- *Corresponding Author:
- Xiao Geng
Department of Oncology, Central Hospital Affiliated to Shandong First Medical University, Jinan, Shandong 250013, China
E-mail: gengxiaogx@163.com
| Date of Received | 24 September 2022 |
| Date of Revision | 05 August 2023 |
| Date of Acceptance | 17 April 2024 |
| Indian J Pharm Sci 2024;86(2):587-592 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To probe the action of platycodin D in the oncogenic phenotypes of esophageal squamous cell carcinoma cells and its possible mechanism. Esophageal squamous cell carcinoma cell line EC9706 was exposed to different doses of platycodin D (2.5, 5 or 10 μmol/l). Untreated cells were adopted as control group. After indicated transfection, functional analyses were carried out. The proliferation, migration and invasion of EC9706 cells were dose-dependently decreased by platycodin D. Moreover, platycodin D treatment dose-dependently increased microRNA-4319 levels in cells. MicroRNA-4319 rise impaired EC9706 cell proliferation, invasiveness and migration. Besides that, the inhibition of microRNA-4319 abolished the action of platycodin D in EC9706 cells. Platycodin D could impair cell proliferation, migration and invasion abilities in esophageal squamous cell carcinoma through elevating microRNA-4319.
Keywords
Platycodin D, esophageal squamous cell carcinoma, microRNA-4319, matrix metalloproteinase, platycodin D
Esophageal Squamous Cell Carcinoma (ESCC) is found to have increasing incidence rate and mortality rate in China in recent years[1]. ESCC is asymptomatic in the early stage; most people don’t notice symptoms until after the cancer has spread. Currently, the surgery and/or radiotherapy remain the main therapies for ESCC, however, the survival time of ESCC patients is still dissatisfactory[2,3]. Thus, it is important to find effective therapeutic methods for ESCC.
Traditional Chinese Medicine (TCM) has anti-tumor, antioxidant stress, anti-inflammatory activity and so on, and is implicated in affecting ESCC development process[4,5]. Platycodin D (PD) is a triterpenoid saponin isolated and separated from the roots of Platycodon grandiflorus. Saponins are a large class of components with low side effects, low cost, easy to obtain and significant antitumor activity, which has become one of the most active and rapidly developing fields in Chinese medicine research, and is worthy of further research and development of anti-tumor new drugs. Recently, the anticancer action of PD has reported. Liu et al.[6] showed that PD could enhance cetuximab-induced metastasis and growth inhibition in colorectal cancer by mediating of Phosphoinositide 3-Kinase (PI3K)/Protein Kinase B (AKT) pathway inactivation. PD hampered the growth of gastric cancer by enhancing ubiquitination-induced c-Myc protein degradation[7]. However, there have been no reports on the relationship between PD and ESCC. MicroRNAs (miRNAs) can degrade or inhibit messenger Ribonucleic Acid (mRNA) translation post transcriptionally by binding with the mRNA, 3' Untranslated Regions (3' UTRs), thereby modulating diverse cellular functions[8,9]. A study exhibited that miR-4319 restrained ESCC growth via targeting NLRC5[10]. However, whether miR-4319 is implicated in modulating ESCC tumorigenesis and has the potential to be the therapeutic target remain unclear.
Therefore, this study mainly probed whether PD and miR-4319 affected the oncogenic phenotypes of ESCC cells, and their relationship in ESCC tumorigenesis.
Materials and Methods
Materials and reagents:
EC9706 cells, Dulbecco's Modified Eagle Medium (DMEM), and 10 % Fetal Bovine Serum (FBS) (American Type Culture Collection (ATCC), United States of America (USA)); PD (purity ≥98 %, Chengdu Yirui Biotechnology Co., Ltd.,); Trizol reagent, PrimeScript RT reagent, SYBR® Green reagent and Lipofectamine™ 3000 (Invitrogen, USA); miR-4319 mimic (miR-4319), inhibitor (anti-miR-4319), miR-NC and anti-miR-NC (RiboBio, Guangzhou, China); Matrigel (50 µg/ml, BD Biosciences, USA); Cell Counting Kit-8 (CCK-8), methanol, 1 % crystal violet, and paraformaldehyde (Beyotime, Shanghai, China); Transwell chamber (Corning Costar Corp., USA); Matrix Metalloproteinase (MMP)-2 (1:2000, ab92536), MMP-9 (1:2000, ab76003), Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) (1:1000, ab8245) primary antibodies, and secondary antibodies (Abcam, USA).
Experimental grouping:
EC9706 cells were cultured with DMEM plus 10 % FNS at 37° with 5 % Carbon dioxide (CO2). For PD treatment, EC9706 cells were exposed to 2.5, 5 or 10 μmol/l PD for 24 h, named PD-L, PD-M and PD-H groups. Untreated cells were used as the control group. Lipofectamine™ 3000 was applied for transient transfection, EC9706 cells were transfected with anti-miR-4319 or anti-miR-NC, followed by 10 μmol/l PD treatment, named PD+anti-miR-NC and PD+anti-miR-4319 groups.
CCK-8 assay:
The each well of a 96-well plate was added with 2×103 EC9706 cells. Following assigned treatment and/or treatment, cells were reacted with CCK-8 10 μl reagent for 2 h, followed by examining the absorbance at 450 nm.
Colony formation assay:
The assigned EC9706 cells were cultured in six-well plate (500 cells/well) with DMEM for about (10-14) d. The medium was discarded, and the each well was added with 500 μl methanol to fix proliferating colonies for 20 min, followed by dyeing for 15 min with 400 μl crystal violet (1 %). Lastly, the colonies (≥50 cells) were photographed and counted.
Scratch assay:
Each group of EC9706 cells was inoculated into a 6-well plate (2×105 cells/well) and cultured in the incubator until per well was filled with cells. Then cells on the 6-well plate were scratched by a 20 μl sterile pipette tip and imaged under a microscope (0 h at the moment) after washing. Thereafter, cells were cultivated for 24 h in media without serum and then imaged. The distance was measured, and cell migration was assessed.
Transwell assay:
About 0.1 ml Matrigel diluent were added onto the plate surface of the upper Transwell chamber and allowed to solidify for 2 h. Then 1×105 assigned EC9706 cells were plated into the upper chamber with 500 μl complete medium on the lower chamber. 24 h later, invaded cells were observed and counted after dying with 0.1 % crystal violet.
Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR):
Total RNA was prepared by the assist of the TRIzol reagent, then complementary Deoxyribonucleic Acid (cDNA) were synthesized, followed by qRT-PCR amplification on a 7500 real-time PCR system. The relative of miR-4319 level was examined using by the comparative method.
Western blot:
EC9706 cells were reacted with 400 μl Radioimmunoprecipitation Assay (RIPA) lysate on ice for 30 min, followed by centrifuging 10 000 r/min for 10 min. The protein samples were denatured, loaded onto Sodium Dodecyl-Sulfate (SDS) gels, and then transferred onto Polyvinylidene Difluoride (PVDF) membranes. Thereafter, primary antibody diluents were used to incubate overnight with membranes at 4°, and following secondary antibody diluents at 37° for 2 h. The gray values were quantified by Quantity One software.
Statistical analyses:
The data were manifested as (x̄±s). Student’s t-test (two tailed) or one-way Analysis of Variance (ANOVA) analysis was adopted for comparisons between two or multiple groups. p<0.05, meant statistically significant.
Results and Discussion
PD treatment dose-dependently elevated EC9706 cell proliferation, evidenced by increased proliferation inhibitory rate and decreased colonies number (Table 1).
| Group | Proliferation inhibitory rate (%) | Colonies number |
|---|---|---|
| Control | 0.00±0.00 | 86.46±6.53 |
| PD-L | 26.34±2.33* | 70.04±5.75* |
| PD-M | 43.68±4.04*# | 51.91±4.87*# |
| PD-H | 65.21±5.53*#& | 36.82±3.26*#& |
| F | 523.348 | 152.318 |
| p | 0.000 | 0.000 |
Note: Control vs. *p<0.05; PD-L vs. #p<0.05 and PD-M vs. &p<0.05
Table 1: The effects of PD on EC9706 cell proliferation (x̄±s, n=9).
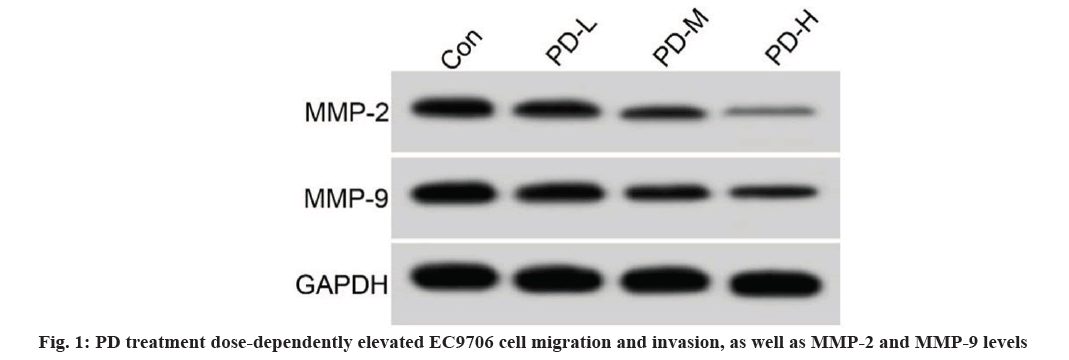
PD treatment dose-dependently elevated EC9706 cell migration and invasion, as well as MMP-2 and MMP-9 levels (fig. 1 and Table 2). As exhibited in Table 3, levels of miR-4319 were dose-dependently increased in PD-L, PD-M, and PD-H groups.
| Group | Migration(%) | Invasion | MMP-2 | MMP-9 |
|---|---|---|---|---|
| Control | 73.11±6.39 | 114.14±11.19 | 0.57±0.05 | 0.74±0.06 |
| PD-L | 57.02±5.08* | 90.56±7.18* | 0.43±0.04* | 0.61±0.05* |
| PD-M | 41.61±3.39*# | 73.65±6.31*# | 0.31±0.03*# | 0.47±0.03*# |
| PD-H | 27.65±2.57*#& | 54.19±5.18*#& | 0.19±0.02*#& | 0.32±0.03*#& |
| F | 163.309 | 95.846 | 176.667 | 149.013 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Table 2: Action of PD on EC9706 cell migration and invasion.
| Group | miR-4319 |
|---|---|
| Control | 1.00±0.00 |
| PD-L | 1.74±0.14* |
| PD-M | 2.55±0.22*# |
| PD-H | 3.32±0.23*#& |
| F | 299.7 |
| p | 0.000 |
Note: Control vs. *p<0.05; PD-L vs. #p<0.05 and PD-M vs. &p<0.05
Table 3: Action of PD on miR-4319 expression in EC9706 cells.
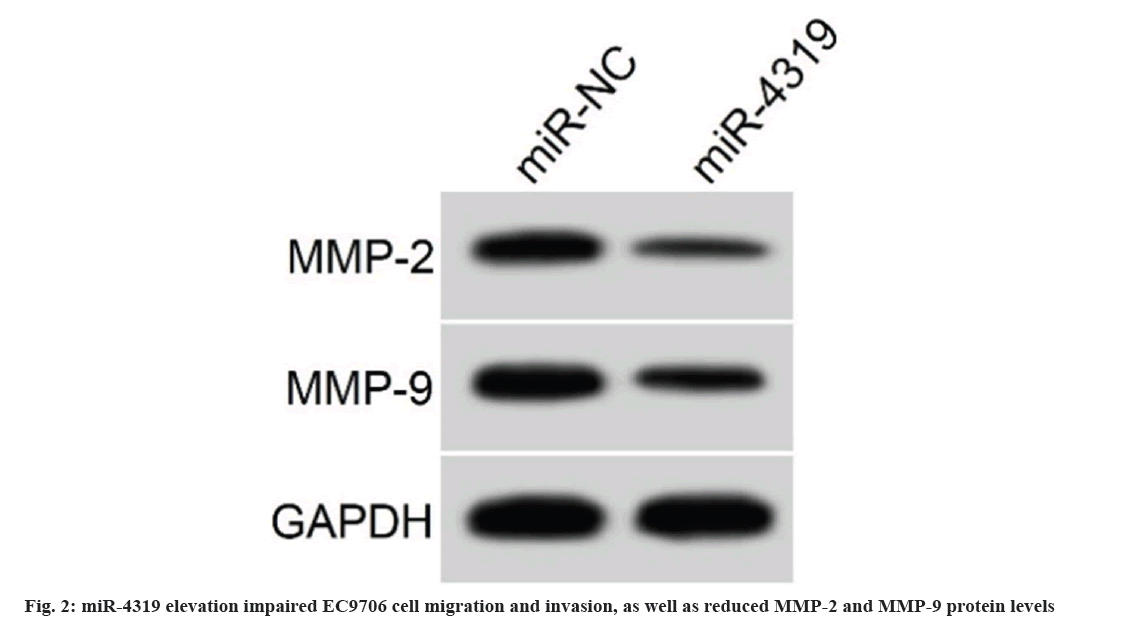
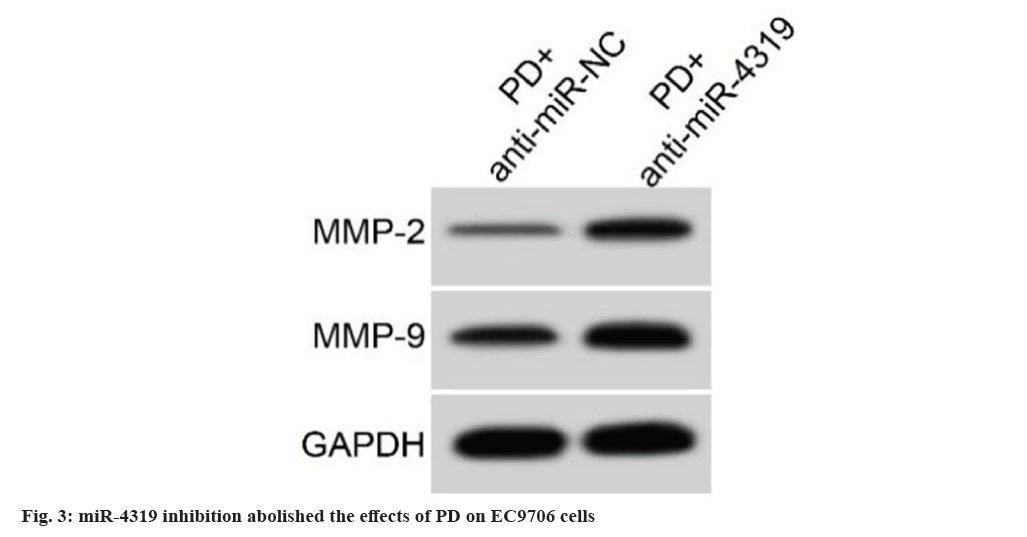
The introduction of miR-4319 mimic in EC9706 cells notably elevated miR-4319 levels, miR-4319 overexpression suppressed cell proliferation, demonstrating by increased proliferation inhibitory rate and declined colonies number (Table 4). miR-4319 elevation impaired EC9706 cell migration and invasion, as well as reduced MMP-2 and MMP-9 protein levels (fig. 2 and Table 5). miR-4319 inhibition reversed PD-mediated inhibition of EC9706 cell proliferation, migration and invasion, as well as the reduction of MMP-2 and MMP-9 protein levels (fig. 3 and Table 6).
| Group | miR-4319 | Proliferation inhibitory rate (%) | Colonies |
|---|---|---|---|
| miR-NC | 1.00±0.00 | 6.16±0.57 | 89.27±6.59 |
| miR-4319 | 3.74±0.28* | 56.74±4.83* | 44.04±4.21* |
| t | 29.357 | 31.200 | 17.352 |
| p | 0.000 | 0.000 | 0.000 |
Note: miR-NC vs. *p<0.05
Table 4: Effects of miR-4319 on EC9706 cell proliferation.
| Group | Migration (%) | Invasion | MMP-2 | MMP-9 |
|---|---|---|---|---|
| miR-NC | 74.86±6.11 | 115.42±11.78 | 0.59±0.05 | 0.76±0.05 |
| miR-4319 | 33.39±3.42* | 62.34±5.12* | 0.26±0.02* | 0.39±0.04* |
| t | 17.768 | 12.397 | 18.384 | 17.335 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: miR-NC vs. *p<0.05
Table 5: Action of miR-4319 on EC9706 cell migration and invasion.
| Group | miR-4319 | Proliferation inhibitory rate (%) | Colonies | Migration (%) | Invasion | MMP-2 | MMP-9 |
|---|---|---|---|---|---|---|---|
| PD+anti-miR-NC | 1.00±0.00 | 57.08±4.97 | 43.70±4.22 | 32.91±4.10 | 61.29±5.13 | 0.24±0.03 | 0.37±0.03 |
| PD+anti-miR-4319 | 0.43±0.04* | 12.85±1.22* | 78.33±6.71* | 66.78±5.59* | 97.51±8.64* | 0.48±0.04* | 0.68±0.05* |
| t | 42.75 | 25.928 | 13.106 | 14.657 | 10.814 | 14.400 | 15.949 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: PD+ anti-miR-NC vs. *p<0.05.
Table 6: miR-4319 inhibition abolished the action of PD on EC9706 cells (x̄±s, n=9).
Currently, chemotherapy and radiotherapy resistance are the main reasons for the reduced treatment efficacy of ESCC patients. TCM has the anticancer activity and can restrain the development process of ESCC by modulating gene expression[11]. At present, saponins isolated from TCM are found to have few toxic side effects and can improve tumors. Saponins can significantly increase the immune response of the body, prolong the immune cycle of vaccines as adjuvants, improve interferon regulatory factors, and swell the expression level of tumor necrosis factor and other immune-related genes, which promote the research prospect of Chinese medicine saponins as vaccine adjuvants and drug administration. PD is one of the widely used traditional herb with multiple pharmacological properties including antiviral, neuroprotective, anti-inflammatory, anti-aging, and anticancer activities[12-14]. PD repressed cell metastasis and proliferation in bladder cancer through XIST/miR-335 axis[15]. Cell growth and mobility could be impaired by PD in gallbladder cancer[16]. In addition, it was also found PD repressed the mobility of multiple myeloma cells via blocking the JAK2/STAT3 pathway[17]. Herein, results of this study showed that PD treatment dose-dependently repressed ESCC cell proliferative, migratory and invasive abilities. MMP-2 and MMP-9 are mobility-related markers, and was found to be elevated in ESCC[18]. In our work, we also showed that MMP-2 and MMP-9 protein levels were dose-dependently restrained by PD treatment, further suggesting the inhibition of PD on ESCC cell mobility.
MiRNAs have been reported to be implicated in ESCC tumorigenesis via modulating the expression of target mRNA[19,20]. However, whether miRNA can serve as a potential target for PD in regulating ESCC remains unclear. miR-4319 is a functional miRNA, it was found to be decreased in colorectal cancer, the up-regulation of its expression could repress the growth of this cancer cells[21]. Thyroid cancer showed decreased miR-4319 levels, which led to cancer cell to migrate and proliferate[22]. In addition, miR-4319 performed antitumor activity in gastric cancer to impede cell growth[23]. In our work, we found PD treatment dose-dependently resulted in the increase of miR-4319 content in ESCC cells. The overexpression miR-4319 was proved to suppress ESCC cell to migrate, invade and proliferate. As expected, MMP-2 and MMP-9 levels were also declined after miR-4319 elevation. Thereafter, we probed whether miR-4319 was implicated in the action of PD in ESCC cells. The result displayed that miR-4319 deficiency abolished the anticancer action of PD on ESCC cells, evidenced by enhanced migration, invasion and proliferation of ESCC cells in high miR-4319 group under PD treatment.
In summary, PD treatment restrained ESCC cell invasion, migration and proliferation. Its mechanism of action might be linked with the promotion of miR-4319 expression, which provide new directions for developing therapeutic drugs for ESCC.
Conflict of interests:
The authors declared no conflict of interests.
References
- Zhu H, Ma X, Ye T, Wang H, Wang Z, Liu Q, et al. Esophageal cancer in China: Practice and research in the new era. Int J Cancer 2023;152(9):1741-51.
[Crossref] [Google Scholar] [PubMed]
- Ye L, Zhang J, Zhang Y, Gu B, Zhu H, Mao X. Isoliquiritigenin suppressed esophageal squamous carcinoma growth by blocking EGFR activation and inducing cell cycle arrest. Biomed Res Int 2020;2020:9259852.
[Crossref] [Google Scholar] [PubMed]
- Zhang R, Hao J, Guo K, Liu W, Yao F, Wu Q, et al. Germacrone inhibits cell proliferation and induces apoptosis in human esophageal squamous cell carcinoma cells. Biomed Res Int 2020;2020:7643248.
[Crossref] [Google Scholar] [PubMed]
- Deng X, Sheng J, Liu H, Wang N, Dai C, Wang Z, et al. Cinobufagin promotes cell cycle arrest and apoptosis to block human esophageal squamous cell carcinoma cells growth via the p73 signalling pathway. Biol Pharm Bull 2019;42(9):1500-9.
[Crossref] [Google Scholar] [PubMed]
- Jin F, Zhu G, Li D, Ni T, Dai X, Wang H, et al. Celastrus orbiculatus extracts induce cell cycle arrest and apoptosis in human esophageal squamous carcinoma ECA-109 cells in vitro via the PI3K/AKT/mTOR signaling pathway. Oncol Lett 2018;15(2):1591-9.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Tian S, Yi B, Feng Z, Chu T, Liu J, et al. Platycodin D sensitizes KRAS-mutant colorectal cancer cells to cetuximab by inhibiting the PI3K/Akt signaling pathway. Front Oncol 2022;12:1046143.
[Crossref] [Google Scholar] [PubMed]
- Xu Q, Pan G, Wang Z, Wang L, Tang Y, Dong J, et al. Platycodin-D exerts its anti-cancer effect by promoting c-Myc protein ubiquitination and degradation in gastric cancer. Front Pharmacol 2023;14:1138658.
[Crossref] [Google Scholar] [PubMed]
- Yao F, Shi W, Fang F, Lv MY, Xu M, Wu SY, et al. Exosomal miR-196a-5p enhances radioresistance in lung cancer cells by downregulating NFKBIA. Kaohsiung J Med Sci 2023;39(6):554-64.
[Crossref] [Google Scholar] [PubMed]
- Yadav N, Sunder R, Desai S, Dharavath B, Chandrani P, Godbole M, et al. Progesterone modulates the DSCAM-AS1/miR-130a/ESR1 axis to suppress cell invasion and migration in breast cancer. Breast Cancer Res 2022;24(1):97.
[Crossref] [Google Scholar] [PubMed]
- Hu X, Wang M, Cao L, Cong L, Gao Y, Lu J, et al. miR-4319 suppresses the growth of esophageal squamous cell carcinoma via targeting NLRC5. Curr Mol Pharmacol 2020;13(2):144-9.
[Crossref] [Google Scholar] [PubMed]
- Wu Z, Zhao Y, Yu F, Shi H, Li J. Qigefang inhibits migration, invasion, and metastasis of ESCC by inhibiting Gas6/Axl signaling pathway. Recent Pat Anticancer Drug Discov 2021;16(2):285-94.
[Crossref] [Google Scholar] [PubMed]
- Khan M, Maryam A, Zhang H, Mehmood T, Ma T. Killing cancer with platycodin D through multiple mechanisms. J Cell Mol Med 2016;20(3):389-402.
[Crossref] [Google Scholar] [PubMed]
- Wang G, Guo H, Wang X. Platycodin D protects cortical neurons against oxygen-glucose deprivation/reperfusion in neonatal hypoxic-ischemic encephalopathy. J Cell Biochem 2019;120(8):14028-34.
[Crossref] [Google Scholar] [PubMed]
- Shi C, Li Q, Zhang X. Platycodin D protects human fibroblast cells from premature senescence induced by H2O2 through improving mitochondrial biogenesis. Pharmacology 2020;105(9-10):598-608.
[Crossref] [Google Scholar] [PubMed]
- Chen D, Chen T, Guo Y, Wang C, Dong L, Lu C. Platycodin D (PD) regulates LncRNA-XIST/miR-335 axis to slow down bladder cancer progression in vitro and in vivo. Exp Cell Res 2020;396(1):112281.
[Crossref] [Google Scholar] [PubMed]
- Zhang X, Zhai T, Hei Z, Zhou D, Jin L, Han C, et al. Effects of Platycodin D on apoptosis, migration, invasion and cell cycle arrest of gallbladder cancer cells. Oncol Lett 2020;20(6):311.
[Crossref] [Google Scholar] [PubMed]
- Wu D, Zhang W, Chen Y, Ma H, Wang M. Platycodin D inhibits proliferation, migration and induces chemosensitization through inactivation of the NF-κB and JAK 2/STAT 3 pathways in multiple myeloma cells. Clin Exp Pharmacol Physiol 2019;46(12):1194-200.
[Crossref] [Google Scholar] [PubMed]
- Xu H, Miao J, Liu S, Liu H, Zhang L, Zhang Q. Long non-coding RNA KCNQ1 overlapping transcript 1 promotes the progression of esophageal squamous cell carcinoma by adsorbing microRNA-133b. Clinics 2021;76(1):e2175-85.
[Crossref] [Google Scholar] [PubMed]
- Sun T, An Q, Yan R, Li K, Zhu K, Dang C, et al. MicroRNA-216a-5p suppresses esophageal squamous cell carcinoma progression by targeting KIAA0101. Oncol Rep 2020;44(5):1971-84.
[Crossref] [Google Scholar] [PubMed]
- Li M, Meng X, Li M. miR-126 promotes esophageal squamous cell carcinoma via inhibition of apoptosis and autophagy. Aging (Albany NY) 2020;12(12):12107.
[Crossref] [Google Scholar] [PubMed]
- Huang L, Zhang Y, Li Z, Zhao X, Xi Z, Chen H, et al. miR-4319 suppresses colorectal cancer progression by targeting ABTB1. United Eur Gastroenterol J 2019;7(4):517-28.
[Crossref] [Google Scholar] [PubMed]
- Bian S. miR-4319 inhibited the development of thyroid cancer by modulating FUS-stabilized SMURF1. J Cell Biochem 2020;121(1):174-82.
[Crossref] [Google Scholar] [PubMed]
- Zhang Z, Wu H, Chen Z, Li G, Liu B. Circular RNA ATXN7 promotes the development of gastric cancer through sponging miR-4319 and regulating ENTPD4. Cancer cell Int 2020;20:25.
[Crossref] [Google Scholar] [PubMed]