- *Corresponding Author:
- S. G. Yang
World Class Smart Lab, Department of New Drug Development, College of Medicine, Inha University, B-308, Chungsuk Bldg, 366, Seohae-Daero, Jung-Gu, Incheon 22332, Republic of Korea
E-mail: Sugeun.Yang@Inha.ac.kr
| Date of Submission | 27 May 2017 |
| Date of Revision | 11 January 2018 |
| Date of Acceptance | 28 July 2018 |
| Indian J Pharm Sci 2018;80(5):837-843 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Recombinant parathyroid hormone (1-34), the drug of choice for treating severe osteoporosis, has a short half-life and requires daily subcutaneous injections. Controlled release formulation of recombinant parathyroid hormone (1-34) might prevent daily injections and improve therapeutic outcome. In this study, recombinant parathyroid hormone (1-34)-loaded poly(D,L-lactic-co-glycolic acid) microspheres using a double emulsion method were prepared. Scanning electron microscopy proved that the microspheres were spherical in shape with 2.0 to 5.0 µm diameter. A loading efficiency up to 84 % was achieved in the optimized formulation. Release study performed using microspheres of 10:1.0 polymer:drug ratio formulation revealed that the release of recombinant parathyroid hormone (1-34) was controlled over 22 days in a biphasic manner with an initial burst and a subsequent slow release. For pharmacokinetic study, recombinant parathyroid hormone (1-34)-loaded poly(D,L-lactic-co-glycolic acid) microspheres were subcutaneously injected to rats at 0.01 mg/kg dose of recombinant parathyroid hormone (1-34). Plasma drug concentration of recombinant parathyroid hormone (1-34)-loaded poly(D,L-lactic-co-glycolic acid) microspheres were maintained for a week whereas free recombinant parathyroid hormone (1-34) was quickly eliminated within a day. These results suggest that recombinant parathyroid hormone (1-34)-loaded poly(D,L-lactic-co-glycolic acid) microspheres appear to have the potential for further clinical development.
Keywords
Recombinant parathyroid hormone (1-34), poly (lactic-co-glycolic acid) (PLGA), microsphere, sustained release, osteoporosis
Osteoporosis is a progressive metabolic bone disease, which decreases the bone mass and deteriorates the microstructure of the bone tissue, increasing the vulnerability of the bone to risk of fracture [1]. Certainly, various treatments have studied for osteoporosis to improve bone density include oestrogen hormone replacement therapy, calcitonin, and bisphosphonate [2]. A recombinant parathyroid hormone (rhPTH 1-34) is the last drug of choice for the treatment of osteoporosis for patients for whom all other drug regimens have failed [3]. It has advantages of activating osteoblasts as an anabolic agent and inhibiting osteoclasts simultaneously [4]. However, rhPTH 1-34 exhibited rapid elimination with a short half-life after subcutaneous injection in the body and requires daily injections in clinical practice [5]. Thus, a new strategy to prolong the therapeutic effect of rhPTH 1-34 is required in patients to avoid repeated injections [6]. A sustained release system for rhPTH 1-34 could improve compliance in osteoporosis patients.
Poly(D,L-lactic-co-glycolic acid) (PLGA)-based microsphere have been studied as one of the materials for controlled release of therapeutic peptides and proteins [7-10]. Although numerous polymers have been tested for the development of controlled release formulation of those peptides and proteins during the last decades, PLGA has been approved polymer for the clinical formulations due to its favorable biocompatibility and biodegradability [7,8,11,12]. Many fabrication techniques have been developed for the efficient microencapsulation of peptides and proteins such as double or multiple emulsions [9], organic phase separation [13], supercritical fluid [14] and spray drying [15]. Especially, for the encapsulation of peptides or proteins, double emulsion (W1/O/W2) method is the most favored to achieve the enhanced loading efficiency and reliable controlled release of drug [9,16-19]. Nafea et al. have studied the PLGA microspheres (MPs) with high incorporation efficiency [20] and Zhao et al. reported the fabrication of magnetic and non-magnetic MPs of PLGA using the W/O/W double emulsion solvent evaporation technique including size control of the MPs [21]. However, this method also required attention to solve problems such as erratic solubility, stability and permeability.
In the current investigation, formulation design of rhPTH 1-34 releasing PLGA MPs (rhPTH 1-34 PLGA MPs) for once a week injection was attempted. rhPTH 1-34 PLGA MPs were prepared using a double emulsion method. The effect of PLGA viscosity, microsphere size, and formulation ratio of PLGA to rhPTH 1-34 were studied in terms of particle size, loading efficiency, and drug release profile. The size and morphology of rhPTH 1-34 PLGA MPs were evaluated using scanning electron microscopy (SEM). Loading efficiencies and release profiles were analyzed using high-performance liquid chromatography (HPLC). The best candidate formulation for the clinical development was introduced to a pharmacokinetic study. In vivo, pharmacokinetic parameters were analysed in a rat model after single subcutaneous injection of rhPTH 1-34 PLGA MPs at 0.01 mg/kg dose of rhPTH 1-34.
Material and Methods
Poly(D,L-lactide-co-glycolide), with 50:50 ratio of lactide to glycolide copolymers were purchased from Boehringer Ingelheim GmbH (Ingelheim, Germany). Poly(vinyl alcohol) (PVA, 3,070 kDa, 87-90 % hydrolysed) and dichloromethane were purchased from Sigma-Aldrich (Milwaukee, WI, USA). rhPTH 1-34 (4,117.72 Da) was obtained from Bachem AG (Bubendorf, Switzerland). Ultrapure water (Millipore, USA) was used. All reagents were purchased from Sigma-Aldrich (Milwaukee, WI, USA) unless otherwise specified, and used without further purification.
Preparation of rhPTH 1-34-loaded PLGA MPs:
rhPTH 1-34 PLGA MPs were prepared using double emulsion (W1/O/W2) method at mass ratios of 10:0.67, 10:1.0, and 10:1.3 (PLGA to rhPTH 1-34). Each PLGA with different intrinsic viscosity values (0.32~0.44 dl/g and 0.61~0.74 dl/g) was dissolved in dichloromethane to 10 % (w/v). rhPTH 1-34 solution was added into the organic phase and emulsified using a probe-type sonicator (Sonosmasher®, Dongseo Science, Korea) at 15 W for 20 s to obtain primary emulsion. The primary emulsion was gradually added into 16 ml of 4 % PVA (w/v) and then homogenized for 1 min at 5000 rpm (Heidolph Silent Crusher-M Homogenizer, Germany) to prepare the secondary emulsion. After homogenization, the emulsion was vortex-mixed and stirred at room temperature for 3 h to completely evaporate the organic solvent. rhPTH 1-34 PLGA MPs were collected by centrifugation and subsequently washed five times with distilled water. After washing the rhPTH 1-34 PLGA MPs were collected and lyophilized.
SEM observation:
The size and morphology of MPs were observed using a SEM (SNE-4,500M, SEC Co., Suwon, Korea). The prepared MPs were mounted on aluminium stubs using double-sided adhesive tape, sputter-coated with a thin layer of gold under vacuum. The coated specimen was observed under the microscope operated at 5 kV of an acceleration voltage. The particle size of the MPs was measured using the Image J software (NIH, USA).
Differential scanning calorimetry (DSC) analysis:
DSC was performed using a Q100 MDSC system (TA Instrument, Leatherhead, UK). Each sample was precisely weighed and put into an aluminium pan. The pans containing samples were hermetically sealed and loaded to the sample compartment. An empty aluminium sealed pan was loaded to the reference compartment. The pans were equilibrated at 20° for 30 min. Samples were heated at a rate of 5°/min between 27 and 170°. rhPTH 1-34 PLGA MPs were analysed, compared to bulk PLGA, a physical mixture of PLGA and rhPTH 1-34.
Estimation of loading efficiency:
The content of rhPTH 1-34 in PLGA MPs was determined using a HPLC with UV detection. rhPTH 1-34 PLGA MPs were dispersed in 1 M NaOH solution, sonicated to achieve full dissolution and then neutralized with 1 M HCl solution. Samples were filtered using 0.45 μm syringe filter and directly injected into the HPLC system (Alliance 2605 system, Milford, MA, USA) connected to column (Capcell Pak C18 UG120 4.6×250 mm, 5 μm, Shiseido, Japan). The mobile phase was a mixture of water and acetonitrile (0.1 % trifluoroacetic acid, TFA) in a gradient manner of 0/80, 1.5/80, 6.5/45, 7/0, 8/0, 8.5/80 and 20/80 as water to acetonitrile (%, v/v). The flow rate of mobile phase was set at 1 ml/min. The rhPTH 1-34 was detected at 210 nm.
In vitro release study:
rhPTH 1-34 PLGA MPs were suspended in phosphate buffered saline (PBS) at pH 7.4 and incubated at 37° with shaking at 50 rpm. At predetermined time intervals, the samples were centrifuged to collect supernatant, and the collected supernatant was replaced with fresh PBS. The supernatant samples were introduced to a HPLC (H02214 808M, Waters, USA) with a mobile phase containing 0.1 % TFA in MilliQ water and 0.1 % TFA in acetonitrile with a gradient program and flow rate at 1 ml/min with a 210-nm UV detector. The released amount of rhPTH 1-34 from the MPs were plotted as a function of time.
Pharmacokinetics study:
rhPTH 1-34 PLGA MPs and free rhPTH 1-34 were subcutaneously injected to male SD rats (6-7 w, 300-350 g, Orient Bio Co., LTD., Korea) at a dose of 0.01 mg rhPTH 1-34/kg. rhPTH 1-34 PLGA MPs were used the formulation ratio of 10:0.67 (w/w) made of low viscosity-PLGA of 0.32~0.44 dl/g. All animal care and procedures were conducted according to the guides and principles in the use of animal establishment by Inha University. Blood samples were collected from the femoral artery and the retro-orbital sinus at set time points up to seven days. Blood concentration of rhPTH 1-34 were determined using an antihuman PTH (1-34) antibody enzyme-linked immunosorbent assay (ELISA) kit (Immutopics, CA, USA). Plasma concentration versus time plot was constructed, and pharmacokinetic parameters (the area under the curve, AUC; peak time, Tmax; and peak concentration, Cmax) were calculated using WinNonlin software (v. 3.0, Pharsight, USA).
Statistical analysis:
The data are expressed as the mean ± standard deviation (SD, n=3). Differences were tested using unpaired and one-sided t-test. Null hypotheses of no difference were rejected if p-values were less than 0.05.
Results and Discussion
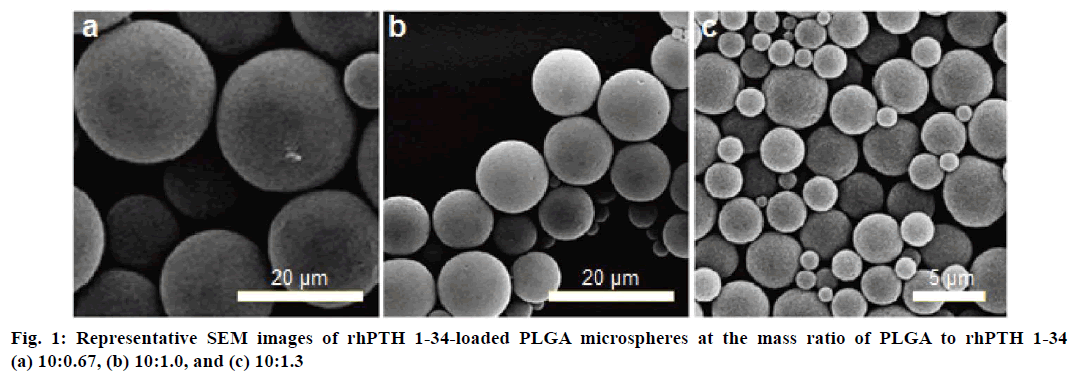
The size and morphology of MPs were observed on a SEM and displayed in Table 1. rhPTH 1-34 PLGA MPs, in all cases, were smooth-surfaced with spherical shape. There was no coalescence of MPs and no irregular shaped particle in all formulations which might affect the release of rhPTH 1-34. Average particle size of MPs with 10:0.67 (polymer:drug) ratio of formulation was 20.8 ± 4.9 μm (Figure 1a). The 10:1.0 and 10:1.3 ratio of formulation showed 13.1 ± 3.9 μm (Figure 1b), and 4.56 ± 1.6 μm (Figure 1c), respectively.
| Formulation ratio (PLGA to rhPTH 1-34, w/w) |
Loading efficiency (%) | |
|---|---|---|
| Low viscosity-PLGA (0.32~0.44 dl/g) |
High viscosity-PLGA (0.61~0.74 dl/g) |
|
| 10:0.67 | 67 ± 1 | 78 ± 12 |
| 10:1.0 | 84 ± 5 | 77 ± 27 |
| 10:1.3 | 67 ± 4 | 84 ± 3 |
Table 1: Loading efficiencies of recombinant parathyroid hormone (1-34) in PLGA microspheres
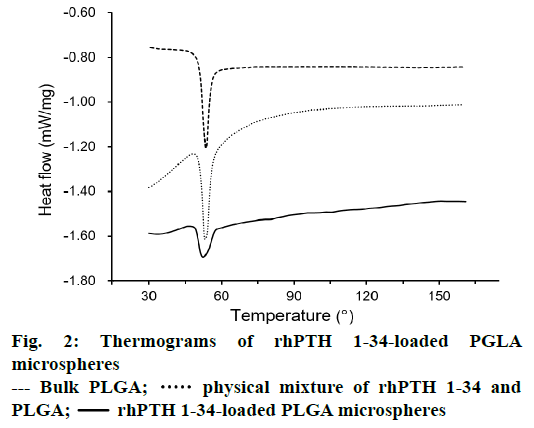
DSC thermograms of PLGA, physical mixture of rhPTH 1-34 and PLGA, and rhPTH 1-34 PLGA MPs were displayed in Figure 2. The result shows the sharp peak of PLGA was obtained at 53°. In physical mixtures of rhPTH 1-34 and PLGA, a sharp PLGA peak also occurred at the same thermal condition. However, the peaks of rhPTH 1-34 and PLGA were smooth and shortened in rhPTH 1-34 PLGA MPs suggesting amorphous states of drug in the MPs.
Table 1 listed the loading efficiencies of rhPTH 1-34 based on viscosity of PLGA and formulation ratios (PLGA to rhPTH 1-34, w/w). Microparticles prepared with PLGA with low viscosity (0.32~0.44 dl/g) showed 67 ± 1, 84 ± 5 and 67 ± 4 % of loading efficiencies by formulation ratios of 10:0.67, 10:1.0, and 10:1.3, respectively. In case of high viscosity, PLGA MPs displayed more predictable tendency of loading efficiency. The 10:0.67 ratio of formulation displayed 78 ± 12 % of loading efficiency and increased up to 84 ± 3 % as the applied amount of drug increased to 10:1.3.
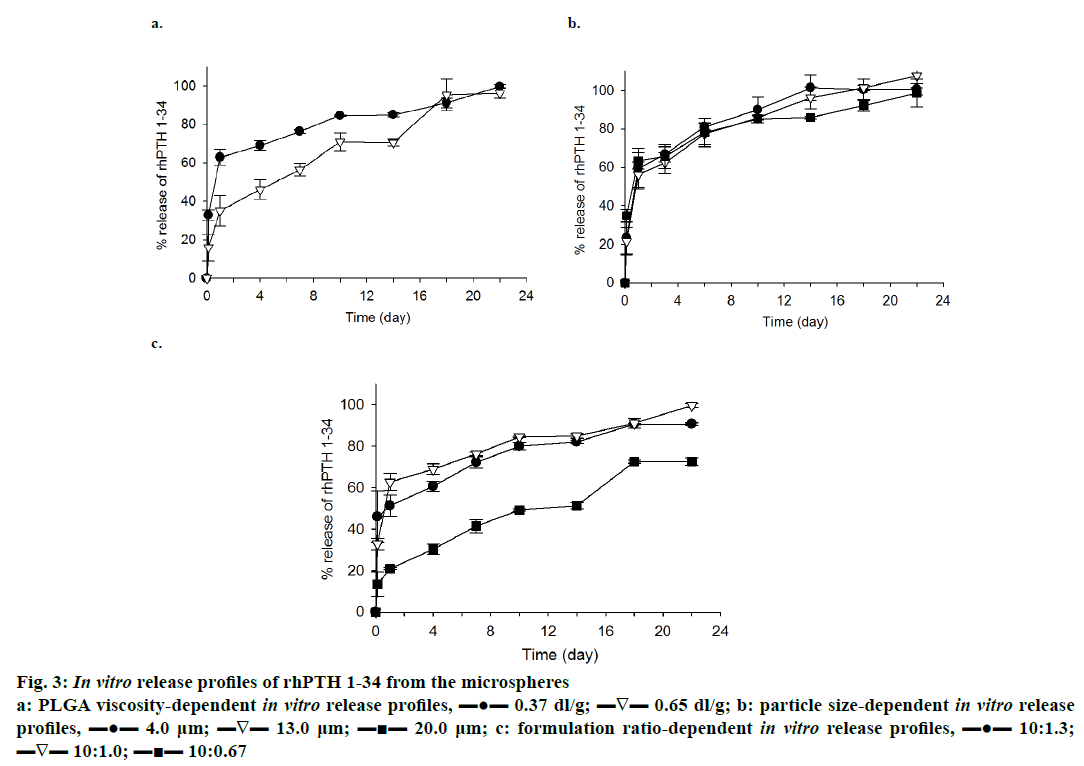
In vitro release profile of rhPTH 1-34 PLGA MPs prepared with different viscosity of PLGA is demonstrated in Figure 3a. The formulation ratio was fixed at 10:1.0, and the size of MPs was set to 4~5 μm. The low viscosity PLGA MPs had a higher release potential of rhPTH 1-34 than the high viscosity PLGA MPs for 18 d of release. Low viscosity PLGA MPs also showed a higher initial burst compared to the high viscosity PLGA MPs. Generally, biphasic release of rhPTH 1-34 with an initial burst on the first day and constant release up to 22 d was observed in both formulations.
Figure 3: In vitro release profiles of rhPTH 1-34 from the microspheres
a: PLGA viscosity-dependent in vitro release profiles, ▬●▬ 0.37 dl/g; ▬∇▬ 0.65 dl/g; b: particle size-dependent in vitro release
profiles, ▬●▬ 4.0 μm; ▬∇▬ 13.0 μm; ▬■▬ 20.0 μm; c: formulation ratio-dependent in vitro release profiles, ▬●▬ 10:1.3;
▬∇▬ 10:1.0; ▬■▬ 10:0.67
The effect of size on the release of rhPTH 1-34 from the PLGA MPs was observed, and the result was displayed in Figure 3b. The large-sized PLGA MPs beneficially decreased the initial burst rate and in case of 10:0.67 formulation, larger size of MPs (20.8 μm) showed minimal initial burst (23.4 ± 8.37 %) in comparison to that (34.9 ± 3.25 %) of preparations with smaller particle size (4.01 μm).
rhPTH 1-34 PLGA MPs were fabricated with different weight ratios of PLGA to rhPTH 1-34 was introduced to release study, and the result of this study presented in Figure 3c. Low viscosity PLGA MPs with 4~5 μm of particle size was used in this study. Drug release from 10:1.0 and 10:1.3 of formulation ratio showed similar trend and achieved maximum release at the end of the study. Especially, MPs made with 1.0:0.67 ratio of formulation displayed more favourable release pattern (like zero-order release profiles) during the release study.
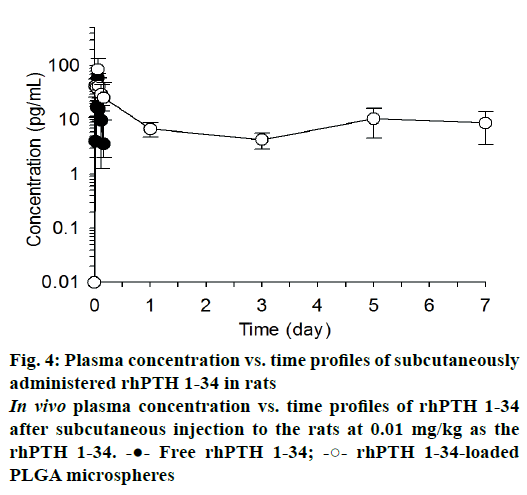
The mean plasma drug concentration vs. time profiles were plotted after single subcutaneous injection of free rhPTH 1-34 and rhPTH 1-34 PLGA MPs and displayed in Figure 4. In case of free rhPTH 1-34, rhPTH 1-34 was quickly reached it is maximum concentration and rapidly declined in the blood after single administration. rhPTH 1-34 was no detectable after one day of the dosing. Meanwhile, rhPTH 1-34 PLGA MPs maintained therapeutic concentration at least for one week. The pharmacokinetic parameters were listed in Table 2. The pharmacokinetics parameters of Cmax and Tmax of rhPTH 1-34 PLGA MPs was 2.1 and 1.7-fold higher than those of free rhPTH 1-34. The AUC0~0.17 (pg·d/ml) of PLGA MPs was 2.6-fold higher than that from free rhPTH 1-34. AUC0.17~7.0 (pg·d/ml) of encapsulated rhPTH 1-34 was 65.15 ± 5.76 pg·d/ml whereas that of free rhPTH 1-34 could not be obtainable. MRT of free rhPTH 1-34 and rhPTH 1-34 PLGA MPs were 0.09 and 3.13 d, respectively.
| Pharmacokinetic parameters | Free rhPTH 1-34 | rhPTH 1-34-loaded PLGA microspheres | p values |
|---|---|---|---|
| Cmax (pg/ml) | 44.23 ± 27.87 | 92.96 ± 36.89 | 0.071 |
| Tmax (day) | 0.06 ± 0.01 | 0.10 ± 0.06 | 0.179 |
| AUC0-0.17 (pg·day/ml) | 2.52 ± 1.15 | 6.53 ± 0.78 | 0.004 |
| AUC0-7 (pg·day/ml) | - | 65.15 ± 5.76 | - |
| MRT (day) | 0.09 | 3.13 | 0.07 |
Cmax, peak concentration; Tmax, peak time; AUC0~0.17, area under the curve at 0.17 day after the administration; AUC0~7, area under the curve at 7 days after the administration; MRT, mean residential time
Table 2: Pharmacokinetic parameters after a single subcutaneous administration of the PLGA microspheres
Previously researchers reported that rhPTH 1-34 had limited therapeutic reliability because of its short halflife (~1 h) [3,22]. Controlled release system of rhPTH 1-34 is necessary to prevent the adverse responses of repeated injections and to improve compliance of osteoporosis patients. To overcome these problems, we introduced the PLGA MPs for a sustained drug release of rhPTH 1-34.
PLGA MPs fabricated with rhPTH 1-34 using low viscosity of PLGA polymer showed high release levels compared to those made with high viscosity PLGA (Figure 3a). However, there was no significant sizedependent release property of rhPTH 1-34 from the PLGA MPs except the initial burst (Figure 3b). The burst release was high in the small-sized microparticles. Especially, formulation ratio was related to the release profiles of rhPTH 1-34 and the PLGA systems of the polymeric MPs have been studied to optimize the release profiles of drugs in clinical applications [23-25]. The low viscosity-PLGA MPs at 10:1.0 and 10:1.3 had higher release rates than those at 10:0.67 (Figure 3c). The release kinetics of rhPTH 1-34 from the PLGA MPs can be explained by diffusion and bulk-eroding characteristics of PLGA [9].
Although the release levels of rhPTH 1-34 were distinguished from the several factors mentioned above, all release profiles of rhPTH 1-34 from the MPs were comparable to show the initial burst and continuous release of rhPTH 1-34. In the case of hydrophilic drug, the drug adheres on the surface of PLGA MPs bursts out initially in the hydrodynamic environment after a short-term incubation [26] and drug is released from the matrix of PLGA MPs, which has a short biological half-life [27-31]. Then, the drug can be slowly released for several weeks as secondary burst release caused by the matrix erosion of the PLGA MPs. As controlling the physicochemical properties such as morphology and drug content of the rhPTH 1-34 PLGA MPs, the polymeric MPs can sustain the drug release without dose dumping and reduce the repeated administration number in vivo [9,26,32]. From the results obtained the release of rhPTH 1-34 from the MPs varied based on the formulation factors. The low viscosity-PLGA MPs at 10:0.67 ratio of formulation was applied for the in vivo pharmacokinetic study.
In this study, the rhPTH 1-34 PLGA MPs showed spherical shapes with smooth surface and narrow size distribution confirmed by SEM analysis. The particle sizes were increased as decreasing the rhPTH 1-34 contents in the microsphere from 10:1.3 to 10:0.67 (polymer: drug, weight ratio). It might be the less quantity of drug was occupied with large amount of polymer matrix, resulting the size increment. In other words, the increase of rhPTH 1-34 content in PLGA MPs could decrease the particle size based on the molecular interaction between rhPTH 1-34 and PLGA polymer. The molecular interaction was identified from the shortened peak at 53° of PLGA as shown in the DSC results (Figure 2). There was no distrusted peak of the fabricated MPs, showing the confirmation of the fertile formulation. Thus, the rhPTH 1-34 and PLGA in the MPs were considered as the amorphous states, suggesting that the rhPTH 1-34 was successfully incorporated into the matrix of PLGA MPs. The loading efficiencies of rhPTH 1-34 in the PLGA MPs were up to 84 % (Table 1).
Blood concentration profiles of rhPTH 1-34 were evaluated after subcutaneous injection of rhPTH 1-34 PLGA MPs. In this study, high dose of rhPTH 1-34 PLGA MPs (0.3 mg/kg as the MPs and 0.01 mg/kg as free rhPTH 1-34) was used to confirm the long-term release which was compared with the conventionally used dose in the clinics (20 μg/d/patient) [33]. Besides the high dose administration of MPs, no significant toxicity was observed after the single injection. rhPTH 1-34 PLGA MPs sustained the plasma concentration of rhPTH 1-34 (Figure 4 and Table 2). AUC0-0.17 (pg·d/ml) increased significantly in rhPTH 1-34 PLGA MPs compared to free rhPTH 1-34 (p=0.004). MRT of rhPTH 1-34 in PLGA MPs was 3.13 ± 0.66 d, suggesting that rhPTH 1-34 PLGA MPs can be applied as a subcutaneous injection once a week [34].
In this study, rhPTH 1-34 PLGA MPs were successfully fabricated using the double emulsion solvent evaporation method. The PLGA MPs showed a spherical shape with a smooth surface. In the formulation factors, PLGA viscosity and formulation ratios of PLGA to rhPTH 1-34 were related to drug release. The rhPTH 1-34, after the subcutaneous injection of rhPTH 1-34 PLGA MPs, showed the prolonged blood circulation compared to free rhPTH 1-34 in a rat model. The rhPTH 1-34 PLGA MPs formulated appeared to have the potential to be developed as an injectable dosage form of rhPTH 1-34.
Acknowledgements:
This work was supported by Inha University Research Grant.
Conflicts of interest:
No conflict of interest was reported by the authors.
References
- Boonen S, Haentjens P, Vandenput L, Vanderschueren D. Preventing osteoporotic fractures with antiresorptive therapy: implications of microarchitectural changes. J Intern Med 2004;255:1-12.
- Anastasilakis AD, Toulis KA, Polyzos SA, Anastasilakis CD, Makras P. Long-term treatment of osteoporosis: safety and efficacy appraisal of denosumab. Ther Clin Risk Manag 2012;8:295-306.
- Langdahl B, Ferrari S, Dempster DW. Bone modeling and remodeling: potential as therapeutic targets for the treatment of osteoporosis. Ther Adv Musculoskelet Dis 2016;8:225-35.
- Lindsay R, Krege JH, Marin F, Jin L, Stepan JJ. Teriparatide for osteoporosis: importance of the full course. Osteoporos Int 2016;27:2395-410.
- Satterwhite J, Heathman M, Miller PD, Marin F, Glass EV, Dobnig H. Pharmacokinetics of teriparatide (rhPTH[1-34]) and calcium pharmacodynamics in postmenopausal women with osteoporosis. Calcif Tissue Int 2010;87:485-92.
- Sugimoto T, Nakamura T, Nakamura Y, Isogai Y, Shiraki M. Profile of changes in bone turnover markers during once-weekly teriparatide administration for 24 weeks in postmenopausal women with osteoporosis. Osteoporos Int 2014;25:1173-80.
- Mirdailami O, Soleimani M, Dinarvand R, Khoshayand MR, Norouzi M, Hajarizadeh A, et al. Controlled release of rhEGF and rhbFGF from electrospun scaffolds for skin regeneration. J Biomed Mater Res A 2015;103:3374-85.
- He J, Li H, Liu C, Wang G, Ge L, Ma S, et al. Formulation and evaluation of poly(lactic-co-glycolic acid) microspheres loaded with an altered collagen type II peptide for the treatment of rheumatoid arthritis. J Microencapsul 2015;32:608-17.
- Ma G. Microencapsulation of protein drugs for drug delivery: strategy, preparation, and applications. J Control Release 2014;193:324-40.
- Marquette S, Peerboom C, Yates A, Denis L, Langer I, Amighi K, et al. Stability study of full-length antibody (anti-TNF alpha) loaded PLGA microspheres. Int J Pharm 2014;470:41-50.
- Katti DS, Robinson KW, Ko FK, Laurencin CT. Bioresorbable nanofiber-based systems for wound healing and drug delivery: optimization of fabrication parameters. J Biomed Mater Res B Appl Biomater 2004;70:286-96.
- Wacker M. Nanocarriers for intravenous injection--the long hard road to the market. Int J Pharm 2013;457:50-62.
- He J, Feng M, Zhou X, Ma S, Jiang Y, Wang Y, et al. Stabilization and encapsulation of recombinant human erythropoietin into PLGA microspheres using human serum albumin as a stabilizer. Int J Pharm 2011;416:69-76.
- Jordan F, Naylor A, Kelly CA, Howdle SM, Lewis A, Illum L. Sustained release hGH microsphere formulation produced by a novel supercritical fluid technology: in vivo studies. J Control Release 2010;141:153-60.
- Wan F, Yang M. Design of PLGA-based depot delivery systems for biopharmaceuticals prepared by spray drying. Int J Pharm 2016;498:82-95.
- Li M, Rouaud O, Poncelet D. Microencapsulation by solvent evaporation: state of the art for process engineering approaches. Int J Pharm 2008;363:26-39.
- Iqbal M, Zafar N, Fessi H, Elaissari A. Double emulsion solvent evaporation techniques used for drug encapsulation. Int J Pharm 2015;496:173-90.
- Nafissi Varcheh N, Luginbuehl V, Aboofazeli R, Peter Merkle H. Preparing Poly (Lactic-co-Glycolic Acid) (PLGA) Microspheres Containing Lysozyme-Zinc Precipitate Using a Modified Double Emulsion Method. Iran J Pharm Res 2011;10:203-9.
- Gasparini G, Kosvintsev SR, Stillwell MT, Holdich RG. Preparation and characterization of PLGA particles for subcutaneous controlled drug release by membrane emulsification. Colloids Surf B Biointerfaces 2008;61:199-207.
- Nafea EH, El-Massik MA, El-Khordagui LK, Marei MK, Khalafallah NM. Alendronate PLGA microspheres with high loading efficiency for dental applications. J Microencapsul 2007;24:525-38.
- Zhao H, Gagnon J, Hafeli UO. Process and formulation variables in the preparation of injectable and biodegradable magnetic microspheres. Biomagn Res Technol 2007;5:2.
- Takasu H, Bringhurst FR. Type-1 parathyroid hormone (PTH)/PTH-related peptide (PTHrP) receptors activate phospholipase C in response to carboxyl-truncated analogs of PTH(1-34). Endocrinology 1998;139:4293-9.
- Wang J, Yang Q, Cheng N, Tao X, Zhang Z, Sun X, et al. Collagen/silk fibroin composite scaffold incorporated with PLGA microsphere for cartilage repair. Mater Sci Eng C Mater Biol Appl 2016;61:705-11.
- Cao H, Chen MM, Liu Y, Liu YY, Huang YQ, Wang JH, et al. Fish collagen-based scaffold containing PLGA microspheres for controlled growth factor delivery in skin tissue engineering. Colloids Surf B Biointerfaces 2015;136:1098-106.
- Zhang HX, Zhang XP, Xiao GY, Hou Y, Cheng L, Si M, et al. In vitro and in vivo evaluation of calcium phosphate composite scaffolds containing BMP-VEGF loaded PLGA microspheres for the treatment of avascular necrosis of the femoral head. Mater Sci Eng C Mater Biol Appl 2016;60:298-307.
- Ramazani F, Chen W, van Nostrum CF, Storm G, Kiessling F, Lammers T, et al. Strategies for encapsulation of small hydrophilic and amphiphilic drugs in PLGA microspheres: State-of-the-art and challenges. Inter J Pharm 2016;499:358-67.
- Wang JJ, Zeng ZW, Xiao RZ, Xie T, Zhou GL, Zhan XR, et al. Recent advances of chitosan nanoparticles as drug carriers. Int J Nanomedicine 2011;6:765-74.
- Lee JO, Youn YS, Lee DK, Cha KH, Lee ES. Development of poly(lactic-co-glycolic acid) microparticles with pH-sensitive drug release behaviors. J Pharm Investig 2015;45:151-6.
- Dhoot NO, Wheatley MA. Microencapsulated liposomes in controlled drug delivery: strategies to modulate drug release and eliminate the burst effect. J Pharm Sci 2003;92:679-89.
- Vilela C, Figueiredo AR, Silvestre AJ, Freire CS. Multilayered materials based on biopolymers as drug delivery systems. Expert Opin Drug Deliv 2017;14:189-200.
- Kim D, MacConell L, Zhuang D, Kothare PA, Trautmann M, Fineman M, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes care 2007;30:1487-93.
- Wu H, Liu J, Wu J, Wan Y, Chen Y. Controlled delivery of platelet-derived growth factor-BB from injectable microsphere/hydrogel composites. Colloids Surf B Biointerfaces 2016;148:308-16.
- Zhou XL, He JT, Du HJ, Fan YY, Wang Y, Zhang HX, et al. Pharmacokinetic and pharmacodynamic profiles of recombinant human erythropoietin-loaded poly(lactic-co-glycolic acid) microspheres in rats. Acta Pharmacol Sin 2012;33:137-44.
- Gopalaswamy V, Dhibar DP, Gupta V, Arya AK, Khandelwal N, Bhansali A, et al. Anabolic bone window with weekly teriparatide therapy in postmenopausal osteoporosis: a pilot study. Endocr Pract. 2017;23:657-61.
 physical mixture of rhPTH 1-34 and
PLGA;
physical mixture of rhPTH 1-34 and
PLGA;  rhPTH 1-34-loaded PLGA microspheres
rhPTH 1-34-loaded PLGA microspheres