- *Corresponding Author:
- M. Singh
GB Pant National Institute of Himalayan Environment, Sikkim Regional Centre, Pangthang, Gangtok, Sikkim 737101, India
E-mail: singmithilesh@gmail.com
| Date of Received | 17 March 2021 |
| Date of Revision | 18 April 2022 |
| Date of Acceptance | 27 January 2023 |
| Indian J Pharm Sci 2023;85(1):207-216 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In the present study, four important ethnomedicinal plants of Sikkim Himalaya viz., Astilbe rivularis Buch- Ham ex. D. Don, Eupatorium adenophorum Spreng., Bergenia ciliata (Haw.) Sternb and Artemisia vulgaris L. were investigated to ensure its ethno pharmacological potency. The methanolic root extracts of the selected plants were investigated for total phenolic content, total flavonoid content, antioxidant and antimicrobial activities. Among the tested extracts, the root methanolic extract of Bergenia ciliata contained the highest total phenolic content (911.4±0.124 mg gallic acid equivalents/g extract) and showed exceptional activity in 2,2-diphenyl-1-picrylhydrazyl and 2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid assays with halfmaximal inhibitory concentration value of 10 μg/ml and 1.0 μg/ml, respectively. The minimum inhibitory concentration of Bergenia ciliata root methanolic extract was also found to be the lowest for gram-negative bacteria Escherichia coli (600 μg/ml) and actinomycetes Streptomyces sp. (800 μg/ml), suggesting high and effective potency against microbes. Bergenia ciliata and Astilbe rivularis root extracts were further investigated for phenolic compounds using high performance liquid chromatography. The extracts of Bergenia ciliata and Astilbe rivularis were found to contain substantial amount of bergenin, gallic acid, chlorogenic acid, catechin, p-coumeric and m-coumeric acid. In conclusion, this study gives scientific evidence on the biological activities of the selected plant species and recommends Bergenia ciliata and Astilbe rivularis as candidate plants for pharmaceutical drug development.
Keywords
Medicinal plants, antioxidant, antimicrobial, phenolic acid, bergenin, Sikkim Himalaya
Herbal medicine is one of the most important traditional legacies, a pioneer component of the traditional trend being followed all over the world for centuries. In recent years, the use of herbal remedies to treat and cure various ailments has been exponentially growing. Traditional knowledge regarding the use of various medicinal plants in primary healthcare practices is abundant in the Indian Himalayan region. Likewise, Sikkim endows many medicinal plants that are used by the local inhabitants for the cure of diseases such as wound healing, gout, diabetes, arthritis, fever, inflammatory disease, immune modulation, respiratory disease, vaginal problems, antimicrobial, etc.,[1-3]. The healing and curing properties of medicinal plants are mainly due to their secondary metabolites such as phenol, terpenoids, alkaloids, etc. Secondary metabolites accumulate in different parts of the plant such as root, stem, leaves, fruit, seeds and the amount of these metabolites vary from species to species depending on its age or various ecological and climatic factors. Of all the plant secondary metabolites, phenolic and polyphenolic groups of compounds are gaining much more importance with their ability to act as redox elements allowing them to act as reducing agents, hydrogen donators and even oxygen quenchers. They inhibit many oxidizing enzymes and counteract free radicals[4]. Recent studies corroborate that antioxidant obtained from medicinal plants are effective against free radicals which are the main cause of cancer, diabetes, inflammatory disease, asthma, cardiovascular diseases, neurodegenerative diseases and premature aging[5,6].
In recent years, aboriginal knowledge regarding locally available medicinal plants of Sikkim is being documented enormously, but a huge gap still exists. So far, very limited efforts have been made towards validation of pharmacological properties of medicinal plants. Moreover, medicinal plants quality with respect to bioactive constituents is also not known. Therefore, the present study was aimed to investigate four medicinal plants of Sikkim viz. Astilbe rivularis (A. rivularis), Artemisia vulgaris (A. vulgaris), Bergenia ciliata (B. ciliata) and Eupatorium adenophorum (E. adenophorum) (Table 1) for their phytochemicals, antioxidant and antimicrobial activities.
| S. no | Botanical name | Common name | Family | Traditional uses and references |
|---|---|---|---|---|
| 1 | Astilbe rivularis Buch-Ham ex. D. Don | Burokhati, Pango | Saxifragaceae | Rhizome is used to treat toothache, body pain, and menstrual disorder. Leaves are used as blood purifier |
| 2 | Artemisia vulgaris L. | Titaypati | Asteraceae | Leaf extract used on cuts and bruises to stop bleeding mostly in nose bleeding. It possesses detergent effect and used as cleansing agent. |
| 3 | Bergenia ciliata (Haw.) Sternb | Pakhanbed | Saxifragaceae | Rhizome is used to treat fractured bones, fresh cuts, wounds, diarrhoea, pulmonary infections, vomiting, fever, cough and boils by locales |
| 4 | Eupatorium adenophorum Spreng. | Banmara, Namnong | Asteraceae | Leaves extract is used in cuts and wounds. |
Table 1: Botanical Name/Authority, Family, Traditional uses and References of Selected Sikkim Himalayan Medicinal Plants used in Traditional Medicine
Materials and Methods
Sample collection:
The root samples of four medicinal plants namely, A. vulgaris, E. adenophorum, A. rivularis and B. ciliata were collected during the flowering season (March 2014) from the plants growing in the arboretum of G. B. Pant National Institute of Himalayan Environment (GBPNIHE), Sikkim Regional Centre, Gangtok, India (latitude 27° 21' 35.7" N; longitude 88° 37' 24.4" E), located at the elevation of 2047 m above sea level. The plant materials were washed thoroughly under running tap water, followed by distilled water and dried on blotting paper at room temperature. Thereafter, washed materials were dried in an oven at 40°±2° until a constant weight was achieved. The dried plant samples were crushed using a grinder into a fine powder, packaged in a polythene bag and kept in a cool dark place for further experimentations.
Chemicals and reagents:
Seven standards namely (+)-catechin, p-coumaric acid, m-coumaric acid, caffeic acid, ferulic acid, gallic acid and bergenin were procured from Sigma Aldrich, India. 2,2-Azinobis-3-Ethylbenzothiazoline-6-Sulphonic Acid (ABTS), Butylatedhydroxytoluene (BHT), chlorogenic acid, 2,2-Diphenyl-1-Picrylhydrazyl (DPPH), High Performance Liquid Chromatography (HPLC) grade methanol, quercetin and Folin and Ciocalteu’s phenol (FC) reagent were purchased from HiMedia, Pvt. Ltd. (Mumbai, India).
Extract preparation:
To prepare the plant extract, 2 g of powdered sample was taken in a conical flask and soaked in 10 ml methanol solution for 24 h. The supernatant was transferred into a new tube and the residue was re-extracted twice with 10 ml solvent. Extracts were filtered using Buckner funnel and Whatman filter paper no.1. The accumulated filtrate was concentrated through drying in an oven maintained at 40°. The concentrated extract was suspended in methanol to yield a 50 mg/ml stock solution.
Determination of Total Phenolic Content (TPC):
TPC was evaluated according to the method of Meda et al.[7]. Briefly, 100 μl of 5 mg/ml extract was transferred in 2.5 ml of 10-fold diluted FC reagent and after 3 min, 2.0 ml of 7.5 % sodium carbonate solution was added. Reaction mixtures were incubated for 30 min at room temperature and then optical density was measured using Ultra Violet (UV)-spectrophotometer (UV- 1800, Shimadzu, Kyoto, Japan) at 760 nm. Gallic acid was used as a standard and TPC of plant extracts was expressed in mg Gallic Acid Equivalents (mg (GAE)/g extract).
Determination of Total Flavonoid Content (TFC):
TFC was estimated by aluminum chloride colorimetric method, with minor modification[8]. In brief, the individual test samples were dissolved in methanol and 1 ml sample solution was properly mixed with 1 ml solution of 2 % aluminium chloride methanolic solution. After 10 min of incubation at ambient temperature, the optical density of the solution was measured at 410 nm using a spectrophotometer. TFC was expressed as milligram Quercetin Equivalent (mg QE/g extract).
Antioxidant Potential:
DPPH free radical scavenging ability: DPPH assay was performed according to the method of Sarikurkcu et al.[9]. Extract dilutions (1 ml) were added to 0.004 % (4 ml) methanolic solution of DPPH. The optical density of the mixture was measured at 517 nm using a spectrophotometer after 30 min of incubation at room temperature. BHT was used as the standard. The ability of a sample to scavenge DPPH radical was calculated by the following equation:
DPPH radical scavenging activity (%)=[(Abscontrol- Abssample)]/[(Abscontrol)×100
Where, Abscontrol is the absorbance of DPPH radical+methanol; Abssample is the absorbance of DPPH radical+extract/standard. The radical scavenging activity of the extracts was determined by the half maximal Inhibitory Concentration (IC50) value. The IC50 value is the concentration of extracts at which DPPH radicals are scavenged by 50 %. The lowest IC50 value indicates higher radical scavenging capacity and vice versa.
Determination of ABTS radical scavenging ability:
The ABTS radical scavenging assay was done by preparing a stock solution in which an equal amount of ABTS (7 mM) and ammonium per sulfate were mixed[10]. The working solution was prepared by diluting the above stock solution with methanol until the absorbance at 734 nm was 0.706±0.02. Fresh ABTS solution was prepared for each assay. Medicinal plant extracts (1.5 ml) were allowed to react with the equal amount of ABTS working solution and after 7 min of incubation, the optical density was taken at 734 using the spectrophotometer. The ABTS scavenging capacity was compared with that of BHT.
ABTS radical scavenging activity (%)=[(Abscontrol- Abssample)]/[(Abscontrol)]×100
Where, Abscontrol is the absorbance of ABTS radical+methanol; Abssample is the absorbance of ABTS radical+extract/standard
The radical scavenging activity of the extracts was determined by IC50 value as mentioned in above DPPH assay.
Antimicrobial potential:
actinomycetes and two fungal phytopathogens) were taken from the microbial culture collection established in the microbiology laboratory of GBPNIHE, Almora, India. Methanolic root extracts were used to determine the antimicrobial activity by the disc diffusion method. The test microorganism i.e. bacteria and actinomycetes were grown in tryptone yeast extract broth whereas fungi in potato dextrose broth. 24 h grown culture of bacteria and 5 d grown culture of actinomycetes and fungi were used for antimicrobial assays. For determination of antimicrobial activity against test microorganisms, 100 μl of broth culture was evenly spread on the respective agar medium in petri plates and sterilized 5 mm disc filters were placed over it. 15 μl of root methanolic extract was then poured over each disc. The plates were incubated at 25° for 2 d in case of bacteria and 5 d for actinomycetes/fungi. A solvent control for each test microorganism was also maintained to nullify any inhibition by it. Following incubation, the zone around the disc was calculated for each test microorganism. All the experiments were performed at least in triplicates and the mean value was calculated.
Minimum Inhibitory Concentration (MIC) of extracts against test microorganisms was determined by following standard protocol of clinical and laboratory standard institute[11,12].
HPLC analysis for bioactive compounds:
Eight bioactive compounds viz. chlorogenic acid, (+)-catechin, p-coumaric acid, m-coumaric acid, caffeic acid, ferulic acid, gallic acid and bergenin detection and quantification were done using reversephase HPLC (Shimadzu, LC-2030 Plus) equipped with Prominence Diode Array Detector. The separation was carried out using a C18 column (Shimadzu–pack solar, 5 μm, 4.6×250 mm). The mobile phase used for the determination of chlorogenic acid, (+)-catechin, p-coumaric acid, m-coumaric acid, caffeic acid, ferulic acid and gallic acid was methanol (A) and 0.1 % orthophosphoric acid in water (B) in 40:60 with a flow rate of 1 ml/min for the total run time of 20 min. A stock solution of 1 mg/ml for each phenolic acid was prepared in methanol. The stock (1 mg/ml) of each known compound was diluted to different concentrations for the preparation of calibration curves. The curve linearity for each standard was determined based on the correlation coefficient. Identification and quantification of the compound were done based on retention time and absorption wavelength spectra profile of each standard.
For the detection and quantification of bergenin, methanol in isocratic mode was used with a flow rate of 0.5 ml/min and injection volume was 20 μl. The detection wavelength for bergenin was 272 nm.
Statistical analysis:
The investigated parameters, except phenolic acid quantification and MIC, were analyzed for Analysis of Variance (ANOVA) and significance was determined at p<0.05 using Statistical Package for the Social Sciences (SPSS) (version 16) software. The significant differences among the mean values were assessed based on Duncan’s multiple range tests. The mean and standard deviation were calculated using MS-Excel. Each experiment was repeated thrice.
Results and Discussion
The content of the extractable phenolic compounds was deduced through a linear gallic acid standard curve (y=2.468x+0.088; R2=0.996). In the investigated root extracts, TPC was detected in the following decreasing order: B. ciliata>A. rivularis>A. vulgaris>E. adenophorum. The highest TPC (911.4±0.124 mg GAE/g extract) was detected in B. ciliata extract as shown in fig. 1.
Fig. 1: Total phenolic and flavonoid contents of root methanolic extracts of selected medicinal plants.
Note: Values are the mean±standard deviation of triplicate experiments. Different letter in a column shows significant differences at p<0.05. AV: Artemisia vulgaris; AR: Astilbe rivularis; BC: Bergenia ciliata; EA: Eupatorium adenophorum; (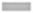 ): TPC and (
): TPC and ( ): TFC
): TFC
TFC was determined through a linear quercetin standard curve (y=28.47x+0.103; R2=0.998). TFC of the studied extracts was found in the following order: A. vulgaris>E. adenophorum>B. ciliata>A. rivularis. The highest TFC of 151.6±1.1 mg QE/g extract was observed in A. vulgaris extract whereas the lowest TFC was detected in A. rivularis extract as shown in fig. 1.
TPC results indicated that among all the studied four plants, the roots of Saxifragaceae family plants contained comparatively higher amount of phenolic compounds than the roots of Asteraceae family plants. Results of flavonoid analysis revealed that root is a reservoir of flavanoids in Asteraceae plants whereas in Saxifragaceae plants root contain comparatively less amount of flavonoids. Earlier, researchers have reported that secondary metabolite accumulation in the medicinal plant is cell and organ-specific and it varies from plant to plant[13].
Previous investigations in Bergenia species showed that Bergenia contained simple phenols, flavonoids, coumarins and tannins making it one of the most potent therapeutic sources which could be used for drug development by pharmaceutical industries in future[14-16]. Likewise, in our study, the root methanolic extract of B. ciliata estimated a high amount of phenolic compounds. Another potent medicinal plant A. rivularis has also exhibited high phenolic content in its root. Other previous studies that have analyzed the total phenolic compounds present in A. rivularis came to the same conclusion depicting high phenolic compounds in the plant which has led to its increased therapeutic potential[17]. All the studied plant species contained a fairly good amount of phenol and flavonoid content, therefore, providing an insight into its pharmacological activity and traditional use.
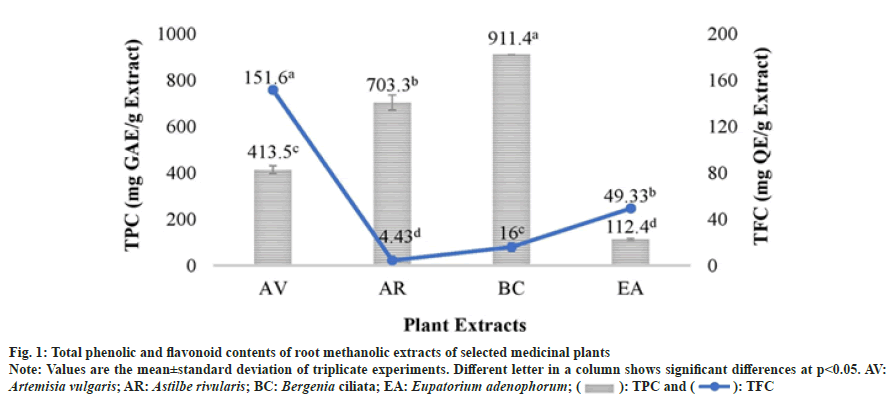
The antioxidant capacities of methanolic extracts of selected plant species were determined using two assays viz. DPPH and ABTS which are based on different reaction mechanisms. The results of the antioxidant assays revealed that the selected plant extracts possessed a significant variability in their inhibitory activity against DPPH and ABTS radicals. In both the assays, B. ciliata and A. rivularis root methanolic extracts showed high radical scavenging activity with DPPH IC50 values of 10.8 μg/ml and 20.0 μg/ml respectively and ABTS IC50 values of 1.0 and 2.0 μg/ml, respectively (fig. 2). The plant extracts which contained higher TPC such as B. ciliata and A. rivularis exhibited better antioxidant activity. A. vulgaris root methanolic extract also exhibited good antioxidant activity which could be due to higher flavonoid content (151.6±2.88 mg QE/g extract). Recently, Subba et al.[18] reported antioxidant activity (DPPH IC50 value 32.05±2.59 μg/ml) of the methanolic root extract of A. vulgaris from the Nepal Himalaya.
Correlation analysis of TPC and TFC with DPPH and ABTS assays revealed that there is a significant correlation between the TPC and antioxidant assays (Table 2). The negative significant correlation was found between TPC and ABTS (R2=-0.97, p<0.05) which suggests that TPC contribution in antioxidant property of the plant is higher than TFC. These results are in well agreement with Kainama et al.[19] work. Moreover, it is well established that antioxidant compounds such as phenolics and flavanoids exert their effect through different mechanism which in turn depend on their structure and functional groups.
| Antioxidant assays | TPC | TFC |
|---|---|---|
| ABTS | -0.97475* | 0.391268 |
| DPPH | -0.84042 | -0.02718 |
Note: *Correlation significance at p<0.05
Table 2: Correlations Coefficient (R2) between the Ic50 Values of Antioxidant Assays and Total Phenolic and Flavonoids Content
Due to the detrimental effects of synthetic antioxidants such as Butylate hydroxyanisole and BHT, traditional medicinal plants are extensively being investigated worldwide in search of novel plant-derived antioxidant compounds. In this study, the root methanolic extracts of B. ciliata and A. rivularis have shown better antioxidant activity than the synthetic antioxidant BHT. These results suggest that natural antioxidants provided by these plants could be used to develop safe drugs with no side effects. Earlier studies on the phytochemical screening and antioxidant activity of B. ciliata and A. rivularis have always shown them to comprise high phenolic compounds and subsequently high antioxidant activity making them highly ethnopharmacologically important[16,17,20].
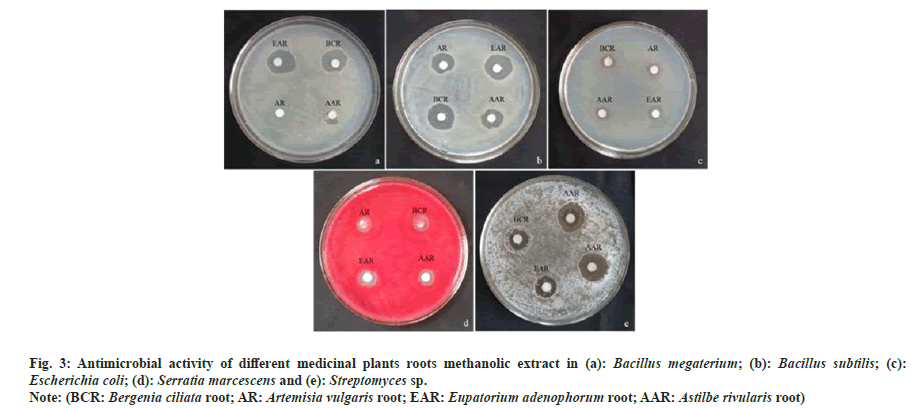
The antimicrobial activity of studied plants (i.e. B. ciliata, A. vulgaris, E. adenophorum and A. rivularis) varied with respect to the test organisms. Generally, bacteria and actinomycetes were found more sensitive to antimicrobial compounds present in methanolic extracts, whereas fungus did not show any zone of inhibition (Table 3). Out of the four plant extracts, E. adenophorum extract showed maximum zone of inhibition against Bacillus megaterium (B. megaterium) (4.17 mm) followed by B. ciliata (4.00 mm) and A. rivularis (3.83). A. vulgaris extract showed no activity against B. megaterium. Similarly, maximum inhibition against Bacillus subtilis (B. subtilis) was observed by root extract of E. adenophorum (6.83 mm) which was followed by B. ciliata, A. rivularis and A. vulgaris extracts. In the case of Gram-negative bacteria, extracts of B. ciliata and E. adenophorum showed maximum inhibition against Serratia marcescens and Escherichia coli (E. coli), respectively. Root extracts of all selected species showed no activity against Nocardia tenerifensis, whereas Streptomyces sp. was inhibited maximum by root extract of A. vulgaris. The antimicrobial activity of medicinal plant extracts are shown in fig. 3. MIC was determined for studied plant root extracts. B. ciliata and A. rivularis showed MIC of 1000 μg/ml and 1800 μg/ml against B. megaterium and Serratia marcescens, respectively. In case of B. subtilis, A. rivularis showed the lowest MIC 600 μg/ml. B. ciliata showed 600 μg/ml MIC for E. coli. B. ciliata exhibited a MIC of 800 μg/ ml against Streptomyces sp. Results of MIC are given in Table 4. Recently, Khan et al.[21] noted B. ciliata root extract antibacterial activity against both gram-positive (B. subtilis and Bacillus atrophoeus) and gram-negative (Kleibsiella pneumonia and Pseudomonas aeruginosa) bacteria with no antifungal activity. The methanolic leaf extract of A. rivularis had also been reported of having good antibacterial activity against gram-positive B. subtilis, B. megaterium, Streptococcus pyogenes and Staphylococcus aureus as well as gram-negative bacteria like E. coli, Pseudomonas aeruginosa, Salmonella typhi and Shigella dysenteriae with MIC value ranged from 8-64 μg/ml against the tested bacterial pathogen[22]. Rai et al.[23] reported antibacterial activity of the rhizome extract of A. rivularis against both gram-positive and gram-negative bacterial pathogens such as B. subtilis, Bacillus amyloliquefaciens, Aeromonas liquefaciens, Flexibactor sp., and Psedomonas sp. Adhikary et al.[24] reported antimicrobial activity of rhizome methanolic extract of A. rivularis against E. coli with MIC of 0.011 mg/ml. As literature indicates, this is the first report on antimicrobial activity of A. rivularis from Sikkim Himalaya. Moreover, so far, very scant information on antimicrobial property of root extracts of selected plants is available.
| Microorganisms | Zone around disc (mm) | |||
|---|---|---|---|---|
| BC | AV | EA | AR | |
| Gram-positive bacteria | ||||
| Bacillus megaterium | 4.00±0.00 | NA | 4.17±0.16 | 3.83±0.16 |
| Bacillus subtilis | 6.17±0.16 | 2.67±0.16 | 6.83±0.43 | 5.17±0.16 |
| Gram-negative bacteria | ||||
| Escherichia coli | 2.17±0.43 | 1.00±0.28 | 4.00±0.00 | 0.67±0.32 |
| Serratia marcescens | 3.5±0.00 | 1.00±0.30 | 3.00±0.30 | 3.20±0.30 |
| Actinomycetes | ||||
| Nocardia tenerifensis | NA | NA | NA | NA |
| Streptomyces sp. | 3.33±0.16 | 4.17±0.16 | 2.33±0.16 | 3.67±0.16 |
| Fungi | ||||
| Fusarium oxysporium | NA | NA | NA | NA |
| Fusarium solani | NA | NA | NA | NA |
Table 3: Antimicrobial Activity of Root Extracts against Test Microorganisms by Disc Diffusion Method
Fig. 3: Antimicrobial activity of different medicinal plants roots methanolic extract in (a): Bacillus megaterium; (b): Bacillus subtilis; (c): Escherichia coli; (d): Serratia marcescens and (e): Streptomyces sp.
Note: (BCR: Bergenia ciliata root; AR: Artemisia vulgaris root; EAR: Eupatorium adenophorum root; AAR: Astilbe rivularis root)
| Microorganisms | MIC (µg/ml) | |||
|---|---|---|---|---|
| BC | AV | EA | AR | |
| Gram positive bacteria | ||||
| Bacillus megaterium | 1000 | - | 1800 | 1000 |
| B. subtilis | 800 | 1200 | 800 | 600 |
| Gram negative bacteria | ||||
| Escherichia coli | 600 | 800 | 1000 | 1000 |
| Serratia marcescens | 1800 | 2000 | 2000 | 1800 |
| Actinomycetes | ||||
| Streptomyces sp. | 800 | 2000 | 1600 | 1400 |
Table 4: MIC of Plant Roots Extracts against Test Microorganisms
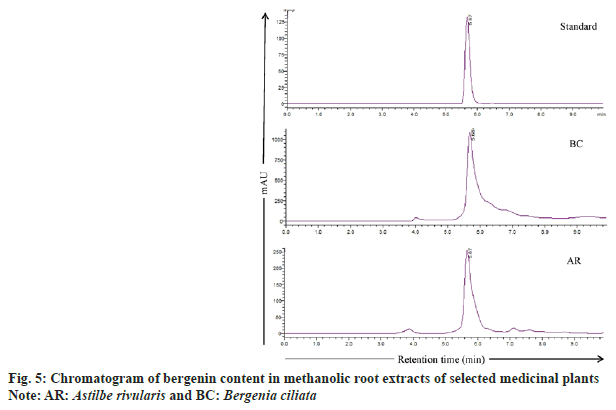
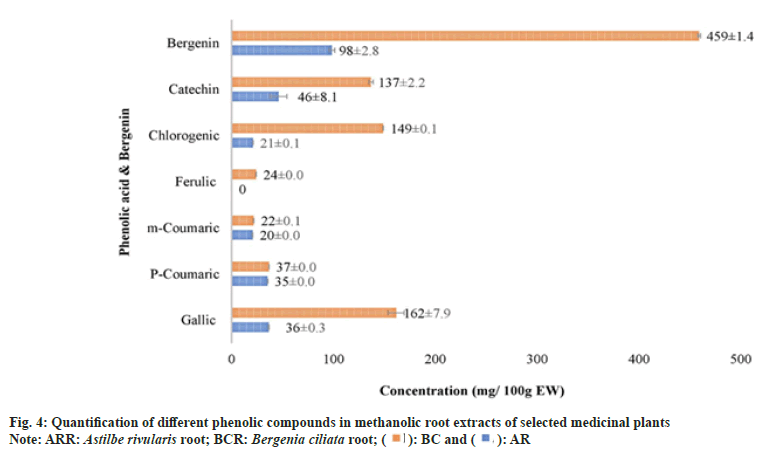
As A. rivularis and B. ciliata root extracts had shown excellent antioxidant and antimicrobial activities, these extracts were further selected for quantification of phenolic compounds namely chlorogenic acid, (+)-catechin, p-coumaric acid, m-coumaric acid, caffeic acid, ferulic acid, gallic acid and bergenin (fig. 4). Phenolic compounds were detected and identified according to their maximum absorption spectra, retention time and the coefficients of determination (R2) of standard compounds (Table 5). The phenolic compounds (chlorogenic acid, (+)-catechin, p-coumaric acid, m-coumaric acid, caffeic acid, ferulic acid and gallic acid) in the root extract of A. rivularis in decreasing order were catechin (46.8±8.1 mg/100 g EW), gallic acid (36±0.3 mg/100 g EW), p-coumaric acid (35±0.0 mg/100 g EW), chlorogenic acid (21±0.1 mg/100 g EW) and m-coumaric acid (20±0.0 mg/100 g EW). Ferulic acid and caffeic acid could not be detected in A. rivularis extract. Earlier, the preliminary investigation of A. rivularis rhizome extract had revealed the presence of alkaloids, flavonoids, coumarins and glycosides[25]. The present study, for the first time, reported presence of bioactive compounds such as catechin, gallic acid, p-coumaric, m-coumaric acid and chlorogenic acid in A. rivularis root extract. Hori et al.[26] isolated seven potent antioxidant constituents i.e. bergenin, 11-ogalloylbergenin, (+)-catechin, (−)-catechin, (−)-afzelechin, (−)-epiafzelechin and 2-(β-D-glucopyranosyloxy)- 4-hydroxylbenzenacetonitrile from the rhizome of A. rivularis. Earlier, GC-MS analysis revealed the presence of terpenoids and fatty acids in the methanolic rhizome extract of A. rivularis[22].
| Compounds | Retention time (min) | Wavelength (nm) | Standard equation | R2 |
|---|---|---|---|---|
| Gallic acid | 3.79 | 278 | y=31527x+-2030 | 0.999 |
| p-Coumeric | 12.38 | 309 | y=112389x-279726 | 0.995 |
| m-Coumeric | 15.47 | 278 | y=22095x-7454 | 0.998 |
| Ferulic acid | 13.53 | 325 | y=64147x-8782 | 0.994 |
| Chlororgenic acid | 5.51 | 325 | y=46335x-12687 | 0.998 |
| Caffeic acid | 7.73 | 325 | y=66668x-2270 | 1 |
| Catechin | 3.79 | 216 | y=86202x-4672 | 0.999 |
| Bergenin | 5.67 | 272 | y=21313x+25411 | 0.988 |
Table 5: Standard Curve Analysis for Quantification of Bioactive Compounds
The decreasing order of phenolic compounds in the methanolic root extract of B. ciliata was recorded as gallic acid (162±7.9 mg/100 g EW), chlorogenic acid (149±0.1 mg/100 g EW), catechin (137±2.2 mg/100 g EW), p-coumaric acid (37±0.0 mg/100 g EW), ferulic acid (24±0.0 mg/100 g EW) and m-coumaric acid (22±0.1 mg/100 g EW).
In spite of high medicinal value and frequent use in traditional medicinal practices, so far, there are very limited reports on the quantification of phenolic acids in B. ciliata. Phenolic compounds like bergenin, arbutin, gallic acid and catechin had been reported since decades in B. ciliata[16,27-30] but efforts to investigate other phenolic compounds such as chlorogenic acid, p-coumaric acid, ferulic acid and m-coumaric acid have never been made.
Bergenin, a C-glucoside of 4-O-methyl gallic acid, is reported in many species such as B. crassifolia, B. cordifolia[29], B. ciliata, Bergenia ligulata (B. ligulata)[31], Corylopsis spicata, Caesalpinia digyna, Mallotus japonicas, Sacaoglottis gabonensis[32] and A. rivularis[25]. In the present study, bergenin quantification was done in the methanolic root extract of B. ciliata and A. rivularis (fig. 4). HPLC analysis results showed that the roots of both the species contain substantial amount of bergenin (fig. 5). B. ciliata root extract accumulated comparatively higher amount of bergenin i.e. 459±1.4 mg/100 g EW than A. rivularis (98±2.8 mg/100 g EW). Boros et al.[29] quantified bergenin in two Bergenia species in three different sampling series and content ranged from 0.32±0.01-5.34±0.03 mg/g in Bergenia crassifolia and 0.20±0.01-4.96±0.02 mg/g in B. ligulata. Srivastava et al.[33] reported bergenin in three different species of Bergenia namely B. ligulata, B. ciliata, and Bergenia stracheyi as 2.42 %, 3.28 %, and 3.28 %. Similarly, these species were also evaluated by Rajbhandari et al.[34] for bergenin content and quantified as B. ligulate (5.73 %), B. ciliata (5.63 %) and Bergenia stracheyi (5.99 %). Bergenin and bergenin-like derivatives have been isolated from the rhizome and aerial parts of A. rivularis[35]. Other species of Astilbe were also reported for bergenin including A. chinensis[36-38], A. myriantha[39] and A. thunbergii[40].
As the literature indicates that bergenin have potential pharmaceutical application including antihepatotoxic, antiulcerogenic, anti-human immunodeficiency virus, neuroprotective, anti-inflammatory, immunomodulatory[41], hepatoprotective[42] and also various biological activities such as antiulcer, antifungal and wound healing effect[43,44]. Therefore, presence of significant amount of bergenin and other phenolic compounds in B. ciliata and A. rivularis root extract might be responsible for the antimicrobial activity against both gram-positive and gram-negative bacterial pathogen and also for their antioxidant properties.
The present study investigated the four ethnomedicinal plants of Sikkim Himalaya viz. A. rivularis, A. vulgaris, B. ciliata and E. adenophorum for phytochemicals, antioxidant and antimicrobial activities. Among the selected species, B. ciliata and A. rivularis exhibited potent antimicrobial activity and antioxidant property. Further, HPLC analysis confirmed that B. ciliata and A. rivularis roots are reservoir of phenolic compounds. The high contents of phenolic compounds including bergenin in root extracts of both the studied species might be responsible for their biological activities. In the light of these results, roots of A. rivularis and B. ciliata are anticipated as prospective source of novel drugs.
Funding:
The authors are thankful to GBPI- In House Project-9 and Department of Biotechnology, New Delhi- India as project ‘Bio prospecting of medicinal plants of Sikkim Himalaya against cancer angiogenesis’ for financial support.
Acknowledgments:
The authors are thankful to Director, G. B. Pant National Institute of Himalayan Environment, India for providing the facilities.
Conflict of interests:
The authors declared no conflict of interests.
References
- Rai L, Sharma E. Medicinal plants of the Sikkim Himalaya: Status, usage and potential. Bishen Singh Mahendra Pal Singh: CABI; 1994.
- Pradhan BK, Badola HK. Ethnomedicinal plant use by Lepcha tribe of Dzongu valley, bordering Khangchendzonga Biosphere Reserve, in north Sikkim, India. J Ethnobiol Ethnomed 2008;4(1):22.
[Crossref] [Google Scholar] [PubMed]
- Panda AK. Medicinal plants use and primary health care in Sikkim. Inte J Ayurvedic Herbal Med 2012;2(2):253-9.
- Pereira DM, Valentão P, Pereira JA, Andrade PB. Phenolics: From chemistry to biology. Molecule 2009;14(6):2202-11.
- Duthie GG, Brown KM. Reducing the risk of cardiovascular disease. In: Functional foods: Designer foods, Pharmafoods, Nutraceuticals. 1994:19-38.
- Milner JA. Reducing the risk of cancer. Functional foods: Designer foods, pharmafoods, nutraceuticals. 1994:39-70.
- Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem 2005;91(3):571-7.
- Lamaison JL, Carnet A. Contents in main flavonoid compounds of Crataegus monogyna Jacq. and Crataegus laevigata (Poiret) DC flowers at different development stages. Pharm Acta Helvetica 1990;65(1):315-20.
- Sarikurkcu C. Antioxidant activities of solvent extracts from endemic Cyclamen mirabile Hildebr. tubers and leaves. Afr J Biotechnol 2011;10(5):831-9.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med 1999;26(9-10):1231-7.
[Crossref] [Google Scholar] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 6th ed. Approved standard 2005; M7-A6.
- Clinical and Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of yeasts. 3rd ed. Approved standard 2008; M27-A3.
- Hussain MS, Fareed S, Ansari S, Rahman MA, Ahmad IZ, Saeed M. Current approaches toward production of secondary plant metabolites. J Pharm Bioallied Sci 2012;4(1):10-20.
[Google Scholar] [PubMed]
- Bagul MS, Ravishankara MN, Padh H, Rajani M. Phytochemical evaluation and free radical scavenging properties of rhizome of Bergenia ciliata (Haw.) Sternb. forma ligulata Yeo. J Nat Rem 2003;3(1):83-9.
- Dhalwal K, Shinde VM, Biradar YS, Mahadik KR. Simultaneous quantification of bergenin, catechin, and gallic acid from Bergenia ciliata and Bergenia ligulata by using thin-layer chromatography. J Food Compos Anal 2008;21(6):496-500.
- Singh M, Pandey N, Agnihotri V, Singh KK, Pandey A. Antioxidant, antimicrobial activity and bioactive compounds of Bergenia ciliata Sternb.: A valuable medicinal herb of Sikkim Himalaya. J Tradit Complement Med 2017;7(2):152-7.
[Crossref] [Google Scholar] [PubMed]
- Subedi L, Timalsena S, Duwadi P, Thapa R, Paudel A, Parajuli K. Antioxidant activity and phenol and flavonoid contents of eight medicinal plants from Western Nepal. J Tradit Chin Med 2014;34(5):584-90.
[Crossref] [Google Scholar] [PubMed]
- Subba B, Thapa S. Analysis of phytoconstituents and biological activities on the selected medicinal plants of Dolakha and Sindhualchowk district of Nepal. J Inst Sci Technol 2018;22(2):140-7.
- Kainama H, Fatmawati S, Santoso M, Papilaya PM, Ersam T. The relationship of free radical scavenging and total phenolic and flavonoid contents of Garcinia lasoar PAM. Pharm Chem J 2020;53:1151-7.
- Rajkumar V, Guha G, Kumar RA, Lazar M. Evaluation of antioxidant activities of Bergenia ciliata rhizome. Record Nat Prod 2010;4(1):38-48.
- Khan A, Jan G, Khan A, Jan FG, Danish M. Evaluation of antioxidant and antimicrobial activities of Bergenia ciliata Sternb (Rhizome) crude extract and fractions. Pak J Pharm Sci 2018;31(1):31-5.
[Google Scholar] [PubMed]
- Ghosh T, Mitra P, Mitra PK. Antibacterial Activity of Astilbe rivularis Buch.–Ham. Ex D. Don leaves: Effect of extraction solvents. Int J pharm 2018;6:2480-5.
- Rai V, Kumar A, Das V, Ghosh S. Evaluation of chemical constituents and in vitro antimicrobial, antioxidant and cytotoxicity potential of rhizome of Astilbe rivularis (Bodho-okhati), an indigenous medicinal plant from Eastern Himalayan region of India. BMC Complement Altern Med 2019;19(1):1-200.
[Crossref] [Google Scholar] [PubMed]
- Adhikary P, Roshan KC, Kayastha D, Thapa D, Shrestha R, Shrestha TM, et al. In vitro evaluation of antimicrobial and cytotoxic potential of dry rhizome extract of Astilbe rivularis. Int J Pharm and Phyt Res 2012;4:122-6.
- Timalsena S, Lamichhane PP. Astible rivularis: Bioactive compounds and pharmacological functions. Chin J Integr Med 2019;25:795-9.
[Crossref] [Google Scholar] [PubMed]
- Hori K, Wada M, Yahara S, Watanabe T, Devkota HP. Antioxidant phenolic compounds from the rhizomes of Astilbe rivularis. Nat Prod Res 2018;32(4):453-6.
[Crossref] [Google Scholar] [PubMed]
- Dix BS, Srivastava SN. Tannin constituents of Bergenia ligulata roots. Ind J Nat Prod 1989;5:24-5.
- Fujii M, Miyaichi Y, Tomimori T. Studies on Nepalese crude drugs. XXII: On the phenolic constituents of the rhizome of Bergenia ciliata (Haw.) Sternb. Nat Med J Physiol 1996;50(6):404-7.
- Boros B, Jakabová S, Madarász T, Molnár R, Galambosi B, Kilár F, et al. Validated HPLC method for simultaneous quantitation of bergenin, arbutin, and gallic acid in leaves of different Bergenia species. Chromatographia 2014;77:1129-35.
- Srivastava N, Singh BN, Srivastava A, Khan AR, Srivastava S, Sharma A, et al. Evaluation of phenolic content recoveries in hydrolyzed extracts of Bergenia ciliata using RP-HPLC, GC–MS after silylation, and validation through antioxidant potential. J Liq Chrom Related Technol 2015;38(19):1722-30.
- Patel DK, Patel K, Kumar R, Gadewar M, Tahilyani V. Pharmacological and analytical aspects of bergenin: A concise report. Asian Pac J Trop Dis 2012;2(2):163-7.
- Singh DP, Srivastava SK, Govindarajan R, Rawat AK. High-performance liquid chromatographic determination of bergenin in different Bergenia species. Acta Chromatograph 2007;19:246-52.
- Srivastava S, Rawat AK. Botanical and phytochemical comparison of three Bergenia species. J Sci Ind Res 2008;7:65-77.
- Rajbhandari M, Lalk M, Mentel R, Lindequist U. Antiviral activity and constituents of the nepalese medicinal plant Astilbe rivularis. Rec Nat Prod 2011;5(2):138-42.
- Ye YP, Sun HX, Pan YJ. Bergenin monohydrate from the rhizomae of Astilbe chinensis. Acta Crystallogr C 2004;60(6):397-8.
[Crossref] [Google Scholar] [PubMed]
- Sun HX, Ye YP, Yang K. Studies on the chemical constituents in radix Astilbes chinensis. Zhongguo Zhong Yao Za Zhi 2002;27(10):751-4.
[Google Scholar] [PubMed]
- Xue Y, Xu XM, Yan JF, Deng WL, Liao X. Chemical constituents from Astilbe chinensis. J Asian Nat Prod Res 2011;13(02):188-91.
[Crossref] [Google Scholar] [PubMed]
- Zou Y, Cui Y. Chemical constituents from Astilbe myriantha. Zhong Yao Ca 2012;35(7):1095-7.
[Google Scholar] [PubMed]
- Kimura Y, Sumiyoshi M, Sakanaka M. Effects of Astilbe thunbergii rhizomes on wound healing: Part 1. Isolation of promotional effectors from Astilbe thunbergii rhizomes on burn wound healing. J Ethnopharmacol 2007;109(1):72-7.
[Crossref] [Google Scholar] [PubMed]
- Singh U, Barik A, Priyadarsini KI. Reactions of hydroxyl radical with bergenin, a natural poly phenol studied by pulse radiolysis. Bioorg Med Chem 2009;17(16):6008-14.
[Crossref] [Google Scholar] [PubMed]
- Chen J, Zhang J, Zhuang Q, Zhang S, Lin X. Electrochemical study of bergenin on a poly (4-(2-pyridylazo)-resorcinol) modified glassy carbon electrode and its determination in tablets and urine. Talanta 2007;72(5):1805-10.
[Crossref] [Google Scholar] [PubMed]
- Nazir N, Koul S, Qurishi MA, Najar MH, Zargar MI. Evaluation of antioxidant and antimicrobial activities of Bergenin and its derivatives obtained by chemoenzymatic synthesis. Eur J Med Chem 2011;46(6):2415-20.
[Crossref] [Google Scholar] [PubMed]
- Maity D, Pradhan N, Maiti GG. Traditional uses of some medicinal plants of Sikkim Himalaya. ECOBIOS 2003;2(1-2):46-56.
- Rai LK, Prasad P, Sharma E. Conservation threats to some important medicinal plants of the Sikkim Himalaya. Biol Conserv 2000;93(1):27-33.
 ): BC and (
): BC and ( ): AR
): AR