- *Corresponding Author:
- Abha Shukla
Department of Chemistry, Kanya Gurukula Campus, Gurukula Kangri Vishwavidyalaya, Haridwar-249 407, India
E-mail: abs.gkv@gmail.com
| Date of Submission | 05 July 2014 |
| Date of Revision | 05 February 2015 |
| Date of Acceptance | 26 September 2015 |
| Indian J Pharm Sci 2015;77(5):640-644 |
This is an open access article distributed under the terms of the Creative Commons Attribution‑NonCommercial‑ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
In the present study, the antioxidant activity of successive leaf extracts of Dracaena reflexa was investigated using the scavenging activity on 1,1-diphenyl-2-picrylhydrazyl and reducing power by ferric reducing antioxidant power assay. Methanol extract was found potent in both the assays. IC50 values of 1,1-diphenyl-2-picrylhydrazyl assay for methanol extract was 0.97 mg/ml and ferric reducing antioxidant power value for the same is 1.19. Phytochemical screening, proximate analysis and total phenolic content were also determined. Qualitative screening for phytochemical showed the presence of alkaloids, flavonoids, terpenoids, glycosides and saponins. Highest phenolic content was shown by methanol extract (49.69 mg gallic acid equivalent/g dry weight). Proximate analysis showed moisture content (3.31%), ash content (8.02%), crude fibre (1.31%), crude fat (0.97%), total protein (3.70%), total carbohydrate (86.01) and nutritive value (367.56 kcal/100 g), which would make it a potential nutraceutical. This study suggested that Dracaena reflexa, a potential natural free radical scavenger, which could find use as an antioxidative.
Keywords
Dracaena reflexa, phytochemical, proximate, total phenolic content, antioxidant activity
Herbal medicine plays vital role in maintaining the health and wealth of mankind. Majority of world population use herbal medicines. The World Health Organization (WHO) reports that approximately 21,000 plants have been used for medicinal purposes [1]. Herbs have stood the test of time for their safety, efficacy, cultural acceptability and minimal side effects [2]. Therapeutic power of some plants is mainly due to the presence of some secondary metabolites, which collectively are referred to as phytochemicals [3]. These phytochemicals have potential to be developed as herbal medicines or could serve as precursors for modern medicine.
It is now widely understiood that free radicals are involved in the pathogenesis of many diseases. Natural defence mecanisms of the human body prevents development of these diseases but in cases of increased onslaught by free radicals and reactive oxygen species bodys defence mechanisms needs to be supplemented by external antioxidants. Synthetic antioxidants are less favoured due to their side effects, hence interest in natural antioxidants of herbal origin is on the increase [4]. Plant-derived phenolic compounds and flavonoids have been reported to be radical scavengers and act as good antioxidant agents.
This study was performed on leaves of Dracaena reflexa, family, Liliaceae. Certain species of Dracaena such as D. cinnabari stem have been investigated for in vitro lipid peroxidation [5], antioxidant activity [6], antiinflammatory activity [7], antimicrobial and cytotoxicity [8]. Investigations on D. draco revealed antimicrobial and antioxidant effect [6], cytotoxic effect and presence of many phenolic compounds [9]. Another species D. cambodiana showed antitumor [10], antioxidant [11] and antimicrobial activity [12]. Similarly D. cochichinensis, D. angustifolia, D. arborea and D. vand were examined by different researchers for medicinal potential. Present study focused on phytochemical screening, proximate analysis, nutritive value and antioxidant activity of leaves of D. reflexa.
Folin Ciocalteu reagent, methanol, sodium acetate and glacial acetic acid were procured from Merck India, Mumbai, sodium carbonate was obtained from Thomas Baker, Mumbai, gallic acid from Hi-Media, Mumbai, 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,4,6-tri-2- pyridyl-1,3,5-triazine (TPTZ) were obtained from Sigma Aldrich, St. Louis, USA, hydrochloric acid from Fischer Scientific, Mumbai and ferric chloride hexa hydrate from S. D. Fine Chem Ltd., Mumbai, India. All other chemicals employed were of standard analytical grade.
Fresh leaves of D. reflexa were collected from Dehradun district of Uttarakhand, India. The plant material was authenticated by the Botanical Survey of India, Dehradun. A herbarium smple (accession No. 114095) was also preserved in the Department of Chemistry, Kanya Gurukula Campus, Gurukula Kangri Vishwavidyalaya, Haridwar, India for further reference. Fresh leaves were washed with water, dried under the shade for 15 days, crushed in a grinder to powder form and then stored in an air tight container for further extraction and various processes.
Proximate analysis of the powdered leaves included estimation of moisture content, ash content, crude fibre, crude fat and protein content [13], whereas total carbohydrate was calculated using the Eqn., total carbohydrate=100−(% ash+% moisture+% crude fibre+% crude protein). Nutritive value of the leaf was expressed in kilocalories/100 g of dry weight of leaves, which was calculated using the formula [14], nutritive value=(4×% protein)+(9×% crude fat)+(4×% total carbohydrate).
One hundred and fifty grams of the dried powdered leaves of D. reflexa were weighed, loaded and extracted in a soxhlet apparatus using 1.5 l each of petroleum ether, dichloromethane, methanol and water successively in accordance to the hierarchy of polarity of solvents. Extraction was continued for 72 h or until the solvent coming out of the siphoning tube was colourless [15]. Extracts were concentrated under reduced pressure in rotary vacuum evaporator and refrigerated for further use.
Phytochemical analysis of the extracts was performed using standard qualitative methods [16,17]. The extracts were analysed for the presence of compounds like alkaloids, flavonoids, tannins, glycosides, terpenoids, steroids, fat and oil, saponins, protein. Total phenol content (TPC) of Dracaena reflexa leaf extract was measured by employed the method of Singleton [18]. To 1 ml of extract (1000 μg/ml) in methanol, 10 ml of 10% Folin Ciocalteu reagent and 8 ml of sodium carbonate (7.5% w/v) solution was added and the reaction volume was made up to 20 ml with distilled water. After 2 h of incubation at room temperature, absorbance was measured at 765 nm on a UV/Vis spectrophotometer. Total phenolics were quantified by calibration curve of gallic acid (25-300 μg/ml). The phenolic content of the sample was expressed as milligram gallic acid equivalents/gram of dry extract (mg GAE/g), which was calculated using the formula [19], T=C×V/M, where, T is the TPC (mg/g of plant extract in GAE), C is the concentration of gallic acid from the calibration curve, V is the volume of the extract in ml and M is the weight of the pure plant extracts.
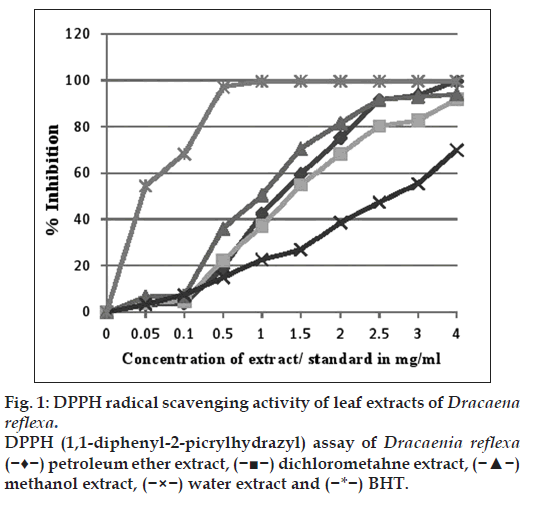
The free radical scavenging activity of the plant extract was determined using Brand and William method with a slight modification [20]. Reaction mixture contained 1 ml of extract of different concentrations (0.1 to 4 mg/ml) and 3 ml of working DPPH in methanol (0.004%). After 30 min of incubation at room temperature in a dark place, the absorbance was measured at 517 nm against methanol as blank on a UV/Vis spectrophotometer. Methanol with DPPH solution was used as control. Percent inhibition of free radical DPPH was calculated as per the formula, % inhibition=(absorbance of the control−absorbance of sample)/absorbance of control)×100. Results were expressed as IC50 the concentration producing 50 % inhibition, which was obtained from the graph plotted between concentrations versus % inhibition.
Ferric reducing antioxidant power (FRAP) value was calculated using the method of Benzie and Strain [21], which was based on reduction of Fe3+TPTZ to Fe2+TPTZ. The FRAP reagent was prepared mixing 300 mM acetate buffer (pH=3.6), 10 mM TPTZ and 200 mM FeCl3.6H2O in a ratio of 10:1:1 at 37°. The reaction mixture contained 1 ml of extract (1500 μg/ml) and 10 ml of working FRAP reagent. The mixture was incubated at 37° for 30 min. The antioxidant potential of samples was determined from standard curve plotted using ascorbic acid in the concentration range between 0-600 μM/ml. One millilitre of methanol in 10 ml of working FRAP reagent was the control and working FRAP reagent served as the blank. Results were expressed in μM/ml and FRAP value of sample is calculated using the formula, FRAP=(absorbance of sample/absorbance of statndard)×100.
Petroleum ether, dichloromethane, methanol and aqueous extracts were prepared to perform phytochemical screening, proximate analysis, total phenolic content and antioxidant activity. Results of proximate analysis are demonstrated in Table 1. The results revealed that leaves of Dracaena reflexa are a good source of mineral elements as they contained a high percentage of ash. Moisture content is in the range of 5-15% indicated that leaves are good for formulating because low moisture content prevented microbial growth [22]. Leaves also appeared to be a good supplement for protein, carbohydrate and fat, which are the major building blocks of nutrition [23]. Leaves also could provide high energy content as the nutritive value is much higher. Collectively proximate analysis showed that leaves of Dracaena reflexa have the potential to be a food supplement, energy drink and a neutraceutical.
| Parameter | Dracaena leaves (percentagedry weight basis) |
|---|---|
| Mean ± SD | |
| Moisture content | 3.31 ± 1.15 |
| Ash content | 8.02 ± 0.25 |
| Crude fibre | 1.31 ± 0.13 |
| Crude fat | 0.97 ± 0.27 |
| Total protein | 3.70 ± 0.39 |
| Total carbohydrate | 86.01 |
| Nutritive value* | 367.56 |
Values are expressed as mean±SD of the three replicates. *Nutritive value is calculated in kcal/100 g dry weight of leaves. SD: Standard deviation
Table 1: Proximate Composition Of Dried Leaves Of Dracaena Reflexa
Phytochemicals present in leaves of D. reflexa were shown in Table 2. Leaves of D. reflexa were found to be a rich source of alkaloids, flavonoids, saponins, glycosides, carbohydrate, amino acids, terpenoids, tannins, steroids, triterpenoids, fat and oil. Some of the major constituents like alkaloids contribute towards analgesic and antimicrobial activity [24]. Flavonoids and tannins are major groups which act as antioxidant. Tannins also reported to have antibacterial properties [25]. Glycosides are likely to possess cardiac activities and might be useful in treating congestive heart failure and cardiac arrhythmia [26]. Saponins are likely to demonstrate antibacterial, antiinflammatory, anticancer, and antidiabetic activities. Terpenoids have been reported to be antibacterial in nature [27]. Presence of all these phytochemical in leaves might contribute towards the therapeutic potential of D. reflexa.
| Phytochemical constituents | Test performed | Extracts | |||
|---|---|---|---|---|---|
| Petroleum ether | Dichloromethane | Methanol | Water | ||
| Alkaloids | Wagner’s test | − | − | + | + |
| Hager’s test | − | − | + | + | |
| Dragendroff’s test | − | − | + | + | |
| Mayer’s test | − | − | + | + | |
| Flavonoids | Alkaline test | − | + | + | + |
| Lead acetate test | − | − | + | + | |
| Carbohydrates | Molisch’s test | − | − | + | + |
| Bendict’s test | − | − | + | + | |
| Barfoed’s test | − | − | + | + | |
| Fehling’s test | − | − | + | + | |
| Tannins | Ferric chloride test | − | − | + | − |
| Glycosides | Borntrager’s test | − | + | + | − |
| Legal’s test | − | − | + | + | |
| Keller–Killiani test | + | + | − | − | |
| Terpenoids | Liebermann burchard test | + | + | − | − |
| Salwoski test | + | + | − | − | |
| Salwoski test (triterpenes) | − | + | + | − | |
| Steroids | Liebermann burchard test | + | + | − | − |
| Fat and oil | Saponification test | + | + | − | − |
| Filter paper test | + | + | − | − | |
| Saponin | Foam test | − | − | + | + |
| Froth test | − | − | + | + | |
| Protein | Ninhydrin | − | − | − | + |
| Biuret | − | − | − | + | |
| +: Present, −: absent | |||||
Table 2: Phytochemical Constituents In Dracaena Reflexa Leaf Extracts
TPC gives a measure of to what extent phenolic compounds are present in the extract. In plants usually tannins, flavonoids, phenolic acids and phenol diterpenes contributes to TPC. Phenol group offers potent antioxidant activity due to its redox potential, which plays an important role in scavenging free radicals, in neutralizing the reactive oxygen species or act as a electron donor to peroxide radical [28]. As the D. reflexa leaf methanol extract showed higher TPC, it is likely to be a potential antioxidant. TPC content of the extracts are summarized in Table 3.
| Extracts | Total phenolic content (mg GAE/gdw)Mean ± SD |
|---|---|
| Petroleum ether | 5.37 ± 0.43 |
| Dichloromethane | 28.25 ± 0.67 |
| Methanol | 49.69 ± 0.70 |
| Water | 10.98 ± 0.80 |
mg GAE/g means milligram of extract equivalent to gallic acid per gram of dry weight of extract. Values are expressed as mean±SD of the three replicates. SD: Standard deviation
Table 3: Total Phenolic Content Of Leaves Extracts Of Dracaena Reflexa
Plants with potent antioxidant properties are reported to exhibit freeradical scavenging activity. DPPH is a stable free radical, which gives purple colour in methanol solutions. This purple colour changes to yellow colour if the DPPH free radical is scavenged by donating either hydrogen radical or any alkyl radical. So plants secondary metabolites which have tendency to donate radical exhibit the good antioxidant power [29]. The D. reflexa leaf methanol extract showed very high free radical scavenging property as evidenced from the IC50 values obtained. Here a direct correlation can be demonstrated between the TPC and free radical scavenging property exhibited by the methanol extract graph of % inhibition versus concentrations showing comparison with standard BHT is demonstrated in fig. 1 and IC50 of different extract is listed in Table 4.
| Extracts/standard | IC50 values in mg/ml |
|---|---|
| Mean ± SD | |
| BHT | 0.050 ± 0.27 |
| Petroleum ether | 1.26 ± 0.34 |
| Dichloromethane | 1.36 ± 0.09 |
| Methanol | 0.97 ± 1.1 |
| Water | 2.66 ± 0.03 |
Values are expressed as mean±SD of the three replicates. BHT: Butylated hydroxytoluene, DPPH: 1,1-dipheny l-2-picrylhydrazyl, SD: standard deviation
Table 4: IC50 Of Leaf Extracts Of Dracaena Reflexa In Dpph Assay
FRAP assay mainly measures the reducing potential of an antioxidant reacting with Fe3+ TPTZ and producing a coloured Fe2+ TPTZ complex. The reducing power of any compound or phytochemical is due to its ability to donate hydrogen atom or an electron to the metal atom. FRAP assay treats the antioxidants in a plant sample as a reducing agent in a colorimetric reaction. The reducing power of a plant is not only due to the presence of H atom donating ability but also due to presence of methoxy and keto groups, triterpenes and acid group [30]. FRAP values are given in Table 5.
| Extracts/ standard | Ferric reducing antioxidant power (μM/ml) Mean ± SD | FRAP value |
|---|---|---|
| Ascorbic acid | 489.75 ± 0.45 | 2.000 |
| Petroleum ether | 330 ± 0.06 | 1.34 |
| Dichloromethane | 238.75 ± 0.90 | 0.97 |
| Methanol | 292.75 ± 1.2 | 1.19 |
| Water | 111.75 ± 3.4 | 0.45 |
Values are expressed as mean±SD of the three replicates. FRAP: ferric reducing antioxidant power, SD: standard deviation
Table 5: Frap Value For Leaf Extracts Dracaena Reflexa
The results obtained in this study clearly showed that leaf extracts of Dracaena reflexa possessed antioxidant activity and the results of proximate analysis support the use of the leaves as a food supplement.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Cathrine L, Nagarajan NP. Preliminary phytochemical analysis and antibacterial activity of leaf extracts of Vitex leucoxylon. Int J Curr Pharm Res 2011;3:71-3.
- Chandan P, Kumar V, Kamthan KP, Singh UB, Srivastava SK, Srivastava RB. Antioxidant and antimicrobial activity of ethanol and water extracts of Cymbopogon jwarancusa leaves. J Appl Pharm Sci 2011;1:68-72.
- Janifer R, Chaurasia AP, Vajpayee PK, Murugan MP, Singh S. Antioxidant activity and phytochemical investigation on a high altitude medicinal plant Dracocephalum heterophyllum Benth. Pharmacogn J 2010;2:112-7.
- Saha MR. In vitro free radical scavenging activity of methanol extract of the leaves of Mimusops elengi linn. Bangladesh J Vet Med 2008;6:197-202.
- Machala M, Kubínová R, Horavová P, Suchý V. Chemoprotective potentials of homoisoflavonoids and chalcones of Dracaena cinnabari: Modulations of drug-metabolizing enzymes and antioxidant activity. Phytother Res 2001;15:114-8.
- Gupta D, Bleakley B, Gupta RK. Dragon’s blood: Botany, chemistry and therapeutic uses. J Ethnopharmacol 2008;115:361-80.
- Ahmed A, Sobarry M, Cherrah Y, Alaoui K. Antiinflammatory and analgesic effects of ethanol extract of Dracaena cinnabari Balf. as endemic plant in Yemen. Int J Pharm Biol Sci 2012;3:96-106.
- Kumar VP, Chauhan NS, Padh H, Rajani M. Search for antibacterial and antifungal agents from selected Indian medicinal plants 2006;107:182-8.
- González AG, Hernández JC, León F, Padrón JI, Estévez F, Quintana J, et al. Steroidal saponins from the bark of Dracaena draco and their cytotoxic activities. J Nat Prod 2003;66:793-8.
- Mei W, Dai H, Wu J, Zhuang L, Hong K. Study on the new use of antitumor of Dracaena cambodiana. Zhong Yao Cai 2005;28:871-3.
- Luo Y, Wang H, Xu X, Mei W, Dai H. Antioxidant phenolic compounds of Dracaena cambodiana. Molecules 2010;15:8904-14.
- Chen HQ, Zuo WJ, Wang H, Shen HY, Luo Y, Dai HF, et al. Two new antimicrobial flavanes from dragon’s blood of Dracaena cambodiana. J Asian Nat Prod Res 2012;14:436-40.
- AOAC. The Official Method of Analysis. 15th ed. Washington, DC: Association of Official Analytical Chemists; 1990.
- Shukla RK, Painuly D, Porval A, Shukla A. Proximate analysis, nutritive value, total phenolic content and antioxidant activity of Litchi chinensis Sonn. Nat Prod Indian J 2012;8:361-9.
- Shukla A, Vats S, Shukla RK. Preliminary phytochemical screening, antibacterial and nitric oxide scavenging activities of Reinwardtia indica leaves extract. Int J PharmTech Res 2013;5:1670-8.
- Evans WC. Trease & Evans’ Pharmacognosy. 16th ed. London: Elsevier Health Sciences; 2009.
- Harborne JB. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. 4th ed. New Delhi: Springer; 1998.
- Singleton VL, Orthofer R, Rosa RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin ciocalteu reagent. Methods Enzymol 1999;299:152-77.
- Sharan SV, Srinivasa RB, Chippada SC, Vangalapati M. In vitro antioxidant activity and estimation of total phenolic content in methanolic extract of Bacopa monnifera. Rasayan J Chem 1999;4:381-6.
- Williams BW, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. Lebenson Wiss Technol 1995;28:25-30.
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem 1996;239:70-6.
- Shellard EJ. Exercises in the Evaluation of Drugs and Surgical Dressing. 1st ed. London: Pitman Medical Publishing Co. Ltd.; 1958.
- Anyasor GN, Aina DA, Olushola M, Aniyikaye AF. Phytochemical constituent, proximate analysis, antioxidant, antibacterial and wound healing properties of leaf extracts of Chromolaena odorata. Ann Biol Res 2011;2:441-51.
- Malu SP, Obochi GO, Edem CA, Nyong BE. Effect of methods of extraction on phytochemical constituents and antibacterial properties of Tetracarpidium conophorum seeds. Glob J Pure Appl Sci 2009;15:373-6.
- Atanassova M, Georgieva S, Ivancheva K. Total phenolic and total flavonoids contents, antioxidant capacity and biological contaminants in medicinal herbs. J Univ Chem Technol Metal 2011;46:81-8.
- Doss A, Parivuguna V, Vijayasanthi M, Surendran S. Antibacterial evaluation and phytochemical analysis of Medicago sativa against some microbial pathogens. Indian J Sci Technol 2011;4:550-2.
- Urzúa A, Rezende MC, Mascayano C, Vásquez L. A structure-activity study of antibacterial diterpenoids. Molecules 2008;13:882-91.
- Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev 2010;4:118-26.
- Dai J, Mumper RJ. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010;15:7313-52.
- Patt DE, Hudson BJ. Natural antioxidants not exploited commercially. In: Hudson BJ, editor. Food Antioxidants. London: Elsevier Applied Science; 1990. p. 171-91.