract (100 mg/kg)+CCl4 (0.8 ml/kg), group 7 received S. tomentosa (100 mg/kg)+CCl4 (0.8 ml/kg), group 8 received chlorogenic acid (5 mg/kg)+CCl4 (0.8 ml/kg), group 9 received hydrangenol-8-O-glucoside (5 mg/kg)+CCl4 (0.8 ml/kg), group 10 received scorzotomentosin-4'-O- β-glucoside (5 mg/kg)+CCl4 (0.8 ml/kg) i.p. daily for seven days.
Blood samples were collected by direct cardiac puncture after seven days treatment and the serum was used for the assay of the marker enzymes AST and ALT. The percentage of daily changes in body weight was measured according to the following Eqn.: change in body weight (%)=100×(weightn–weightinitial)/ weightinitial, where, weightinitial: measurement on the first day, weightn: measurement after 2., 3., … 8th d.
Histopathological examination of the liver
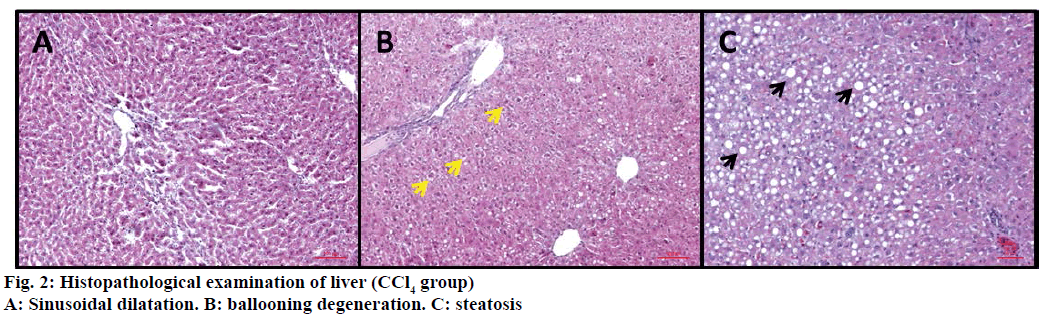
The livers of the experimental animals were fixed in 10% neutral buffered-formalin prior to routine processing in paraffin-embedded blocks. Sections (5 μm thick) were cut and stained using Hematoxylineosin (HE). Axio V 16 microscope was used to take photographs. Histological damage was measured as one-blind experiment and was expressed using the following score system; 0: absent; +: mild; ++: moderate; +++: severe [18,19]. Ballooning degeneration, sinusoidal dilatation, vascular congestion and steatosis were used for evaluation of the histopathological results.
Statistical analyses
Results are reported as mean±SEM (standard error of mean) and as percentage (%). Kruskal-Wallis test (post hoc Mann-Whitney U with Bonferroni adjustment and Moses Extreme Reactions Test) and one-way analysis of variance (One-Way ANOVA, post hoc Scheffe) were used for statistical analyses. Probability levels of less than 0.05 (P<0.05) were considered significant.
Results and Discussion
Plasma AST and ALT levels were given in Table 2. Differences in plasma AST levels of CCl4 group and Scorzonera sp. extracts as well as chlorogenic acid, hydrangenol-8-O-β-glucoside and scorzotomentosin- 4'-O-β-glucoside were not significant. However plasma ALT levels were measured higher than CCl4 group after treatment of S. mollis ssp. szowitsii, S. tomentosa and S. cana var. jacquiniana root exracts. Chlorogenic acid, hydrangenol-8-O-β-glucoside and scorzotomentosin-4'-O-β-glucoside groups serum ALT levels were reduced when compared to CCl4 group.
| Groups | ALT | AST |
|---|---|---|
| Serum (U/l) | Serum (U/l) | |
| Control (ISS) | 40.45 | 96.80 |
| CCl4 | 51.15a | 110.25 |
| S. canavar.jacquiniana | 66.70ab | 114.10 |
| S. latifolia | 52.20a | 97.00 |
| S. mollisssp. szowitsii | 56.30ab | 103.90 |
| S. parviflora | 50.60a | 102.00 |
| S. tomentosa | 62.20ab | 108.00 |
| Chlorogenic acid | 58.70 | 329.20 |
| Hydrangenol-8-O-glucoside | 39.35b | 111.20 |
| Scorzotomentosin-4'-O-β-glucoside | 33.25ab | 124.25 |
| P value | 0.034 | 0.457 |
Table 2: Effects of Scorzonera sp. Extracts and Compounds on Serum Levels of AST and ALT
Results of the body weight changes of animals are given in Table 3. Body weight of the animals was measured in the beginning and at the end of the study. While animals in control group (ISS) were gaining weight as measured by 2.64%, in all other groups animals had weight loss in different percentage as given in Table 3.
| Groups | Mean | Std. deviation | Std. error | Difference (%)* |
|---|---|---|---|---|
| Control (ISS) | 2.67 | 1.37 | 0.56 | 2.64 |
| CCl4 | –0.93 | 2.93 | 1.20 | –0.95 |
| S. canavar. jacquiniana | –6.44a | 4.64 | 2.07 | –6.58 |
| S. latifolia | –4.72 | 1.21 | 0.54 | –4.68 |
| S. mollis ssp. szowitsii | –3.78 | 3.31 | 1.48 | –3.85 |
| S. parviflora | –2.46 | 1.56 | 0.70 | –2.43 |
| S. tomentosa | –6.98a | 5.14 | 2.30 | –6.91 |
| Chlorogenic acid* | –11.38 | – | – | –11.38 |
| Hydrangenol–8–O–glucoside | –9.15a | 3.80 | 1.90 | –5.91 |
| Scorzotomentosin–4'–O–β–glucoside | –14.14ab | 0.98 | 0.70 | –10.08 |
| P value | 0.001 |
Table 3: Body Weight Values of the Study Groups
Histopathological examination results using score system were exhibited in Table 4. Significant differences between control (ISS) group and treatment groups were observed (P<0.05). Ballooning degeneration, sinusoidal dilatation, vascular congestion and steatosis were observed by severe score in CCl4 treated group. Histopathological results have revealed CCl4 treated group has high histopathological score as shown in Table 4 and remarkable results were assigned between all other treatment groups except hydrangenol-8-O- β-glucoside and scorzotomentosin-4'-O-β-glucoside treated groups (P>0.05).
| Groups | Sinusoidal dilatation | Vascular congestion | Ballooning degeneration | Steatosis | Rate of damage | Median |
|---|---|---|---|---|---|---|
| Control (ISS) | 0 | 1 | 0 | 0 | 1/5=0.20 | 0 |
| CCl4 | 13 | 14 | 14 | 15 | 56/5=11.20 | 11.5a |
| S. canavar.jacquiniana | 2 | 5 | 7 | 1 | 15/3=5.00 | 3ab |
| S. latifolia | 3 | 3 | 12 | 13 | 31/5=6.2 | 3ab |
| S. mollisssp. mollis | 1 | 5 | 5 | 4 | 15/5=3.00 | 3ab |
| S. parviflora | 3 | 5 | 5 | 1 | 14/5=2.8 | 3ab |
| S. tomentosa | 4 | 4 | 5 | 4 | 17/5=3.40 | 3ab |
| Chlorogenic acid | 2 | 2 | 2 | 2 | 8/5=1.60 | 2ab |
| Hydrangenol-8-O-glucoside | 11 | 11 | 12 | 13 | 47/5=9.40 | 9a |
| Scorzotomentosin-4'-O-β-glucoside | 12 | 12 | 14 | 14 | 52/5=10.40 | 11.5a |
Table 4: Histopathological Examination Results
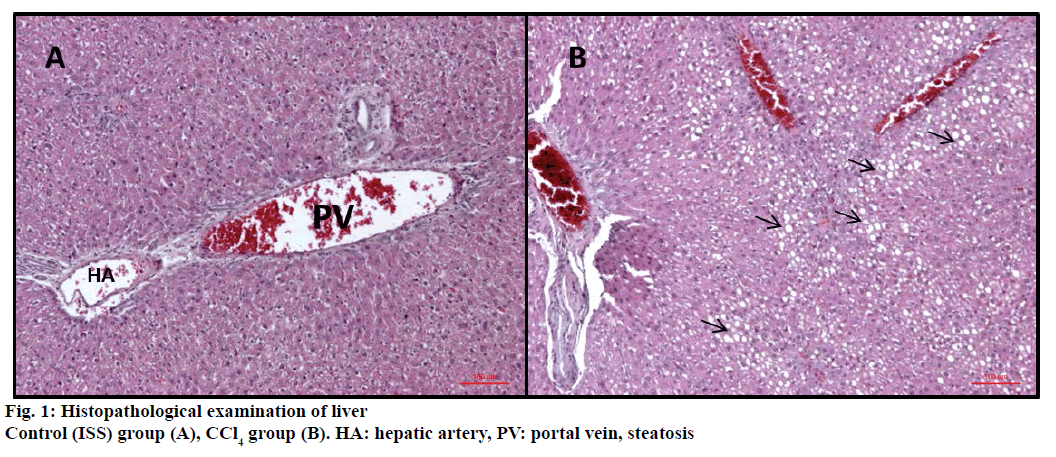
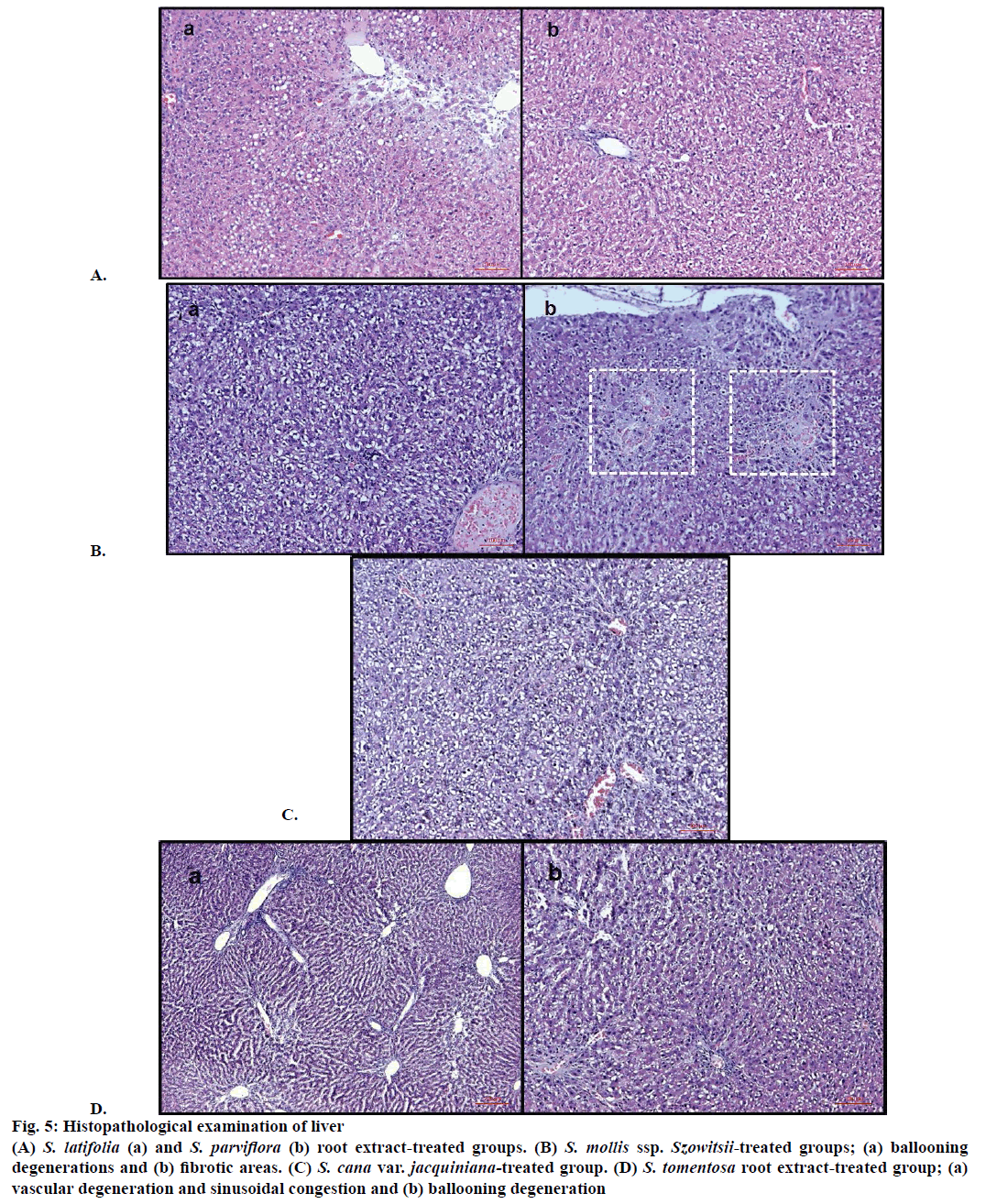
In control (ISS) group no degeneration was observed in hepatocytes, sinusoids and vascular cells (Figure 1A). On the other hand CCl4 treated group were displayed severe damage; ballooning degeneration, vascular congestion, sinusoidal dilatation and steatosis (Figures 1B and 2).
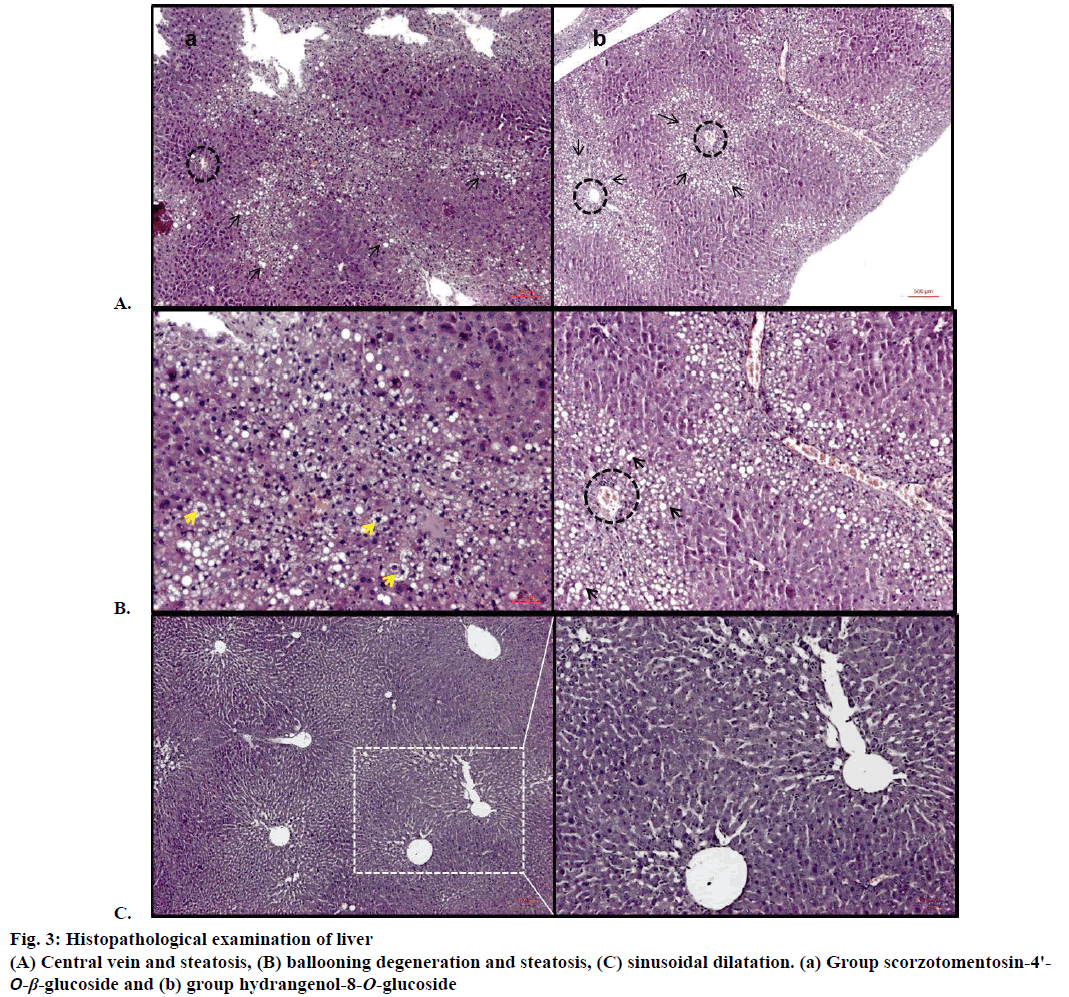
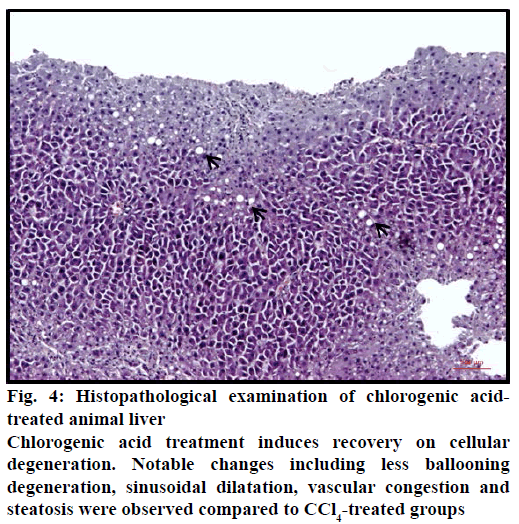
Ballooning degeneration, vascular congestion, sinusoidal dilatation and steatosis were also occasionally observed in groups after treatment by hydrangenol-8-O-β-glucoside and scorzotomentosin- 4'-O-β-glucoside. Steatosis areas were observed generally around central vein. Sinusoidal dilatation was also observed mainly around central vein (Figure 3). Chlorogenic acid treatment and S. latifolia, S. parviflora; S. mollis ssp. szowitsii; S. cana var. jacquiniana; S. tomentosa root extracts induce recovery on cellular degeneration (Figures 4 and 5). Notable changes including less ballooning degeneration, sinusoidal dilatation, vascular congestion and steatosis were observed compared to CCl4 treated groups. Figure 5A displayed S. latifolia, S. parviflora root extract treated groups liver. Vascular congestion and sinusoidal dilatation were detected rarely when compared as CCl4 treated animal livers. Nevertheless more ballooning degeneration, sinusoidal dilatation, vascular congestion and steatosis were seen compared to control (ISS) treated groups.
Figure 4: Histopathological examination of chlorogenic acidtreated animal liver
Chlorogenic acid treatment induces recovery on cellular degeneration. Notable changes including less ballooning degeneration, sinusoidal dilatation, vascular congestion and steatosis were observed compared to CCl4-treated groups
Figure 5: Histopathological examination of liver
(A) S. latifolia (a) and S. parviflora (b) root extract-treated groups. (B) S. mollis ssp. Szowitsii-treated groups; (a) ballooning degenerations and (b) fibrotic areas. (C) S. cana var. jacquiniana-treated group. (D) S. tomentosa root extract-treated group; (a) vascular degeneration and sinusoidal congestion and (b) ballooning degeneration
S. mollis ssp. szowitsii and S. cana var. jacquiniana treated groups histopathological results have revealed that ballooning degeneration was seen, however vascular congestion, sinusoidal dilatation and steatosis were scarcely as shown in Figures 5B and C. Fibrosis or fibrotic areas were also detected rarely in S. mollis ssp. szowitsii treated group animal livers (Figure 5B). Figure 5D exhibited histopathological results of liver treated by S. tomentosa root extract. Ballooning degeneration, vascular congestion and sinusoidal dilatation were frequently seen. On the other hand steatosis was observed rarely (Figure 5D).
Acute liver toxicity model induced by CCl4 was used to evaluate hepatoprotective activities of S. latifolia, S. tomentosa, S. mollis ssp. szowitsii, S. parviflora and S. cana var. jacquiniana root extract as well as chlorogenic acid, hydrangenol-8-O-β-glucoside and scorzotomentosin-4'-O-β-glucoside obtained from S. latifolia roots as main components in current study.
CCl4-induced hepatotoxicity test model has been extensively used in animal studies to evaluate drugs can be used for treatment of liver diseases [16,19]. CCl4 caused to severe damages in liver such as steatosis, inflammation, apoptosis and cell necrosis [19,20]. Giving CCl4 to animals as a single dose mediated to necrosis and steatosis in a short time. Hepatocytes functional defects have been occurred by injuring plasma membrane, endoplasmic reticulum (ER), mitochondria and Golgi apparatus [20,21] resulted in elevation of serum AST and ALT levels indicating hepatocellular damage [19]. Movement of fat from the cell is blocked by disruption of mechanism for coupling triglycerides to the appropriate apoprotein to form the lipoprotein carrier molecule is caused steatosis [21]. The acute hepatotoxic effect of CCl4 is due to its metabolite trichloromethyl radical (CCl3 •) which occurred by metabolization of CCl4 via cytochrome P-450 system. CCl3 • radical is converted to trichlromethyl peroxy radical CCl3COO• in the presence of oxygen, is more active than the first one. These free radicals are react with different substances including proteins, nucleic acids, lipids and lead to dysfunction. Alkylation of macromolecules including cellular proteins by simultaneous attack on polyunsaturated fatty acids starts the process of lipid peoxidation leading to liver cell necrosis [16,20]. Therefore free radical scavengers or generation inhibitors could be useful in CCl4- induced liver injury [20]. CCl4 also caused activation of many catabolic enzymes which disrupt cytoskeletal construction and cell death via apoptosis or necrosis by increasing the levels of Ca2+ in cells [20].
Chlorogenic acid an ester of caffeic acid and quinic acid (3-O-caffeoylquinic acid (CGA)) possess significant antioxidant activity as well as antibacterial, anticarcinogenic, antiinflammatory, antihypertension activities [22,23]. Chlorogenic acid is one of the most abundant hydroxycinnamic acid derivative in human diet and is widely distributed in medicinal plants, a number of fruits and vegetables as well as daily beverages like coffee, tea, wine, and tobacco [22,24]. Related to its hepatoprotective activity many studies have been reported besides its other biological activities. Lipid peroxidation induced by CCl4 has been supressed by chlorogenic acid. 200 μmol/kg administration of chlorogenic acid resulted in significant protection against the liver damage [23]. According to the Shi et al. [14] chlorogenic acid treatment inhibited development of hepatic fibrosis in pericentral region. Small degree of bridging fibrosis was observed. Lower severity score for liver fibrosis was determined in chlorogenic acid (60 mg/kg) treatment group when compared to CCl4 group. It has been reported that chlorogenic acid has protective activity against liver fibrosis due to its inhibitory activity on hepatic stellate cells (HSCs) activation and production of transforming growth factor (TGF-β1) and vascular endothelial growth factor (VEGF) as well as ER stress. Mitochondrial pathway of apoptosis in liver fibrosis could be regulated by chlorogenic acid is suggested [14]. Serum AST, ALT and TB levels were lowered significantly by administration of chlorogenic acid at 300 or 500 mg/kg dose while ALT levels were comparable with CCl4 treated group. Any mortality has not been observed up to 5000 mg/kg [15]. Wu et al. reported that [25] chlorogenic acid administration at 75 mg/kg and 150 mg/kg dose for 3 w provided significant recovery in liver fibrosis induced by bile duct ligation. Serum alanine transaminase, AST, ALP, total bilirubin, direct bilirubin and total bile acid levels were decreased. Furthermore collagen I, collagen III, TGF-β1 and VEGF mRNA increasing induced by BDL treatment were also suppressed by chlorogenic acid [25]. Free radicals generated by CCl4 metabolization via cytochrome P450 2E1 induce liver cell apoptosis and necrosis as well as upregulation of TNF-α, IL-10 and TGF-β in necrotic hepatocytes. Especially TGF-β caused progression of liver injury to chronic liver disease by activating local leucocytes and promoting the circulation of leucocytes to the necrotic area. Chlorogenic acid down regulates TGF-β1 protein expression and decreased NFkB expression. Furthermore pretreatment of chlorogenic acid protected the PC12 cells from β-amyloid (Aβ) which are reported to reduced viability of PC12 cells by reducing the level of intracellular Ca+2 and apoptosis related of proteins. Chlorogenic acid was reported to potentiate the antiapoptotic and antifibrogenic effects of silymarin and this combination increased the expression of Bcl-2 down regulated by CCl4 [22]. In current study chlorogenic acid was also investigated for its hepatoprotective activity in acute hepatotoxicity test model induced by CCl4 together with hydrangenol-8-O- β-glucoside and scorzotomentosin-4'-O-β-glucoside. Scorzonera species were evaluated for their possible hepatoprotective activities due to their chlorogenic acid contents in present study. Biochemical results exhibited that plasma AST levels were not significant between CCl4 group and Scorzonera sp. extracts as well as hydrangenol-8-O-β-glucoside and scorzotomentosin- 4'-O-β-glucoside. On the other hand high levels of ALT were measured in S. mollis ssp. szowitsii, S. tomentosa ve S. cana. var. jacquiniana root extract treated groups while lower levels of ALT were detected in hydrangenol-8-O-β-glucoside and scorzotomentosin- 4'-O-β-glucoside than CCl4 group. Histopathological examinations clearly displayed all Scorzonera species tested and chlorogenic acid had less cellular damage than CCl4 treated groups. S. latifolia, S. parviflora; S. mollis ssp. szowitsii; S. tomentosa, S. cana var. jacquiniana root extracts and chlorogenic acid treatment induce recovery on cellular degeneration. Chlorogenic acid treatment at 5 mg/kg dose did not lowered AST and ALT levels significantly. However histopathological examinations have revealed that recovery on cellular damage has been improved and less sinusoidal dilatation, vascular congestion, balloning degeneration as well as steatosis were detected. All Scorzonera species used in current study were investigated for their chlorogenic acid contents by HPLC analyses qualitatively and quantitatively previously. S. latifolia roots contain higher chlorogenic acid (1246.78±3.20 μg/g) and this was followed by S. tomentosa (734.72±1.04 μg/g), S. parviflora (509.96±6.64 μg/g), S. cana var. jacquiniana (331.028±2.83 μg/g) and S. mollis ssp. szowitsii (159.25±0.24 μg/g) [6,7]. According to the current study Scorzonera root extracts displayed hepatoprotective activities probably due to their chlorogenic acid contents. Derivatives of chlorogenic acid 3,4 and 3,5 dicaffeoylquinic acids were also reported to have protective activity in CCl4 induced hepatotoxicity [26] which have also been isolated from some Scorzonera species previously [11,12].
In conclusion, hepatoprotective activities of the tested Scorzonera species could be attributed to their chlorogenic acid content and its derivatives. Usage of Scorzonera species could be useful for hepatic diseases, furthermore chlorogenic acid is promising agent in treatment of hepatic diseases which needs further studies.
Acknowledgements
Authors thank Prof. Hayri Duman, Plant Taxonomist, Department of Biological Sciences, Faculty of Art and Sciences, Gazi University for identifying the plant species used in this study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Baytop T. Türkiye’deBitkilerileTedavi (Theraphy with Medicinal Plants in Turkey). İstanbul: Nobel Publishers; 1999.
- Sezik E, Yesilada E, Tabata M, Honda G, Takaishi Y, Fujita T, et al. Traditional medicine in Turkey VIII. Folk medicine in East Anatolia; Erzurum, Erzincan, Agrı, Kars, Igdir Provinces. Econ Bot 1997;51:195-211.
- Bahadır Ö, SaltanÇitoğlu G, Smejkal K, Dall’Acqua S, Özbek H, Cvacka J, et al. Analgesic compounds from Scorzoneralatifolia(Fisch. and Mey.) DC. J Ethnopharmacol 2010;131:83-7.
- Bahadir Ö, Saltan HG, Özbek H. Antinociceptive activity of some ScorzoneraL. Species. Turk J Med Sci 2012;42:861-6.
- BahadırAcıkara Ö, SaltanÇitoğlu G, Dall’Acqua S, Özbek H, Cvacka J, Zemlicka M, et al. Bioassay-guided isolation of the antinociceptive compounds motiol and beta-sitosterol from Scorzoneralatifoliaroot extract. Pharmazie2014;69:711-4.
- KüpeliAkkol E, BahadırAcıkara Ö, Süntar İ, SaltanÇitoğlu G, Keleş H, Ergene B. Enhancement of wound healing by topical application of Scorzonera species: Determination of the constituents by HPLC with new validated reverse phase method. J Ethnopharmacol 2011;137:1018-27.
- Süntar İ, BahadırAcıkara Ö, SaltanÇitoğlu G, Keleş H, Ergene B, KüpeliAkkol E. In vivoand in vitroevaluation of the therapeutic potential of some Turkish Scorzonera species as wound healing agent. Curr Pharm Design 2012;18:1421-33.
- KüpeliAkkol E, BahadırAcıkara Ö, Süntar İ, Ergene B, SaltanÇitoğlu G. Ethnopharmacological evaluation of some Scorzonera species: In vivoantiinflammatory and antinociceptive effects. J Ethnopharm 2012;140:261-70.
- BahadırAcıkara Ö, Süntar İ, SaltanÇitoğlu G, Keleş H, Ergene B, AkkolKüpeli E. Determination of phenolic acids and flavonoids and antiinflammatory activity of Scorzonera species. Int J PharmacogPhytochem Res 2014;6:59-65.
- BahadırAcıkara Ö, SaltanÇitoğlu G, Dall’Acqua S, Smejkal K, Cvacka J, Zemlicka M. A new triterpene from Scorzoneralatifolia(Fisch. and Mey.) DC. Nat Prod Res 2012;26:1892-7.
- BahadırAcıkara Ö, Hosek J, Babula P, Cvacka J, Budesinsky M, Dracinsky M, et al. Turkish Scorzonera Species Extracts Attenuate Cytokine Secretion via NF-κB Inhibition Showing Promising Anti-inflammatory Effect. Molecules 2016;21:1-14.
- Sarı A. Phenolic compounds fromScorzoneralatifolia(Fisch. &Mey.) DC. Nat Prod Res 2012;26:50-5.
- Sarı A, Zidorn C, Spitaler R, Ellmerer EP, Ozgokce F, Orgama KH, et al. Phenolic compounds fromScorzoneratomentosaL. HelvChimActa 2007;90:311-31.
- Shi H, Dong L, Bai Y, Zhao J, Zhang Y, Zhang L. Chlorogenic acid against carbontetrachloride-induced liver fibrosis in rats. Eur J Pharm 2099;623:119-24.
- Sun Z, Liu S, Zhao Z, Su R. Protective Effect of Chlorogenic Acid against Carbon Tetrachloride-induced Acute Liver Damage in Rats. Chinese Herbal Medicine 2014;6:36-41.
- Citoğlu GS, BahadırAcıkara Ö, Sever Yılmaz B, Ozbek H. Evaluation of analgesic, antiinflammatory and hepatoprotective effects of lycorine from Sternbergiafisheriana (Herbert) Rupr. Fitoterapia 2012;83:81-7.
- Mistry S, Dutt KR, Jena J. Protective effect of Sidacordataleaf extract against CCl4 induced acute liver toxicity in rats. Asian Pac J Trop Med 2013;6:280-4.
- Abdel-Wahhab MA, Nada SA, Arbid MS. Ochratoxicosis: prevention of developmental toxicity by L- methionine in rats. J ApplToxicol 1999;19:7-12.
- Zhang Z, Liu Y, Lu L, Luo P. Hepatoprotective activity of GentianaveitchiorumHemsl. Against carbon tetrachloride-induced hepatotoxicity in mice. Chin J Nat Med 2014;12:488-94.
- Toori MA, Joodi B, Sadeghi H, Sadeghi H, Jafari M, Talebianpoor MS, et al. Hepatoprotective activity of aerial parts of Otostegiapersicaagainst carbon tetrachloride-induced liver damage in rats. Avicenna J Phytomed 2015;5:238-46.
- Tiwari P, Ahirwae D, Chandy A, Ahirwar B. Evaluation of hepatoprotective activity of alcoholic and aqueous extracts of Selaginellalepidophylla. Asian Pas J Trop Dis 2014;4:81-6.
- Al-Rasheed NM, Fadda LM, Al-Rasheed NM, Ali HM, Yacoub HI. Down-Regulation of NFkB, Bax,TGF-β, Smad-2mRNA expression in the Livers of Carbon Tetrachloride Treated Rats using Different Natural Antioxidants. Braz Arch BiolTechnol 2016;59:1-10.
- Zhou J, Ashoori F, Suzuki S, Nishigaki I, Yagi K. Protective Effect of Chlorogenic Acid on Lipid Peroxidation Induced in the Liver of Rats by Carbon Tetrachloride or Co-Irradiation. J ClinBiochemNutr 1993;15:119-25.
- El-Seedi HR, El-Said AMA, Khalifa SAM, Göransson U, Bohlin L, Borg-Karlson AK, et al. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties and biological activities of hydroxyl cinnamic acids. J Agric Food Chem 2012;60:10877-95.
- Wu D, Bao C, Li L, Fu M, Wang D, Xie J, et al. Chlorogenic acid protects against cholestatic liver injury in rats. J Pharm Sci 2015;129:177-82.
- Basnet P, Matsushige K,Hase K,Kadota S,Namba T. Four di-O-caffeoylquinic acid derivatives from propolis potent hepatoprotective activity in experimental liver injury models. Biol Pharm Bull 1996;19:1479-84.