- *Corresponding Author:
- Ayusman Swain
Department of Mathematics and Science, Government Polytechnic Kendrapara, Odisha 754289, India
E-mail: ayusman.iitd@gmail.com
| Date of Received | 14 September 2021 |
| Date of Revision | 04 July 2023 |
| Date of Acceptance | 01 September 2023 |
| Indian J Pharm Sci 2023;85(5):1198-1207 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Psoromic acid is a natural depsidone compound, often found in the lichen sources such as Usnea complanata and Rhizoplaca melanophthalma. It was reported to have versatile antioxidative, enzyme inhibitory and therapeutic properties. Studies on the biological activities of psoromic acid in the last decade have explored its potential in treating diseases like cancer, tuberculosis and cardiovascular disease. The present study extensively reviewed relevant works of literature from the sources like Scopus, Elsevier, MEDLINEPubMed, SpringerLink and Google Scholar. Biological activities such as antioxidant, gastroprotective effect, cardiovascular protection, anticancer, antitumor, antiviral response and enzyme inhibition were exclusively summarized. The proven therapeutic properties of psoromic acid suggested future clinical research in a larger sample size for the pharmacological acceptance of its antioxidant, antimicrobial and anticancer effects. The review may broaden the scope of knowledge of researchers to study pharmacokinetics, molecular mechanisms, efficacy and drug safety of psoromic acid and its derivatives.
Keywords
Psoromic acid, Rab geranylgeranyl transferase inhibitor, lichen metabolite, anticancer, enzyme inhibition, antimycobacterial
Natural products or their derived compounds have been extensively used as potential drugs in clinical trials. Natural sources such as plants and microorganisms provide a rich source of active ingredients of medicines[1]. Plants and microorganisms are endowed with various classes of molecules/metabolites, which have been playing a dominant role in discovering and developing effective drug ingredients for managing most human diseases/disorders. Some metabolites such as sulforaphane, benzoxazolinone, apratoxin, vincristine, etoposide, topotecan and other natural compounds having potent anticancer, antibacterial and antioxidative properties were traced to be the proven drugs or the derivatives of drugs used to treat most human diseases[2–4]. At present, pharmacological research has been inclined towards natural herbal supplements in managing degenerative diseases. Many literature findings suggested the consumption of antioxidant-rich foods of natural origin to retard the occurrence of diseases associated with oxidative stress, such as neurodegenerative, cancer, diabetes, cardiovascular and Alzheimer's diseases[5,6]. Among the available natural sources, lichens have been of pharmacological importance due to their usage as folk medicine and the discovery of new health-beneficial metabolites[7,8].
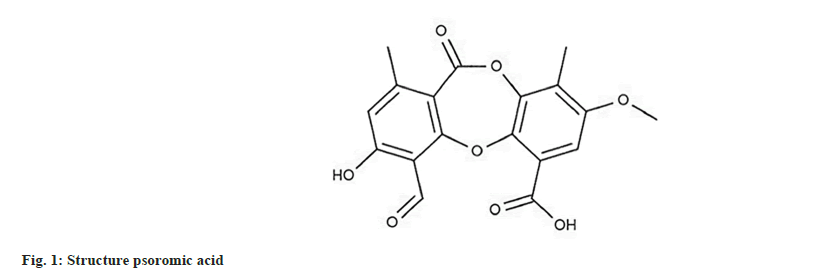
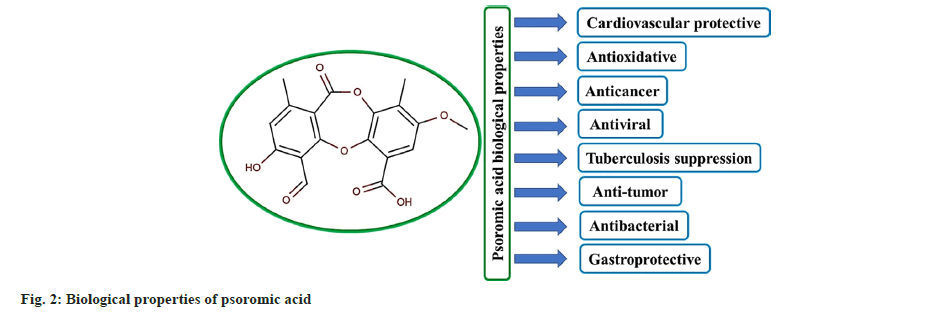
Lichens are the symbiotic association between a photobiont (an inhabitant) comprising an algae or a cyanobacterium and a fungal partner mycobiont (an exhabitant). Even in some lichen species, particular yeast was reported as another symbiont[7,9]. They are found in their natural habitats but often adapted to extreme environments and produce characteristic secondary metabolites because of the symbiotic nature of organisms. Lichens have been used in different folk medicines as antibiotics, antiasthmatics, anticonvulsives, astringents and anti-inflammatories. Several classes of lichen metabolites have been also reported for the treatment of respiratory and gastric disorders and have therapeutic potential[7,10]. Lichens have their own morphological and physiological characteristics different from the algal and fungal constituents and, thus, are capable of producing new metabolites as therapeutic agents against various diseases[11]. Depsides, depsidones, depsones, dibenzofurans and chromones are the most common compounds isolated from lichens[12]. Different lichen extracts were shown to have antioxidative and antibacterial characteristics and are endowed with many bioactive health-beneficial metabolites. The isolation and biological evaluation of such metabolites have become a major area of natural product research in the last few decades[13,14]. Psoromic acid, a natural depsidone class compound, is often found in lichens such as Usnea complanata and Rhizoplaca melanophthalma. It possesses versatile antioxidative, enzyme inhibitory and other therapeutic properties[15,16]. Psoromic acid is a depsidone class compound having a carboxylic acid (C6') and an aldehyde group (C3) attached to aromatic rings 2 and 1, respectively (fig. 1). Phenolic compounds are exclusively produced in lichens, apart from plant sources and other organisms. The structural diversity of phenolics is associated with many beneficial biological and pharmacological properties. Many research findings suggest that psoromic acid could be a candidate molecule for further pharmaceutical safety and efficacy studies in managing diseases like cancer, tumor, cardiovascular disease, tuberculosis and oxidative stress[13,17,18]. The present review aims to make a synopsis of all the major research works on biological activities (fig. 2) and therapeutic studies of psoromic acid in the last decade. Such a synopsis may make a suitable platform for researchers to include the small molecule psoromic acid and its derivatives for further studies on infectious and chronic diseases and establish its structure-activity relationships.
For review purposes, the internet sources such as Scopus, Elsevier, MEDLINE-PubMed, SpringerLink and Google Scholar were used to search for relevant information and pieces of literature. The databases were extensively searched for significant experimental works and studies on the biological activities of psoromic acid reported till June 2021. The keywords such as 'biological activities', 'anticancer', 'antioxidative', 'antibacterial', 'enzyme inhibiting potential', 'antiviral' and 'Rab-Prenylation inhibition', were searched by combining them with 'psoromic acid' in the above search engines. The works of literature concerning the biological studies and therapeutic potentials were reviewed to outline the effectiveness of psoromic acid as a lead molecule in controlling the biomolecules responsible for the occurrence of diseases such as tuberculosis, cancer, tumor and cardiovascular diseases.
Antioxidative and Cardiovascular Protecting Activities
Behera et al. studied the antioxidative and cardiovascular properties of usnic acid and psoromic acid, which were isolated from lichen Usnea complanata[13]. The antioxidant assays were performed using cultured lichen extract, usnic acid and psoromic acid. The purified compounds usnic acid and psoromic acid efficiently inhibited lipid peroxidation and showed significant scavenging activity against free radicals (2,2-Diphenylpicrylhydrazyl (DPPH) and Nitric Oxide (NO) radicals) in different antioxidant assays. Psoromic acid inhibited free radical, nitric oxide radical and lipid peroxidation with half maximal Inhibitory Concentration (IC50) values 0.271, 0.21 and 0.174 mg/ml respectively which is comparable to that of usnic acid which showed inhibition with IC50 values 0.195, 0.188 and 0.214 mg/ml respectively. The standard BHA showed inhibition with IC50 0.031, 0.04 and 0.037 respectively. The cardiovascular protective activity was measured in terms of β-Hydroxy-β-Methylglutaryl Coenzyme A (HMG-CoA) Reductase (HMGR) and angiotensin-converting enzyme (ACE) inhibitions and fibrinolytic activity. HMGR catalyses endogenous cholesterol synthesis through reductive deacylation of HMG-CoA to mevalonate. The increased blood cholesterol levels may lead to coronary heart disease or hyperlipidemia. Therefore, controlling the action of HMGR is very important to hyperlipidemia prevention. The results showed that usnic acid and psoromic acid exhibited a non-competitive and competitive type HMGR inhibition respectively. The ACE was also inhibited by usnic acid and psoromic acid with un-competitive and mixed type inhibition respectively. Psoromic acid showed a very poor zone of hydrolysis of fibrinogen (0.8 cm² at 100 µg) compared to the positive control plasmin which showed hydrolysis of fibrinogen 1.6 cm² at 12.5 µg.
Recent work of literature also found psoromic acid as a phytochemical in the Canna indica rhizome extract[6]. The acetone extract of the rhizome exhibited scavenging activity against DPPH radical, 2,2′-Azino-Bis-3-ethylbenzthiazoline-6-Sulphonic acid (ABTS) cation radical and superoxide anion radical with IC50 values 21, 23 and 170 µg/ml, respectively compared to one of the positive controls BHA which inhibited those radicals with IC50 7, 12 and 164 µg/ml respectively. Further, the extract showed biomolecular protection activities. The extract was found to have psoromic acid as one of the major components analyzed through High Resolution Liquid Chromatograph tandem Mass Spectrometery (HRLC-MS/MS). Psoromic acid was also studied among other phenolic natural compounds for its α-amylase and α-glycosidase inhibition potential in in vitro enzyme inhibition assays envisaging its possible role as an antidiabetic agent[19]. Honda et al. worked on the radical scavenging potential of nine different lichen-derived metabolites[20]. Psoromic acid was isolated from lichen Urobatis jamaicensis. The compound was characterized and studied for DPPH radical-scavenging activity. Psoromic acid recorded scavenging activity of half maximal Effective Concentration (EC50) 17.36 mM against 0.1 mM DPPH and showed comparable results with other lichen metabolites such as salazinic acid, hypostictic acid and lichexanthone. Like quercetin and chlorogenic acid, psoromic acid has good bioavailability and polyphenolic structure[21,22]. The antioxidant properties of psoromic acid may lead to its potential applications in nutraceuticals and drug formulations. Further preclinical and clinical studies are necessary for psoromic acid to explore its efficacy and pharmaceutical applications. All the reported biological activities (Table 1) of psoromic acid reveal it as a potential multifunctional drug candidate.
| Biological function/activities | Results/mechanism of action | References |
|---|---|---|
| Apoptotic activities in hepatocytes | API values 21 and 28 for Caspase-3 activation and DNA fragmentation, respectively (Better activity compared to many tested depsides and depsidones) | [11] |
| Cytotoxicity (IC50: 11 µg/ml in 24 h) assessed by LDH release is lower than tested depside compounds | ||
| Antioxidative Properties | Radical scavenging activities with IC50 0.271, 0.21 and 0.174 mg/ml in terms of FRSA, NORSA and LPI activity respectively (Moderate activities compared to the standard:BHA) | [13] |
| EC50 17.36 mM against 0.1mM DPPH (Moderate activity compared to other depsidones) standard:gallic acid | [20] | |
| Cardiovascular Protection concerning inhibition of HMGR and ACE | Inhibition of ACE: Mixed type Inhibition of HMGR: Competitive type |
[13] |
| Antibacterial activity | Antibacterial activity (MIC 11.72 and 5.86 μg/ml) against S. gordonii (MBC 3000 μg/ml) and P. gingivalis (MBC 11.72 μg/ml) respectively | [14] |
| Strongest activity among the studied lichen-derived compounds | ||
| Antitumor potential; Treatment of Glioblastoma Multiforme (GBM) |
Psoromic acid inhibited PRCC cells with IC50 79.40 mg/l (better activity than olivetoric acid and physodic acid) | [15] |
| Inhibition of U87MG cells with IC50 56.22 mg/l (comparable activity with olivetoric acid) | ||
| Antiviral properties Inhibitory activity against HSV-1, HSV-2 and HSV-1 DNA polymerase |
High inhibition of HSV-1 (IC50: 1.9µM) and HSV-2 (EC50 2.7µM) | [16] |
| Better activity compared to the standard drug: Acyclovir | ||
| Competitive inhibition of HSV-1 DNA polymerase with IC50: 0.7µM. | ||
| Better activity compared to the reference drugs aphidicolin and acyclovir triphosphate | ||
| Selective inhibition of RabGGTase (Anticancer potential) | Strong inhibition of RabGGTase (400 nM) with IC50 1.4 μM | [18] |
| Selective inhibition of RabGGTase over FTase and GGTase-I | ||
| Competitive type of inhibition of RabGGTase | ||
| Depsidones having psoromic acid core structure also displayed appreciable inhibitory activity | ||
| α-amylase and α-glucosidase inhibition activity | IC50 416.96 nM and 443.11 nM for inhibition of α-amylase and α-glucosidase respectively (Comparable activity with usnic acid) | [19] |
| Gastroprotective activity | Psoromic acid dose of 30 mg/kg induces 65 % inhibition of gastric lesion index in HCl/ethanol-induced gastric lesions in mice | [23] |
| Comparable result with the standard: lansoprazole | ||
| Antiparasitic activities | Inhibition (IC50 31.6 μM) of liver stage parasites of P. berghei (Moderate activity compared to usnic acid) | [29] |
| Inhibition of plasmodial FAS-II enzymes PfFabI, PfFabG and PfFabZ with IC50 71.4, 183 and 35.2 μM respectively | ||
| Glutathione-S-transferase inhibition by psoromic acid (IC50 16.90 µM) Comparable activity with usnic acid | [19] | |
| Antimycobacterial activities | Inhibition of nine strains of M. tuberculosis with psoromic acid MIC range 3.2 to 4.1 μM and selectivity indices ranging between 18.3 and 23.4 Comparable activity with the standard drug:Isoniazid 85.8 % inhibition of UGM at 20 mM psoromic acid Comparable result with the standard:UDP Inhibition of TBNAT with IC50 8.7µM psoromic acid Comparable result with the standard drug:Isoniazid |
[17] |
| Inhibition activity (MIC 122.9 μM) against M. tuberculosis (Moderate activity compared to usnic acid) | [29] | |
| Antiviral response against cytomegalovirus (MCMV-GFP) | Strong inhibition of MCMV-GFP | [33] |
| Better potential than 25-hydroxycholesterol | ||
| Pre-mRNA splicing inhibition | IC50: 56 µM for inhibition of pre-mRNA splicing | [37] |
| Comparable activity with norstictic acid |
Table 1: Reported Biological Activities and Therapeutic Potentials of Psoromic Acid.
Gastroprotective Activities
Sepulveda et al. worked on the effectiveness of selected lichen metabolites for gastroprotective activities[23]. The compounds such as lobaric acid, atranorin, psoromic acid, variolaric acid, diffractaic acid and perlatolic acid were extracted from different lichen species and studied for their action to prevent gastric ulcers on the HCl/ethanol-induced gastric lesion model mice. In the experiment, the above six lichen compounds were orally administered to the mice groups at the time of gastric lesion induction. Lansoprazole and Tween 80 were taken as the positive and negative control, respectively. Potential reduction in the gastric lesions was observed by lobaric acid (76 % inhibition), psoromic acid (65 % inhibition) atranorin (63 % inhibition) at 30 mg/kg. The lesion index for the three compounds was observed to be 8.42, 12.86 and 13.57 mm respectively. Their activities were comparable to positive control lansoprazole (69 % inhibition, lesion index: 11.14 mm). HCl/ethanol causes hemorrhages, acute tissue damage, oxidative damage, generation of ROS and other complications[24]. Thus, by inhibiting those effects, the lichen metabolites envisaged an important pharmaceutical application. The gastric lesion inhibition activity by psoromic acid was found to have comparable results with metabolites such as epitaondiol, sargaol, quercetin, rutin, chalcone compounds and other flavonoid compounds studied earlier[25,26]. The gastroprotective activities of flavonoids were linked with the antioxidant properties (inhibition lipid peroxidation, enhancement of superoxide dismutase activities and reduction of protein carbonyl compounds)[25]. Hence the antioxidant character of the molecule psoromic acid could promote the healing of gastric ulceration.
Antibacterial Activities
As bacterial resistance has been reducing the effect of many antibiotics, searching for a new class of compounds of natural origin with combined effects of antioxidant and antibacterial properties is challenging these days.Many flavonoid compounds and their synthetic derivatives have been studied to have antibacterial activities[27,28]. However, the search of a new class of natural compounds is still an ongoing research interest. Sweidan et al. worked on several depsidone compounds for their antibacterial properties[14]. Psoromic acid, extracted from lichen Squamarina cartilaginea, showed the best result with the lowest Minimum Inhibitory Concentration (MIC) of 11.72 and 5.86 μg/ml against the bacteria Streptococcus gordonii DL1(Minimum Bactericidal Concentration (MBC) 3000 μg/ml)and Porphyromonas gingivalis ATCC 33277(MBC 11.72 μg/ml) respectively. The results revealed psoromic acid as a potential new candidate molecule among the other lichen metabolites against oral pathogens without cytotoxicity against gingival epithelial carcinoma cells. Psoromic acid showed significant antibacterial activity, indicating the importance of lichen metabolites in designing potential analogous compounds against multidrug-resistant bacteria.
Antiparasitic Activities
The anti-plasmodial activity and prophylactic potential were reported for few lichen metabolites. Lauinger et al. studied four lichen metabolites such as evernic acid, vulpic acid, psoromic acid, and (+)-usnic acid against the liver stage of the malaria parasite of Plasmodium berghei (P. berghei) and selected pathogens (Staphylococcus aureus, Escherichia coli, and Mycobacterium tuberculosis(M. tuberculosis)[29]. The lichen metabolites usnic acid and psoromic acid recorded high malaria prophylactic potential. Usnic acid showed the highest liver stage activity and stage specificity, followed by psoromic acid. Psoromic acid significantly inhibited (IC50 31.6 Μm) liver-stage parasites of P. berghei and showed strong inhibition of Plasmodial FAS-II Enzymes and M. tuberculosis (Table 1). The significant in vitro glutathione-S-transferase inhibition by psoromic acid (IC50 16.90 µM) among other natural antioxidant compounds was reported, which revealed the molecule as a potential antiparasitic agent[19].
Antimycobacterial Activities
The antimycobacterial study by Hassan et al. reported the effect of psoromic acid on M. tuberculosis strains which affects alveolar macrophages leading to tuberculosis[17]. Nine strains of M. tuberculosis were tested with psoromic acid and another standard drug isoniazid. The results showed that the antimycobacterial activity (in terms of MIC) of psoromic acid was in the range 3.2 to 4.1 Μm (selectivity indices range 18.3-23.4) whereas that for the isoniazid was in the range 5.4 to 5.8 Μm (selectivity indices range 13.0-13.9). Psoromic acid lowered the MIC significantly and showed and no cytotoxicity up to 75 mM on the human liver hepatocellular carcinoma cell line. Further, in the antimycobacterial experiment, the target enzymes for inhibition studies were Uridine-5’-Diphosphate (UDP)-Galactopyranose Mutase (UGM) and arylamine-N-acetyltransferase (TBNAT). Psoromic acid at 20 mM exhibited 85.8 % anti-UGM activity compared to 99.3 % inhibition by the standard inhibitor UDP. As a profound UGM inhibitor, psoromic acid can significantly block mycobacterial growth. Psoromic acid also inhibited TBNAT with IC50 value 8.7 µM (77.4 % inhibition), showing comparable results with the standard isoniazid (IC50 6.2 µM and 96.3 % inhibition). In molecular docking studies (Autodock VINA software) with UGM (PDB code: 4RPJ) active sites, the docking scores (binding affinities) for psoromic acid and UDP were -7.4 and -6.2 kcal/mol, respectively. The binding affinity of psoromic acid with the active pocket of TBNAT (PDB code: 4BGF) was found to be -7.6 kcal/mol. The docking study showed significant H-bonding in the active pockets of UGM and TBNAT. Both the enzymes were significantly inhibited by psoromic acid and the molecular docking result supported the interaction mechanism, the psoromic acid may be considered as a potential inhibitor to target M. tuberculosis. It could be a better alternative to drugs such as isoniazid, dapsone and sulfamethazine.
Antiviral Activities
The Herpes Simplex Virus type 1 (HSV-1) is associated with facial infection and encephalitis, whereas HSV type-2 (HSV-2) can cause genital infection[30]. HSV is involved in many ocular diseases, such as endotheliitis, neurotrophic keratopathy, and stromal keratitis. HSV enters the host's nerve cells and affects the cell during the immunity deficiency. In this context, the Herpesvirus Deoxyribonucleic Acid (DNA) polymerases, which have a significant role during the viral replication cycle, have been recognized as the primary targets for antiherpetic drug development[31]. The commercially available drug acyclovir and other related nucleoside analogs are being used to target HSV DNA polymerases. However, the acyclovir-resistant HSV has been noticed, particularly among bone marrow transplant patients and human immunodeficiency virus patients[16,32]. The natural compounds having a lower occurrence of resistance and lower adverse effects are of increasing interest for controlling HSV growth.
Hassan et al., studied the antiviral properties of psoromic acid by experimenting with its inhibitory potential against HSV-1 and HSV-2, and HSV-1 DNA polymerase[16]. A plaque reduction assay was performed with infected Vero cells with HSV-1. Psoromic acid showed higher anti-HSV-1 activity (IC50: 1.9 µM) with a selectivity index of 163.2 compared to the drug acyclovir (IC50: 2.6 µM and selectivity index 119.2). Psoromic acid when combined with acyclovir even showed the most potent antiviral activity against HSV-1 (IC50: 1.1 µM and selectivity index 281.8). The antiviral activity of psoromic acid against HSV-2 was tested through the titer reduction (cytopathic end-point assay) method with HSV-2 infected in Vero cells. Psoromic acid inhibited HSV-2 with EC50 2.7 µM and a selectivity index 114.8 whereas the drug acyclovir exhibited EC50 2.8 µM and a selectivity index 110.7. Even the anti-HSV-2 activity enhanced when psoromic acid was combined with acyclovir (EC50: 1.8 µM and selectivity index 172.2). Psoromic acid competitively inhibited HSV-1 DNA polymerase with IC50: 0.7 µM and showed better inhibition potential than the standard inhibitors aphidicolin (IC50: 0.8 µM) and acyclovir triphosphate (IC50: 0.9 µM). In this study, psoromic acid was found to have low cytotoxicity and remarkable antiherpetic activities.
Blanc et al.[33] studied the antiviral response of 25-hydroxycholesterol in an experiment where psoromic acid was also used as a control. Psoromic acid was tested to have a high antiviral response against the murine cytomegalovirus (MCMV-GFP) as it inhibited the viral growth infection with more potent than the human metabolite 25-hydroxycholesterol. Many studies reported the antiviral effects of naturally occurring metabolites such as apigenin, luteolin, quercetin, catechin derivatives, kaempferol and rutin against a range of viruses[34]. But efficacy and bioavailability of the antiviral agents in the human body need to be enhanced. As psoromic acid was found to have shown better results than the standard in certain cases, its efficacy needs to be tested in next-level preclinical experiments.
Anticancer and Antitumor Activities
Emsen et al., studied the in vitro action of lichen-derived metabolites such as olivetoric acid, physodic acid and psoromic acid on high-grade glioma tumors, Glioblastoma Multiforme (GBM) cell lines and Primary Rat Cerebral Cortex (PRCC) cells[15]. As GBM occurs in the nervous system and is a grade IV tumor, it affects the central nervous system resulting in headaches, dizziness, speech disorders, loss of sensation and visual disturbances[35,36]. The search for natural or plant-based products having a therapeutic role is the major challenge in treating diseases to avoid the side effects of chemotherapy and radiotherapy[15]. Psoromic acid was extracted from lichen Rhizoplaca melanophthalma. The lichen Pseudevernia furfuracea (L.) was the source for the isolation of olivetoric acid and physodic acid. All these metabolites were tested for their biological activities on human brain GBM cell line U87MG and PRCC cells from rats. Physodic acid showed the highest total antioxidant activity in PRCC cells followed by psoromic acid, whereas psoromic acid showed the highest total antioxidant activity in the U87MG cell line. Olivetoric acid showed the highest cytotoxic activity and highest oxidative DNA damage in U87MG cells. All three metabolites have almost the same genotoxicity effect on the PRCC cells. However, psoromic acid exhibited the highest antiproliferative activity on PRCC cells (IC50 79.40 mg/l). It also inhibited U87MG cells (IC50 56.22 mg/l), indicating its better overall activities compared to the other two metabolites olivetoric acid (IC50 125.71 and 17.55 mg/l for two cells, respectively) and physodic acid (IC50 698.19 and 410.72 mg/l for two cells respectively). The total oxidant status of both cells was decreased after treatment with all three metabolites, wherein psoromic acid had a pronounced effect on PRCC cells. The cytotoxicity and genotoxicity effects of all three metabolites were concentration-dependent. The binary correlation analysis showed decreased viable cell numbers in both PRCC and U87MG cells after treatment of the three lichen-derived metabolites, but the effects of olivetoric acid and psoromic acid was more pronounced. The apoptotic effect of psoromic acid along with many other lichen-derived depsides and depsidones was analyzed by Correche et al in terms of caspase-3 activation and DNA fragmentation analysis[11]. Psoromic acid showed Apoptotic Potential Index (API) 21 and 28 for Caspase-3 activation and DNA fragmentation respectively at an effective cytotoxic concentration 10µg/ml (Table 1). The significant apoptotic activities shown by psoromic acid were comparable with other tested lichen metabolites such as salazinic acid and stictic acid. Along with a good apoptotic effect, psoromic acid also showed low cytotoxicity when analyzed in terms of the Lactate Dehydrogenase (LDH) release from the hepatocytes (Table 1).
Misregulation or disruption of alternative and constitutive splicing can cause cancer, neurodegenerative and autoimmune diseases in humans. Samatov et al. studied the lichen secondary metabolite psoromic acid in an in vitro high-throughput splicing assay using HeLa nuclear extract[37]. They reported psoromic acid as a small molecule pre-m-RNA splicing inhibitor with IC50 56 µM, having comparable results with norstictic acid (IC50 28 µM )[37]. Complete inhibition of splicing was observed at 250 mM psoromic acid and 100 mM norstictic acid. It was reported that the 3-aldehyde group and 4-hydroxy group positions in psoromic acid favored inhibition activities. The structural feature of psoromic acid is envisaged to be the framework for synthesizing many derivative compounds for studying inhibitory activities, and their mechanistic interactions.
The natural compound luteolin has been extensively reported for its apoptosis activity and cell cycle arrest in various cancer cells, which again has advanced its anticancer studies at the molecular level as well as in encapsulated nanoparticle form[38,39]. The antioxidant and apoptotic activity of psoromic acid may lead to its anti-carcinogenic effect due to phenolic and depsidone core structure, in a similar approach as of the studied bioactive flavonoids. The polyphenolic nature and health-beneficial properties of psoromic acid could be advantageous for further clinical studies following other polyphenols like curcumin, indole-3-carbinol, 3,3-diindolymethane and tea polyphenols, which have been in clinical trials for safety and efficacy in preventing cancer[40].
Inhibition of Rab Geranylgeranyl Transferase (RabGGTase):
Rab Geranylgeranyl Transferase (RabGGTase) is the protein that mediate the prenylation of Rab proteins. Rab GTPases are such enzymes that regulate specific intracellular membrane trafficking and recruit specific effector proteins that are responsible for vesicular transport. However, they do not contain a specific recognition sequence for prenylation. Prenylation of geranylgeranyl isoprenoids to Rab GTPases is necessary for its effective function[41]. The prenylation is mediated by RabGGTase. Overexpression of RabGGTase and a few other Rab proteins like Rab25 has been reported in several cancer types and genetic diseases. Prenylation is essential for the function of many oncogenic Rab proteins and therefore, the catalytic enzyme could be a target for drug design and development[18,42]. Recent works of literature have shown that apoptosis induction in tumor cells occurs due to the inhibition of RabGGTase. Thus, the enzyme can be a potential target for cancer chemotherapy. Phosphonocarboxylate, a specific RabGGTase inhibitor, has been a lead drug molecule for antitumor therapy and treatment of thrombotic disorders and osteoporosis. As RabGGTase has structural and functional similarities with many other protein prenyltransferases, developing RabGGTase inhibitors has been a great challenge[43]. A potent and selective RabGGTase inhibitor could be a possible drug candidate.
Deraeve et al. studied in vitro prenylation of Rab protein and Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) assay to evaluate the selectivity of psoromic acid as an inhibitor of RabGGTase and other related enzymes[18]. NBD-Farnesyl Pyrophosphate (NBD-FPP), a fluorescent analog of the lipid substrate geranylgeranyl pyrophosphate was prenylated to Rab protein and was detected by the change of fluorescence. Competitive type inhibition of RabGGTase was observed by psoromic acid inhibitor as recorded in the fluorometric assay. Psoromic acid inhibited RabGGTase (400 Nm) with IC50 1.4 Μm selectively over other enzymes Ftase and GGTase-I. Though many structural analogs of psoromic acid were synthesized by changing different substituents to test the inhibition activities, none were as potent as the psoromic acid. The in silico molecular docking study of a complex of psoromic acid and apo-RabGGTase (PDB Id: 1DCE) showed that psoromic acid covalently bonded to the N-terminal amine of the RabGGTase α subunit and associated with the lipid-binding site. The major hydrophobic interactions were of the two phenyl rings with the residues βTrp244 and βPhe289. The docking results led to a proposed drug model of a minimal structure of depsidone core and a 3-hydroxyl and 4-aldehyde motif for selective inhibition of RabGGTase.
Synthetic compounds such as phosphonopropionate derivatives, phosphonocarboxylates were reported to have inhibited the prenylation of RabGGTase[42,44]. A peptide-based synthetic RabGGTase inhibitor was also studied to have shown selective interaction with the enzyme[45]. By looking at the natural origin compounds, significant development has been made using the small molecule psoromic acid. Hence studies on the efficacy of psoromic acid in selective inhibition of RabGGTase prenylation need further preclinical and clinical research.
Conclusion
From the works of relevant literature, it was found that psoromic acid has high antioxidant and radical scavenging activities that may lead to its protective effect on cardiovascular and other physiological components. It has shown a significant gastroprotective effect in HCl/ethanol-induced mice. The depsidone core structure may have a pivotal role in its beneficial bioactivities. Significant inhibition of UGM, TBNAT, HGMR, ACE and M. tuberculosis indicated its effectiveness against tuberculosis disease. The antiviral properties were produced by inhibiting the herpes simplex virus and MCMV-GFP. Most significantly, psoromic acid as a small molecule inhibitor of RabGGTase among the reported inhibitors, could be the best candidate molecule for the study of cancer chemotherapy due to its natural origin. The potent antitumor activity through the inhibition of PRCC and U87MG cells, apoptotic activities, inhibition of pre-mRNA splicing and selective inhibition of RabGGTase suggested further research on mechanisms of action of psoromic acid core structure and for the synthesis and designing of safe and effective anticancer drugs. Further pharmacokinetic and in vivo studies with clinical phenotypes may pave the way for pharmacological and medicinal acceptance of psoromic acid as a potential antimicrobial and anticancer drug molecule.
Conflict of interest:
Authors declare that there is no conflict of interest.
References
- Harvey AL. Natural products in drug discovery. Drug Discov Today 2008;13(19-20):894-901.
[Crossref] [Google Scholar] [PubMed]
- Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981− 2002. J Nat Prod 2003;66(7):1022-37.
[Crossref] [Google Scholar] [PubMed]
- Cornblatt BS, Ye L, Dinkova-Kostova AT, Erb M, Fahey JW, Singh NK, et al. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis 2007;28(7):1485-90.
[Crossref] [Google Scholar] [PubMed]
- Kingston DG, Newman DJ. Natural products as anticancer agents. Nat Prod Chem Biol 2012:325-49.
- Lotito SB, Frei B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon. Free Radic Biol Med 2006;41(12):1727-46.
[Crossref] [Google Scholar] [PubMed]
- Ayusman S, Duraivadivel P, Gowtham HG, Sharma S, Hariprasad P. Bioactive constituents, vitamin analysis, antioxidant capacity and α-glucosidase inhibition of Canna indica L. rhizome extracts. Food Biosci 2020;35:100544.
- Shukla V, Joshi GP, Rawat MS. Lichens as a potential natural source of bioactive compounds: A review. Phytochem Rev 2010;9:303-14.
- Korkmaz AI, Akgul H, Sevindik M, Selamoglu Z. Study on determination of bioactive potentials of certain lichens. Acta Alimentaria 2018;47(1):80-7.
- Tuovinen V, Ekman S, Thor G, Vanderpool D, Spribille T, Johannesson H. Two basidiomycete fungi in the cortex of wolf lichens. Curr Biol 2019;29(3):476-83.
[Crossref] [Google Scholar] [PubMed]
- Müller K. Pharmaceutically relevant metabolites from lichens. Appl Microbiol Biotechnol 2001;56:9-16.
[Crossref] [Google Scholar] [PubMed]
- Correché ER, Enriz RD, Piovano M, Garbarino J, Gómez-Lechón MJ. Cytotoxic and apoptotic effects on hepatocytes of secondary metabolites obtained from lichens. Altern Lab Anim 2004;32(6):605-15.
[Crossref] [Google Scholar] [PubMed]
- Schmitt I, Lumbsch HT. Molecular phylogeny of the Pertusariaceae supports secondary chemistry as an important systematic character set in lichen-forming ascomycetes. Mol Phylogenet Evol 2004;33(1):43-55.
[Crossref] [Google Scholar] [PubMed]
- Behera BC, Mahadik N, Morey M. Antioxidative and cardiovascular-protective activities of metabolite usnic acid and psoromic acid produced by lichen species Usnea complanata under submerged fermentation. Pharm Biol 2012;50(8):968-79.
[Crossref] [Google Scholar] [PubMed]
- Sweidan A, Chollet-Krugler M, Sauvager A, van de Weghe P, Chokr A, Bonnaure-Mallet M, et al. Antibacterial activities of natural lichen compounds against Streptococcus gordonii and Porphyromonas gingivalis. Fitoterapia 2017;121:164-9.
[Crossref] [Google Scholar] [PubMed]
- Emsen B, Aslan A, Togar B, Turkez H. In vitro antitumor activities of the lichen compounds olivetoric, physodic and psoromic acid in rat neuron and glioblastoma cells. Pharm Biol 2016;54(9):1748-62.
[Crossref] [Google Scholar] [PubMed]
- Hassan ST, Šudomová M, Berchová-Bímová K, Šmejkal K, Echeverría J. Psoromic acid, a lichen-derived molecule, inhibits the replication of HSV-1 and HSV-2, and inactivates HSV-1 DNA polymerase: Shedding light on antiherpetic properties. Molecules 2019;24(16):2912.
[Crossref] [Google Scholar] [PubMed]
- Hassan ST, Šudomová M, Berchová-Bímová K, Gowrishankar S, Rengasamy KR. Antimycobacterial, enzyme inhibition, and molecular interaction studies of psoromic acid in Mycobacterium tuberculosis: Efficacy and safety investigations. J Clin Med 2018;7(8):226.
[Crossref] [Google Scholar] [PubMed]
- Deraeve C, Guo Z, Bon RS, Blankenfeldt W, DiLucrezia R, Wolf A, et al. Psoromic acid is a selective and covalent Rab-prenylation inhibitor targeting autoinhibited RabGGTase. J Am Chem Soc 2012;134(17):7384-91.
[Crossref] [Google Scholar] [PubMed]
- Gulçin İ, Taslimi P, Aygün A, Sadeghian N, Bastem E, Kufrevioglu OI, et al. Antidiabetic and antiparasitic potentials: Inhibition effects of some natural antioxidant compounds on α-glycosidase, α-amylase and human glutathione S-transferase enzymes. Int J Biol Macromol 2018;119:741-6.
- Honda NK, Lopes TI, da Silva Costa RC, Coelho RG, Yoshida NC, Rivarola CR, et al. Spielmann AA. Radical-scavenging potential of phenolic compounds from Brazilian lichens. Orbital 2015;7(2):99-107.
- Santana-Gálvez J, Cisneros-Zevallos L, Jacobo-Velázquez DA. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017;22(3):358.
[Crossref] [Google Scholar] [PubMed]
- Yang D, Wang T, Long M, Li P. Quercetin: Its main pharmacological activity and potential application in clinical medicine. Oxid Med Cell Longev 2020;2020:8825387.
[Crossref] [Google Scholar] [PubMed]
- Sepulveda B, Chamy MC, Piovano M, Areche C. Lichens: Might be considered as a source of gastroprotective molecules. J Chil Chem Soc 2013;58(2):1750-2.
- Olaleye SB, Farombi EO. Attenuation of indomethacin‐and HCl/ethanol‐induced oxidative gastric mucosa damage in rats by kolaviron, a natural biflavonoid of Garcinia kola seed. Phytother Res 2006;20(1):14-20.
[Crossref] [Google Scholar] [PubMed]
- de Lira Mota KS, Dias GE, Pinto ME, Luiz-Ferreira Â, Monteiro Souza-Brito AR, Hiruma-Lima CA, et al. Flavonoids with gastroprotective activity. Molecules 2009;14(3):979-1012.
[Crossref] [Google Scholar] [PubMed]
- Areche C, San-Martín A, Rovinosa J, Sepúlveda B. Gastroprotective activity of epitaondiol and sargaol. Nat Prod Commun 2011;6(8):1934578X1100600805.
[Crossref] [Google Scholar] [PubMed]
- Martelli G, Giacomini D. Antibacterial and antioxidant activities for natural and synthetic dual-active compounds. Eur J Med Chem 2018;158:91-105.
[Crossref] [Google Scholar] [PubMed]
- Farhadi F, Khameneh B, Iranshahi M, Iranshahy M. Antibacterial activity of flavonoids and their structure–activity relationship: An update review. Phytother Res 2019;33(1):13-40.
[Crossref] [Google Scholar] [PubMed]
- Lauinger IL, Vivas L, Perozzo R, Stairiker C, Tarun A, Zloh M, et al. Potential of lichen secondary metabolites against Plasmodium liver stage parasites with FAS-II as the potential target. J Nat Prod 2013;76(6):1064-70.
[Crossref] [Google Scholar] [PubMed]
- Johnston C, Corey L. Current concepts for genital herpes simplex virus infection: Diagnostics and pathogenesis of genital tract shedding. Clin Microbiol Rev 2016;29(1):149-61.
[Crossref] [Google Scholar] [PubMed]
- Zarrouk K, Piret J, Boivin G. Herpesvirus DNA polymerases: Structures, functions and inhibitors. Virus Res 2017;234:177-92.
[Crossref] [Google Scholar] [PubMed]
- Morfin F, Thouvenot D. Herpes simplex virus resistance to antiviral drugs. J Clin Virol 2003;26(1):29-37.
[Crossref] [Google Scholar] [PubMed]
- Blanc M, Hsieh WY, Robertson KA, Kropp KA, Forster T, Shui G, et al. The transcription factor STAT-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity 2013;38(1):106-18.
[Crossref] [Google Scholar] [PubMed]
- Zakaryan H, Arabyan E, Oo A, Zandi K. Flavonoids: Promising natural compounds against viral infections. Arch Virol 2017;162:2539-51.
[Crossref] [Google Scholar] [PubMed]
- Zhang X, Zhang WE, Cao WD, Cheng G, Zhang YQ. Glioblastoma multiforme: Molecular characterization and current treatment strategy. Exp Ther Med 2012;3(1):9-14.
[Crossref] [Google Scholar] [PubMed]
- Urbańska K, Sokołowska J, Szmidt M, Sysa P. Glioblastoma multiforme: An overview. Contemp Oncol 2014;18(5):307-12.
[Crossref] [Google Scholar] [PubMed]
- Samatov TR, Wolf A, Odenwälder P, Bessonov S, Deraeve C, Bon RS, et al. Psoromic acid derivatives: A new family of small‐molecule pre‐mRNA splicing inhibitors discovered by a stage‐specific high‐throughput in vitro splicing assay. Chembiochem 2012;13(5):640-4.
[Crossref] [Google Scholar] [PubMed]
- Kang KA, Piao MJ, Hyun YJ, Zhen AX, Cho SJ, Ahn MJ, et al. Luteolin promotes apoptotic cell death via upregulation of Nrf2 expression by DNA demethylase and the interaction of Nrf2 with p53 in human colon cancer cells. Exp Mol Med 2019;51(4):1-4.
[Crossref] [Google Scholar] [PubMed]
- Kollur SP, Prasad SK, Pradeep S, Veerapur R, Patil SS, Amachawadi RG, et al. Luteolin-fabricated ZnO nanostructures showed PLK-1 mediated anti-breast cancer activity. Biomolecules 2021;11(3):385.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Jiang YF. Natural compounds as anticancer agents: Experimental evidence. World J Exp Med 2012;2(3):45.
[Crossref] [Google Scholar] [PubMed]
- Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG. Post-translational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat Rev Drug Discov 2007;6(7):541-55.
[Crossref] [Google Scholar] [PubMed]
- Coxon FP, Helfrich MH, Larijani B, Muzylak M, Dunford JE, Marshall D, et al. Identification of a novel phosphonocarboxylate inhibitor of Rab geranylgeranyl transferase that specifically prevents Rab prenylation in osteoclasts and macrophages. J Biol Chem 2001;276(51):48213-22.
[Crossref] [Google Scholar] [PubMed]
- Stigter EA, Guo Z, Bon RS, Wu YW, Choidas A, Wolf A, et al. Development of selective, potent RabGGTase inhibitors. J Med Chem 2012;55(19):8330-40.
[Crossref] [Google Scholar] [PubMed]
- Kusy D, Marchwicka A, Małolepsza J, Justyna K, Gendaszewska-Darmach E, Błażewska KM. Synthesis of the 6-Substituted Imidazo [1, 2-a] Pyridine-3-Yl-2-Phosphonopropionic Acids as Potential inhibitors of rab geranylgeranyl transferase. Front Chem 2021;8:596162.
[Crossref] [Google Scholar] [PubMed]
- Tan KT, Guiu-Rozas E, Bon RS, Guo Z, Delon C, Wetzel S, et al. Design, synthesis, and characterization of peptide-based rab geranylgeranyl transferase inhibitors. J Med Chem 2009;52(24):8025-37.
[Crossref] [Google Scholar] [PubMed]