- *Corresponding Author:
- Zhou Zhang
Department of General Surgery, Shidong Hospital, Yangpu, Shanghai 200438, China
E-mail: zhouzhang9911@gmail.com
| Date of Received | 12 August 2021 |
| Date of Revision | 10 March 2022 |
| Date of Acceptance | 02 September 2022 |
| Indian J Pharm Sci 2022;84(5):1171-1177 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Piperine is a dietary alkaloid with huge pharmacological importance and was first isolated from pepper extract. It has been reported with anti-cancer, anti-amoebic, anti-mycobacterial, anti-mutagenic, anticonvulsant, anti-asthmatic, immunomodulatory, anti-inflammatory and anti-oxidant activity. Therefore, the current investigation was designed to unveil the anticancer effects of piperine molecule against human breast carcinoma. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay was executed for determination of effects of piperine on breast cancer cell viability. Clonogenic assay was implemented for checking the effect of piperine on clonogenic potency of breast cancer cells. To monitor morphology of cancerous breast cells, phase contrast microscopy was performed after piperine exposure. Apoptosis was checked by acridine orange/ethidium bromine staining assay and by execution of Western blotting analysis. Cell migration and invasion was monitored by transwell chambers assay. Muse flow cytometric analysis was performed for assessing of different cell cycle phases. Western blotting assay was implemented to check the levels of phosphatidylinositol 3-kinase and protein kinase B. Results revealed that piperine induced dose reliant cytotoxicity in MCF-7 human breast cancer cells. Further, application of piperine on cancer cell colonies remarkably reduced the number of cell colonies to minimum. Phase contrast microscopy evidenced some specific morphological modifications indicating apoptosis allied cytotoxicity of piperine like apoptotic bodies, condensed nucleus and membrane blabbing. Acridine orange/ethidium bromine staining assay revealed that piperine induced apoptosis in MCF-7 cells which was further investigated by Western blotting. This revealed increased expression of B-cell lymphoma 2 associated X and reduced expression of B-cell lymphoma 2, indicating apoptosis induction by piperine. Cell migration as well as cell invasive ability of MCF-7 cells was reduced to minimum by the application of piperine in dose dependent-manner. Flow cytometric analysis evidenced that piperine arrested the cell cycle at growth 2/mitosis phase, hence suppressed the breast cancer progression. Finally, Western blotting assay predicted constant expression of phosphatidylinositol 3-kinase and protein kinase B and reduced expression of phosphorylated phosphatidylinositol 3-kinase. Hence, evidenced the blocking of phosphatidylinositol 3-kinase/protein kinase B signaling pathway. In conclusion, the current investigation regarding anticancer effects of piperine against breast cancer revealed remarkable suppression of cancer cells. It induced apoptosis, suppressed cell migration and invasion, blocked cell cycle and phosphatidylinositol 3-kinase/ protein kinase B signaling pathway.
Keywords
Breast cancer, alkaloids, piperine, apoptosis, cell migration, cell invasion
Breast Cancer (BC) is a dangerous and most frequent malignancy prevailing in women globally. The incidences of developing BC have gone up with nearly 3.1 % increase annually over last 30 y. Alone in the year of 2010, nearly 1.6 million cases of BC were registered[1]. BC comprises of variant subtypes and those subtypes arise from basal progenitor or luminal progenitor cells undergoing different genetic mutations[2]. BC is both histopathologically as well as genetically heterogeneous, thus underlying mechanisms of developing BC still remains uncertain[3], which cause hurdles in BC diagnosis as well as treatment, owing to this conventional treatment for BC like surgery, radiation and chemotherapy does not hold good efficiency. Currently, subtypes of BC are categorized on the basis of activity of Hormone Receptors (HR) and Human Epidermal Growth Factor Receptor 2 (HER2)[4]. These subtypes show difference in metastasis, biology, treatment as well as prognosis. It has been reported in various studies that the reproductive pattern (breastfeeding and pregnancy) has a direct relationship with developing BC[5-7]. Before the introduction of modern medicine for treatment of human ailments, medicinal plants were extensively used and were effective too[8,9]. Afterwards, the knowledge of medical properties of medicinal plants were explored, which lead to the formation of variant therapies and traditional medicine systems including Traditional Chinese Medicine (TCM), indigenous medicine, Ayurvedic medicine, aromatherapy and naturopathy[10-12]. Apart from conventional medicine, modern medicine comprises of numerous plant derived chemical entities including anti-cancer drugs like vinblastine and taxol[13,14]. Naturally occurring alkaloids have long been serving as a pool of drug discovery and few of these have already been permitted by United States Food and Drug Administration (US FDA) like camptothecin. Piperine, an alkaloid is a key dietary phytochemical due to its pharmacological importance and prevalence in spicy foods. It acts as anti-cancer, antimetastatic, larvicidal, anti-inflammatory, immunosuppressive, leishmanicidal and antiparasitic[15-22]. Orsted first isolated piperine from the extract of pepper[23]. The main purpose of the current study was to investigate anticancer and apoptotic effects of piperine alkaloid against human BC cells which was found to be mediated via cell migration and cell invasion inhibition, Growth 2 (G2)/Mitosis (M) phase cell cycle arrest and targeting Phosphatidylinositol 3-Kinase/Protein Kinase B (PI3K/ AKT) signaling Pathway.
Materials and Methods
Cellular viability measurement:
Viability of human breast carcinoma cells (MCF-7) was assessed by 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl Tetrazolium Bromide (MTT) assay after piperine exposure. In brief, MCF-7 cells were cultured onto 96-well plate at 2×104 cells/well of density for 24 h at 37°. After culturing of the cells, treatment with varying piperine doses (0, 10, 20, 50 and 100 μM) was initiated for 24 h and 48 h. Afterwards, MCF-7 cells were incubated at 37° in a Carbon dioxide (CO2) incubator (5%). Viability of piperine treated cells was then observed by performing MTT assay as designated by Mosmann et al.[24]. For Optical Density (OD) measurements absorbance was recorded with micro plate Enzyme Linked Immunosorbent Assay (ELISA) reader (Tecan, United States) at 570 nm.
Assessment of clonogenic potency:
Human MCF-7 BC cells were plated onto 6-well plate (3500 cells each well) containing Fetal Bovine Serum (FBS) 10 %. Seeding of MCF-7 cells was followed by piperine exposure at changing doses viz. 0, 20, 50 and 100 μM for 10 d. Piperine treated cells were then stained with crystal violet after fixation in paraformaldehyde (Beyotime, China). Thereafter, cell colonies were studied under a digital camera (Olympus, Japan) followed by further investigations.
Cell morphology determination by phase contrast microscopy:
Morphological changes induced by piperine in MCF-7 BC cells were recorded by performing phase contrast microscopy. Briefly, MCF-7 cells were cultured in 6 mm cultural dishes containing 2×103 cells each. After culturing, cells were treated with piperine at changing doses viz. 0, 20, 50 and 100 μM for 24 h each dish. Piperine treatment was followed by removal of cultural media and washing of treated MCF-7 cells with Phosphate Buffered Saline (PBS). Finally, MCF-7 cells were observed and studied with a phase contrast inverted microscope (Leica, Germany) at 200x magnification.
Apoptosis assessment by Acridine Orange/Ethidium Bromine (AO/EB) staining:
Ariffin et al. method of AO/EB staining was employed to assess the effect of piperine on apoptotic cell morphology[25]. MCF-7 BC cells were harvested at 80 % confluence of growth and subjected to piperine exposure at varying doses viz. 0, 20, 50 and 100 μM. Thereafter, AO/EB staining was performed followed by visualization. Visualization was performed with a confocal laser-scanning microscope (Olympus Fluoview FV1000) under 40x of magnification using 1.4 Numerical Aperture (NA) as an objective.
Transwell chambers assay for cell migration and cell invasion:
Transfection of MCF-7 BC cells was executed with piperine drug at changing concentrations of 0, 20, 50 and 100 μM. The lower transwell chambers were filed with Roswell Park Memorial Institute (RMPI)-1640 cultural medium and FBS (10 %) (Corning Incorporated, Corning, New York, United States). Transfected cells were filled in upper chambers of transwell bearing cultural medium at a density of 1×105 cells/well. These transwell chambers were incubated for 12 h and then incubated for 10 min at 4°. The cells which didn’t migrate were cleared with a cotton swab followed by staining with crystal violet with 5 min of incubation. Under a light microscope (TS100; Nikon Corporation, Tokyo, Japan) with 200x of magnification different sections were pictured. The cell invasion potency of MCF-7 BC cells was determined by following similar procedure except transwell chambers coated with Matrigel was used.
Examination of different cell cycle phases:
For cell cycle analysis, MCF-7 cells were first treated with varying piperine doses (0, 20, 50 and 100 μM) for 24 h. Then these cells were subjected to washing, once with PBS followed by 5 min of centrifugation. After centrifugation, cells were fixed in 70 % ethanol and repeatedly washed twice with PBS. Cell suspensions were supplemented with Ribonucleic Acid (RNA) 50 μl and Propidium Iodide (PI) 25 μl and then incubated for 15 min. Finally, Deoxyribonucleic Acid (DNA) content was determined through Muse flow cytometry (Millipore, Billerica, Massachusetts, United States).
Western blotting analysis:
Human MCF-7 BC cells were collected at 80 % confluence of growth followed by lysing with Radioimmunoprecipitation Assay (RIPA) buffer (Sigma-Aldrich). The protein content within each lysate was determined with Bicinchoninic Acid (BCA) protein assay kit (Beyotime, China). Thereafter, 10% Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) was used for uniformly arranging protein amounts (40 μg) followed by loading these over Polyvinylidene Difluoride (PVDF) membranes (Millipore, Billerica, Massachusetts, United States). PVDF membranes were then subjected to primary antibodies treatment (antibodies against B-Cell Lymphoma Protein-2 (BCL-2), Bcl-2 Associated X (BAX), PI3K and AKT) (1:1000; Epitomics, Burlingame, California, United States). Primary antibody exposure was followed by secondary antibody exposure (anti-rabit Immunoglobulin G (IgG) conjugated to Human Resource Planning (HRP)) with incubation overnight at 4°. Finally, protein bands were envisaged through Enhanced Chemiluminescence (ECL) detection system (Pierce, Rockford, IL, United States).
Statistical analysis:
Significant difference was evaluated by execution of Student’s t-test using Statistical Package for Social Sciences (SPSS) for Windows software package (release 11.0.0, SPSS Inc., United States). Variance was considered significant if p<0.05.
Results and Discussion
Cellular viability of MCF-7 BC cells was testified by MTT assay after piperine (fig. 1A) drug exposure at varying doses (0, 10, 20, 50 and 100 μM). Cellular viability was found to be retarding with increasing drug doses as well as with the extent of drug exposure. The cell viability percentage decreased from nearly 95 % to 15 % after 24 h of exposure and reduced from 90 % to 10 % after 48 h of drug exposure (0-100 μM) (fig. 1B). Hence, evidencing the cytotoxicity effects of piperine in MCF-7 BC cells.
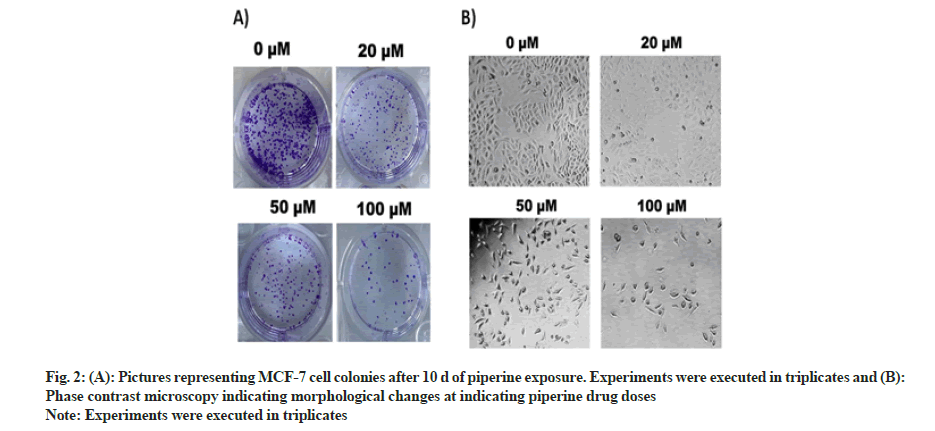
Fig. 1: (A): Chemical structure of piperine molecule and (B): Results representing cellular viability of MCF-7 cells after piperine treatment. MCF-7 cells were treated with piperine for 24 h and 48 h at changing doses of 0, 10, 20, 50 and 100 μM.
Note: All the experimental data are revealed as mean±standard deviation. Experiments were executed in triplicates with p<0.05,
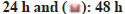
Clonogenic potency of MCF-7 BC cells was determined by execution of clonogenic assay. Along with cell viability inhibition the MCF-7 cell colonies were also inhibited in a dose-dependent manner. The number of cell colonies pictured under a digital camera at varying drug doses (0, 20, 50 and 100 μM) of MCF-cell colonies can be seen reduced to minimum (fig. 2A).
To assess the morphological changes in MCF-7 cells after exposure with piperine, phase contrast microscopy was executed. The results evaluated that the piperine induced cell shrinkage, apoptotic bodies, bubbling, condensed nuclei, membrane blabbing and echinoid spikes (fig. 2B). These morphological modifications evidenced induction of apoptosis mediated cytotoxicity by piperine in MCF-7 cells.
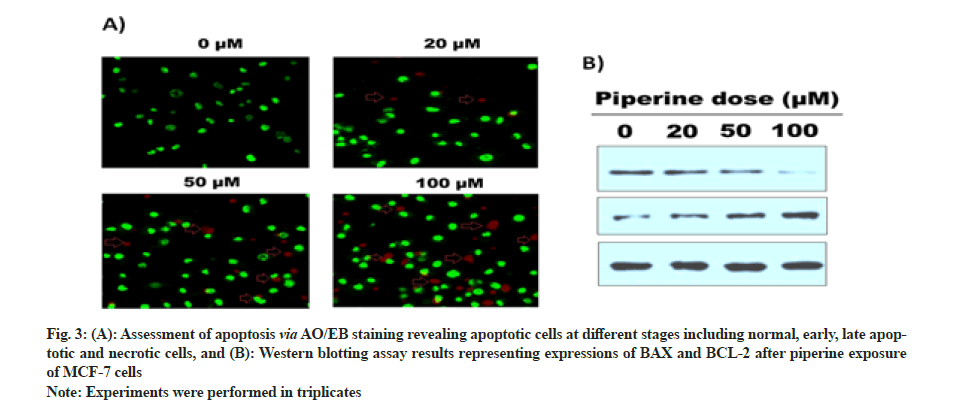
AO/EB staining was performed to monitor the apoptotic cells after piperine exposure. Results indicated regular tumor cells, late and early apoptotic cells, and necrotic cells were inspected via fluorescent microscopy, granular yellow-green or crescent-shaped AO nuclear staining marked early-stage apoptotic cells. Unevenly localized and concentrated orange nuclear ethidium bromide staining represents late-stage apoptotic cells. Uneven orange-red fluorescence represents necrotic cells with increased volume. Cells seemed to be in the progression of disintegration (fig. 3A). Further, Western blotting assay unveiled that the activity of BAX increased significantly with increasing piperine doses and activity of BCL-2 reduced at higher drug doses (fig. 3B).
Fig. 3: (A): Assessment of apoptosis via AO/EB staining revealing apoptotic cells at different stages including normal, early, late apoptotic and necrotic cells, and (B): Western blotting assay results representing expressions of BAX and BCL-2 after piperine exposure of MCF-7 cells.
Note: Experiments were performed in triplicates.
Therefore, AO/EB staining and Western blotting assay represent that the anti-proliferative effects of piperine drug are mediated via induction of apoptosis in MCF-7 BC cells.
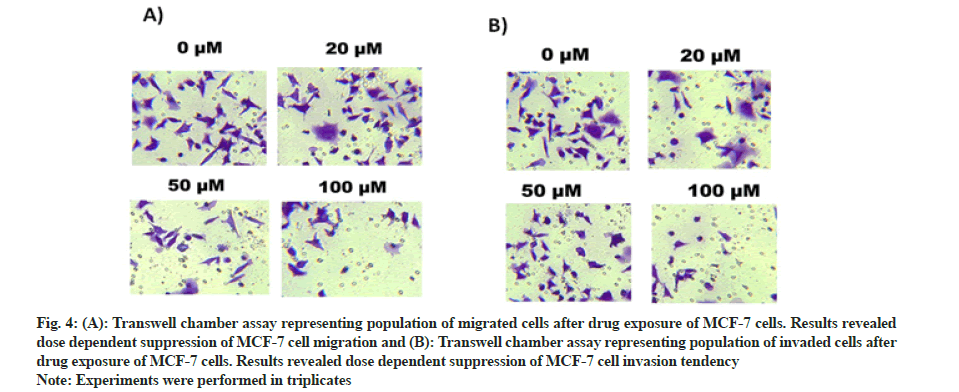
Transwell chambers were used to monitor cell migration as well as cell invasive tendency of piperine treated MCF-7 cells. After 48 h of exposure in transwell chambers migrated cells were examined. It was established from the results that number of migrated cells reduced from maximum at controls to minimum after piperine drug exposure (fig. 4A). The number of invasive cells also reduced dose dependently from high to low after piperine exposure (fig. 4B). Thus transwell chamber assays unveiled that along with inducing cytotoxicity in MCF-7 cells, piperine significantly retarded the cell migratory and cell invasive potency of MCF-7 cells.
Fig. 4: (A): Transwell chamber assay representing population of migrated cells after drug exposure of MCF-7 cells. Results revealed dose dependent suppression of MCF-7 cell migration and (B): Transwell chamber assay representing population of invaded cells after drug exposure of MCF-7 cells. Results revealed dose dependent suppression of MCF-7 cell invasion tendency.
Note: Experiments were performed in triplicates.
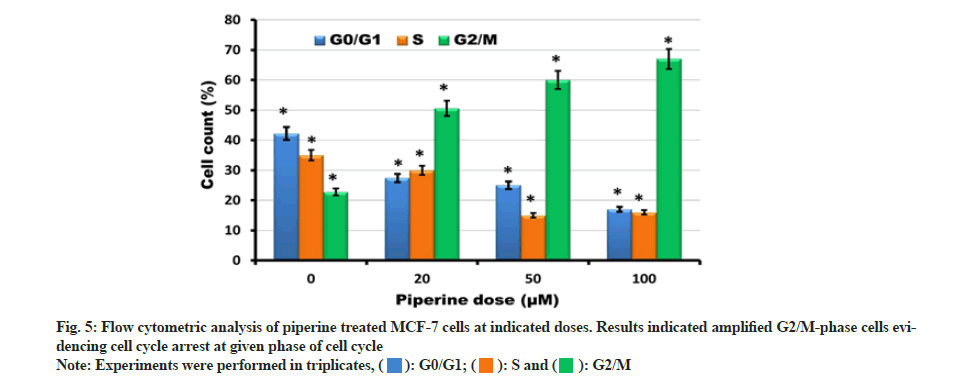
Muse flow cytometry was executed to investigate DNA content for determination of different cell cycle phases. After exposure with varying piperine drug doses (0, 20, 50 and 100 μM) for 24 h different cell cycle phases were determined. The results revealed that the number of G0/G1 and S-phase cells reduced significantly and dose-reliantly to minimum after piperine exposure. In contrast with this the number of G2/M phase cells accumulated and reached to higher concentrations at higher drug doses (fig. 5). This indicated that piperine induced cell cycle arrest at G2/M phase of cell cycle. Hence, evidencing that piperine allied cytotoxicity in MCF-7 cells is also mediated via induction of cell cycle arrest.
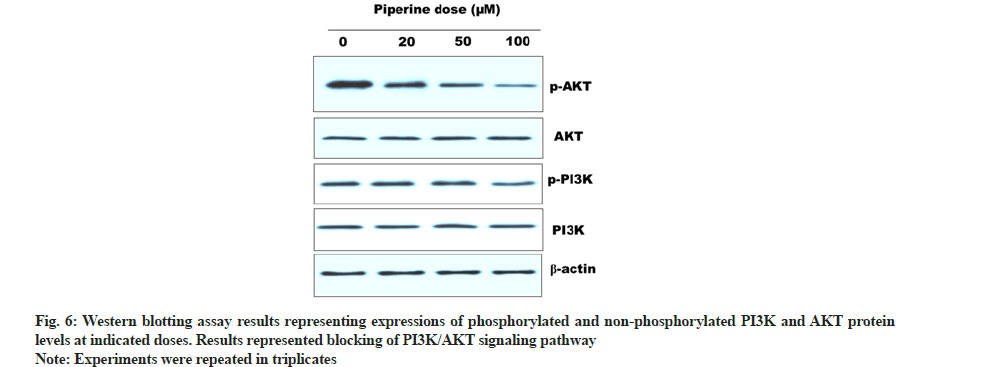
The effect of piperine over the PI3K/AKT signaling pathway was investigated via Western blotting assay. This pathway plays a vital role in cancer cell development and progression. Hence, hampering this pathway leads to suppression of cancer. After, piperine treatment cellular protein was quantified and subjected to Western blotting. Results indicated that the activity of phosphorylated PI3K and AKT reduced remarkably with increased piperine doses. In contrast with this PI3K and AKT activity remained unchanged (fig. 6). Thus indicated blocking of PI3K/AKT signaling pathway in MCF-7 cells by piperine.
Different chemotherapeutics induce anti-cancer effects via induction of apoptosis in cancer cells. Apoptosis is accomplished via two different pathways; extrinsic (death receptor allied) and intrinsic (mitochondria allied)[26]. Piperine has a tendency to alter the expressions of apoptosis engaged proteins thus has a capability of inducing both intrinsic as well extrinsic apoptosis. Piperine has been reported with anti-tumor activity against mouse BC 4T1 model and also suppressed tumor metastasis[27]. The anti-cancer effects of piperine in 4T1 cells were due to induction of caspase-3-reliant intrinsic apoptosis and cell cycle arrest at G2/M phase via reduction of cyclin B1 activity. Piperine was also reported with blocking of Sterol Regulatory Element-Binding Protein 1 (SREBP-1), Extracellular Signal-Regulated Kinases (ERK) 1/2 and Fas Cell Surface Death Receptor (FAS) expression in over expressive HER-2 BC[28]. Piperine inhibits Epidermal Growth Factor (EGF) allied Matrix Metallopeptidase 9 (MMP-9) activity and acts via suppression of Nuclear Factor Kappa B (NF-kB) and Activator Protein 1 (AP-1) activation and by intervening with p38 Mitogen-Activated Protein Kinase (MAPK), AKT and ERK1/2 signaling pathways which leads to suppression of cellular migration[29]. In the current investigation, piperine alkaloid was testified for induction of anticancer and apoptotic effects in human BC cells, along with investigating its effects on cell migration and cell invasion, cell cycle and PI3K/AKT signaling pathway. It was observed that piperine induced cytotoxicity in MCF-7 cells in a dose dependent manner along with suppression of cancer cell colonies. Further phase contrast microscopy predicted specific morphological modifications that pointed induction of cytotoxicity by piperine may be mediated via induction of apoptosis. It was evidenced by performing AO/EB staining that piperine induced apoptotic cell death in MCF-7 cells and further supported by Western blotting (showed increased BAX and decreased BCL-2 levels). Cell migration as well as cell invasion ability was reduced to minimum by application of piperine on MCF-7 cells. Next, piperine arrested the cell cycle at G2/M phase depicted after execution of flow cytometric analysis. Finally, Western blotting assay revealed blocking of PI3K/AKT signaling pathway.
In conclusion, the current investigation evidenced that piperine alkaloid induces anticancer and apoptotic effects in human BC cells. The effects of BC suppression were found to be mediated via suppression of cell migration and cell invasion, G2/M phase cell cycle arrest and targeting PI3K/AKT signaling pathway.
Funding support
This study was supported by the project of Shanghai Municipal Commission of Health and Family Planning (No. 20164Y0276)
Conflict of interests:
The authors declared no conflict of interest.
References
- Forouzanfar MH, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJ, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: A systematic analysis. Lancet 2011;378(9801):1461-84.
[Crossref] [Google Scholar] [PubMed]
- Sims AH, Howell A, Howell SJ, Clarke RB. Origins of breast cancer subtypes and therapeutic implications. Nat Clin Pract Oncol 2007;4(9):516-25.
[Crossref] [Google Scholar] [PubMed]
- Hedenfalk IA, Ringnér M, Trent JM, Borg A. Gene expression in inherited breast cancer. Adv Cancer Res 2002;84:1-34.
[Crossref] [Google Scholar] [PubMed]
- Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, et al. Tailoring therapies-improving the management of early breast cancer: St Gallen International expert consensus on the primary therapy of early breast cancer 2015. Ann Oncol 2015;26(8):1533-46.
[Crossref] [Google Scholar] [PubMed]
- Bernstein L, Ross RK. Endogenous hormones and breast cancer risk. Epidemiol Rev 1993;15(1):48-65.
[Crossref] [Google Scholar] [PubMed]
- Azim HA, Partridge AH. Biology of breast cancer in young women. Breast Cancer Res 2014;16(4):427.
[Crossref] [Google Scholar] [PubMed]
- Key TJ, Pike MC. The role of oestrogens and progestagens in the epidemiology and prevention of breast cancer. Eur J Cancer Clin Oncol 1988;24(1):29-43.
[Crossref] [Google Scholar] [PubMed]
- Yu F, Takahashi T, Moriya J, Kawaura K, Yamakawa J, Kusaka K, et al. Traditional Chinese medicine and Kampo: A review from the distant past for the future. J Int Med Res 2006;34(3):231-9.
[Crossref] [Google Scholar] [PubMed]
- Azaizeh H, Saad B, Khalil K, Said O. The state of the art of traditional Arab herbal medicine in the Eastern region of the Mediterranean: A review. Evid Based Complement Alternat Med 2006;3(2):229-35.
[Crossref] [Google Scholar] [PubMed]
- Scartezzini P, Speroni E. Review on some plants of Indian traditional medicine with antioxidant activity. J Ethnopharmacol 2000;71(1-2):23-43.
[Crossref] [Google Scholar] [PubMed]
- Cheng JT. Drug therapy in Chinese traditional medicine. J Clin Pharmacol 2000;40(5):445-50.
[Crossref] [Google Scholar] [PubMed]
- Lev E. Ethno-diversity within current ethno-pharmacology as part of Israeli traditional medicine: A review. J Ethnobiol Ethnomed 2006;2(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- Volkov SK, Grodnitskaya EI. Application of high-performance liquid chromatography to the determination of vinblastine in Catharanthus roseus. J Chromatogr B Biomed Sci Appl 1994;660(2):405-8.
- Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel anti-leukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 1971;93:2325-7.
- Freire-de-Lima L, Ribeiro TS, Rocha GM, Brandão BA, Romeiro A, Mendonça-Previato L, et al. The toxic effects of piperine against Trypanosoma cruzi: Ultrastructural alterations and reversible blockage of cytokinesis in epimastigote forms. Parasitol Res 2008;102(5):1059-67.
[Crossref] [Google Scholar] [PubMed]
- Lu JJ, Bao JL, Chen XP, Huang M, Wang YT. Alkaloids isolated from natural herbs as the anticancer agents. Evid Based Complement Alternat Med 2012;2012:485042.
[Crossref] [Google Scholar] [PubMed]
- Sahi S, Tewatia P, Ghosal S. Leishmania donovani pteridine reductase 1: Comparative protein modeling and protein–ligand interaction studies of the leishmanicidal constituents isolated from the fruits of Piper longum. J Mol Model 2012;18(12):5065-73.
[Crossref] [Google Scholar] [PubMed]
- Rafiq RA, Ganai BA, Tasduq SA. Piperine promotes ultraviolet (UV)-B-induced cell death in B16F10 mouse melanoma cells through modulation of major regulators of cell survival. RSC Adv 2015;5(16):11884-94.
- Rodgers G, Doucette CD, Soutar DA, Liwski RS, Hoskin DW. Piperine impairs the migration and T cell-activating function of dendritic cells. Toxicol Lett 2016;242:23-33.
[Crossref] [Google Scholar] [PubMed]
- Samuel M, Oliver SV, Coetzee M, Brooke BD. The larvicidal effects of black pepper (Piper nigrum L.) and piperine against insecticide resistant and susceptible strains of Anopheles malaria vector mosquitoes. Parasit Vectors 2016;9(1):238.
- Philipova I, Valcheva V, Mihaylova R, Mateeva M, Doytchinova I, Stavrakov G. Synthetic piperine amide analogs with antimycobacterial activity. Chem Biol Drug Des 2018;91(3):763-8.
[Crossref] [Google Scholar] [PubMed]
- Soutar DA, Doucette CD, Liwski RS, Hoskin DW. Piperine, a pungent alkaloid from black pepper, inhibits B lymphocyte activation and effector functions. Phytother Res 2017;31(3):466-74.
[Crossref] [Google Scholar] [PubMed]
- Gorgani L, Mohammadi M, Najafpour GD, Nikzad M. Piperine-The bioactive compound of black pepper: From isolation to medicinal formulations. Compr Rev Food Sci Food Saf 2017;16(1):124-40.
[Crossref] [Google Scholar] [PubMed]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65(1-2):55-63.
[Crossref] [Google Scholar] [PubMed]
- Zainal Ariffin SH, Wan Omar WH, Zainal Ariffin Z, Safian MF, Senafi S, Megat Abdul Wahab R. Intrinsic anticarcinogenic effects of Piper sarmentosum ethanolic extract on a human hepatoma cell line. Cancer Cell Int 2009;9(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Tanaka T. Role of apoptosis in the chemoprevention of cancer. J Exp Clin Med 2013;5(3):89-91.
- Lai LH, Fu QH, Liu Y, Jiang K, Guo QM, Chen QY, et al. Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharm Sin B 2012;33(4):523-30.
[Crossref] [Google Scholar] [PubMed]
- Do MT, Kim HG, Choi JH, Khanal T, Park BH, Tran TP, et al. Antitumor efficacy of piperine in the treatment of human HER2-overexpressing breast cancer cells. Food Chem 2013;141(3):2591-9.
[Crossref] [Google Scholar] [PubMed]
- Pradeep CR, Kuttan G. Piperine is a potent inhibitor of nuclear factor-κB (NF-κB), c-Fos, CREB, ATF-2 and proinflammatory cytokine gene expression in B16F-10 melanoma cells. Int Immunopharmacol 2004;4(14):1795-803.
[Crossref] [Google Scholar] [PubMed]
 .
.