- *Corresponding Author:
- P. Nandesh Mohan
Department of Roga Nidana, Parul Institute of Ayurveda and Research, Parul University, Vadodara, Gujarat 391760, India
E-mail: dr.srinanden@gmail.com
| Date of Received | 25 December 2023 |
| Date of Revision | 19 May 2024 |
| Date of Acceptance | 22 October 2024 |
| Indian J Pharm Sci 2024;86(5):1791-1799 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Healthcare-associated infections are a major concern among healthcare professionals and hospitalized individuals. These infections can be significantly controlled through proper hand hygiene. Conventionally, alcohol-based agents are used for hand sanitization, but they often cause severe skin dryness. Maintaining skin moisture is essential to prevent damage that could potentially lead to chronic infections. This can be achieved by using non-alcoholic hand sanitizers. The primary objective of this study was to develop such a non-alcoholic hand sanitizers. To achieve this, a novel Ayurveda-based herbal non-alcoholic hand sanitizer was formulated using aqueous distillate (Arka) prepared from the dried flowers of an Indian medicinal herb, Woodfordia fruticosa Kurz (commonly known as fire flame bush or Dhathaki). Phytochemical analysis and antimicrobial activity testing were conducted on this preparation, termed Dhathaki Arka. Additionally, its hand sanitization efficacy was compared among healthcare professionals at Sri Dharmasthala Manjunatheshwara College of Ayurveda and Hospital, Hassan, India. Results indicated that the novel non-alcoholic herbal hand sanitizer, formulated based on Ayurvedic principles, demonstrated effective antimicrobial activity, making it a competent and skin-safe hand sanitizer. In conclusion, the herbal-based Dhathaki Arka hand sanitizer is effective for hand hygiene while avoiding the adverse effects on skin caused by alcohol-based alternatives. Therefore, Dhathaki Arka warrants further large-scale evaluation for development as a commercial herbal-based hand sanitizer.
Keywords
Woodfordia fruticosa Kurz, Arka, antimicrobial activity, phytochemical analysis, reduction factor
Healthcare-Associated Infections (HCAIs), also known as hospital-acquired or nosocomial infections, occur among Healthcare Professionals (HCPs) and hospitalized individuals alike[1,2]. HCPs can acquire these infections during interactions with individuals, individual samples/specimens, medical equipment, and other daily activities in the hospital. Individuals may develop HCAIs during their hospital stay (typically within 48-72 h) or after discharge, within 10 d[2]. All individual groups, regardless of gender, age, or medical condition, are susceptible to acquiring HCAIs. However, elderly and immunocompromised individuals, as well as those in the Intensive Care Unit (ICU), are at a higher risk of developing HCAIs. These infections are caused by bacteria, fungi, viruses, and parasites that are present and circulate within the hospital environment[3]. The list of common HCAIs and the worldwide estimate of their incidence rate are provided in Table 1[4]. These nosocomial infections are primarily spread through direct surface-to-body contact or via vectors. Some common vectors include flies, cockroaches, and ants found in medical centers[5]. Indirect transmission of HCAIs typically occurs through contact with contaminated medical components such as needles, syringes, gloves, dressings, vials, bags, medical equipment, and more[6]. Additionally, HCPs can serve as significant vectors of HCAIs, as their hands often harbor microorganisms that are transmitted between individuals[7]. Several control measures are implemented in hospital settings worldwide to prevent and reduce HCAIs. The most crucial and effective measure is the maintenance of hand hygiene through regular hand washing or the use of sanitizers, which is mandatory for HCPs at the start and end of every activity. This includes physicians, surgeons, nursing staff, microbiologists, lab technicians, pharmacists, and others. Additional control measures include maintaining hospital hygiene, vaccination, proper sample handling and transportation, disposal of medical waste, use of protective coverings (such as clothing and gloves), use of antiseptics and disinfectants, regular infection surveillance in hospital environments, and educating HCPs[6]. However, all these control measures will only be effective if they are rigorously implemented and consistently followed by every HCP.
| S. No | Hospital acquired infections | Worldwide estimated rate (%) |
|---|---|---|
| 1 | Urinary tract infection | 12.9 |
| 2 | Surgical site infections | 21.8 |
| 3 | Pneumonia | 21.8 |
| 4 | Bloodstream infections | 9.9 |
| 5 | Ear, nose, tongue and mouth infections | 5.6 |
| 6 | Skin infections | 3.2 |
| 7 | Cardiovascular infections | 1.2 |
| 8 | Bone and joint infections | 1.0 |
| 9 | Central nervous system related infections | 0.8 |
| 10 | Reproductive tract infections | 0.6 |
| 11 | Systemic infections | 0.2 |
Table 1: The Commonly Encountered Hospital-Acquired Infections among HCPS and Individuals with their Worldwide Estimated Rate of as per Recent Reports
In practice, less than 50 % of HCPs are reported to effectively and consistently practice handwashing or sanitization[8-10]. Several global studies on HCP- selected populations have confirmed that their knowledge, practices, and attitudes towards HCAIs are of significant concern[3,11-13]. In 2013, Danzmann et al.[14] conducted an extensive global systematic review on the role of HCPs in the outbreak of nosocomial infections. The study covered 152 outbreaks across 26 countries. Of these, 59 outbreaks were caused by physicians (30 of which were from surgeons), 56 by nursing staff, 9 by technical staff, and 5 each by kitchen staff and midwives. Appropriate and regular education for HCPs has shown a significant reduction in the occurrence of such infections[12,13].
Hand hygiene or sanitization in hospital settings is primarily achieved through two techniques; soap, water hand washing and alcohol-based hand sanitization. In the former method, transient microbes on the hands are washed off but not killed. In contrast, Alcohol-Based Hand Sanitizers (ABHS) effectively kill both resident and transient microbes[15]. Repeated hand washing is not practically feasible for HCPs every time they perform a medical activity. As a result, hand sanitizers are the preferred method for achieving hand hygiene in healthcare settings. In most cases, chemically formulated ABHS are used. However, with regular use, the chemicals present in these sanitizers may induce dermatitis. The alcohol component (60 %-95 %, v/v; ethanol, isopropanol, or n-propanol) in hand sanitizers dehydrates the skin, leading to skin infections and other sensitivity issues such as redness and rashes. The dryness caused by alcohol can cause the upper skin layer to peel off, making the hands more sensitive to Ultraviolet (UV) light. In some hand sanitizers, alcohol is replaced by triclosan, a potent antimicrobial agent. However, triclosan, when applied to wounds, can enter the bloodstream and cause allergic reactions[16]. Additionally, ABHS are not effective against bacterial spores, non-enveloped viruses, or protozoa[17]. Owing to these limitations, there is a pressing need for skin-friendly and NAHS that can effectively replace ABHS for use in hospital settings as well as for domestic applications.
NAHS available in the market are often substituted with chemicals or antiseptics instead of alcohol. Some examples include chlorhexidine (highly effective against gram-positive bacteria, but moderately effective against gram-negative bacteria), chloroxylenol (effective against all bacteria except Pseudomonas aeruginosa), iodine (a potent antimicrobial, but causes skin irritation and discoloration), quaternary ammonium compounds (highly effective against gram-positive bacteria and lipophilic viruses only), triclosan (a potent antimicrobial with a risk of allergic reactions if it enters the bloodstream), and benzalkonium chloride (less effective compared to alcohol)[17]. Several groups have explored the use of herbal or natural ingredients as alternatives to chemicals in the development of gentle NAHS[18-22]. The adoption of these plant- and herbal-based hand sanitizers is being promoted due to their antimicrobial activity and other beneficial biological properties, offering a safer alternative to mitigate the adverse effects associated with chemical-based and ABHS[19]. In the present investigation, we have developed a novel, medicinal plant-based Ayurvedic non-alcoholic hand sanitizer.
The Ayurvedic medicinal plant of choice in the present investigation is Woodfordia fructicosa (W. fruticosa) Kurz, commonly called fire flame blush. In Ayurveda, this plant is named as Dhathaki. In Sanskrit, Dhathaki is named as tamrapushpi, as the flowers are bright red in colour. It is an indigenous deciduous flowering shrub of India belonging to the family of Lythraceae. The plant grows up to 3.6 m in height in the hilly regions of India. It is also distributed in other South East and far East Asian countries. The parts of plant including leaves, flowers, and bark are used in Ayurveda in form of decoctions, churnas, ghritas, and others to treat several diseases such as dysentery and diarrhoea as it is highly astringent[23]. Of the 18 arishtas (fermented formulations) mentioned by the Indian Ministry of Health and Family Welfare for medicinal use, 17 contain Dhathaki as an active ingredient[24]. In folk medicine of India and Nepal, a decoction of Dhathaki leaves, known as dawai, is used to treat fever. Additionally, a tonic prepared from the dried flowers of Dhathaki is used to treat liver disorders, mucosal membrane issues, and hemorrhoids[25]. The acrid and pungent extract of dried Dhathaki flowers is astringent, a property attributed to the tannins present in them, including woodfordins, oenotheins, gemin D, tellimagrandin, heterophyllin, and isoschimawalin. Other phytochemicals found in the flowers include carbohydrates, chrysophanol- 8-O-Beta (β)-d-glucopyranoside, myricetin glycosides, naringenin 7-glucoside, kaempferol 3-O-glucoside, pelargonidin 3,5-diglucoside, cyanidin 3,5-diglucoside, phenolic compounds (such as ellagic acid), flavonoids (quercetin and myricetin glycosides), sterols, steroids, gums, meso- inositol, octacosanol, and β-sitosterol[24,26,27]. These components make the flower extract a potent agent with anti-hemorrhagic, styptic, stimulant, laxative, sedative, anti-parasitic, wound-healing, alexiteric, and antibacterial properties[28]. In various Southeast Asian countries, traditional medical practitioners have long utilized the therapeutic properties of Dhathaki flowers, leading to high demand for these flowers in the herbal product market both domestically and internationally[24]. A medication named Sidowava, made from dried Dhathaki flowers, is used in traditional medicine in Indonesia and Malaysia to treat sprue, bowel disorders, and infertility in women[29,30]. The biological activities of Dhathaki flower extracts include antimicrobial, antitumor, antiulcer, antifertility, immunomodulatory, hepatoprotective, cardioprotective, antioxidative, analgesic, anthelmintic, antihyperlipidemic, and antihyperglycemic effects[24]. With this background, Dhathaki flowers were selected as a promising candidate for developing a novel non-alcohol- based hand sanitizer. The method of hand sanitizer preparation was also carefully chosen. The Arka kalpana method is a popular Ayurvedic technique for preparing pharmaceutical and therapeutic formulations, as it preserves the active and volatile compounds of the herbal medicine for extended periods. This method involves creating a liquid herbal formulation by distilling water-soaked herbal products using an Arka yantra (distillation apparatus) [31]. In this study, dried Dhathaki flowers were distilled to prepare a novel hand sanitizer, named Dhathaki Arka. This formulation was evaluated for its phytochemical content and antimicrobial activity against clinical isolates at a laboratory scale. Additionally, its efficacy as a hand sanitizer was assessed through a comparative study involving healthcare professional volunteers in a hospital setting.
Materials and Methods
Sample collection:
1 kg of dried Dhathaki (W. fruticosa) flowers was collected from the campus of Sri Dharmasthala Manjunatheshwara College of Ayurveda and Hospital (SDMAH) in Hassan, Karnataka, India. The plant sample was authenticated as W. fruticosa Kurz flowers through 3rd party verification at the Department of Botany, Government Science College, Hassan. Detailed morphological features of the sample were carefully noted and documented in-house for future reference.
Microbial cultures:
Confirmed clinical isolates of Escherichia coli, Klebsiella pneumoniae, Pseudomonas sp., Staphylococcus aureus, and Candida albicans were used in this study.
Test subjects:
A total of 30 healthy volunteers, comprising HCPs (postgraduate residents, consultants, and hospital staff) from SDMAH, Hassan, were enrolled in this study after obtaining written consent. HCPs with cuts, wounds, skin infections, a history of hypersensitivity, or recent illness recovery were excluded. Enrolled participants were instructed not to use any topical or systemic antimicrobial agents or medications known to affect the skin's microbial flora within 5 hours prior to swab collection.
Preparation of Dhathaki drug extracts using dried flowers:
For the preparation of the Dhathaki drug extract, the volatile fraction of the dried flowers was extracted using the cold maceration method, with slight modifications as described elsewhere[31]. Briefly, 50 g of W. fruticosa dried flowers was placed in 250 ml of distilled water in a conical flask, which was then sealed with a stopper. The flowers were soaked in water on a rotary shaker for 7 d with constant shaking. After 7 d, the filtrate was filtered using a 0.22 µm filter paper, and the resulting filtrate was collected. An aliquot was separately preserved for antimicrobial activity experiments. The remaining filtrate was subjected to evaporation at 60°- 80° to obtain the concentrated extract. This evaporated extract was then dissolved in Dimethyl Sulfoxide (DMSO) at a concentration of 2000 µg/ml (w/v) and stored at room temperature in aliquots until use. The drug extract was specifically prepared in this study for the determination of phytochemical components and antimicrobial activity of Dhathaki flowers.
Preparation of Dhathaki Arka using dried flowers:
For the specific purpose of application as a hand sanitizer, Dhathaki dried flowers were subjected to the preparation of Arka kalpana following the classical method in the Department of Rasashastra and Bhaishajya Kalpana, SDMAH, Hassan. Briefly, 50 g of W. fruticosa dried flowers were cut into smaller pieces and soaked overnight in 250 ml of distilled water. The following day, 10 parts of distilled water were added to the soaked solution and subjected to distillation. The initial few drops of the distillate were discarded, and the rest were collected in sterile 15 ml containers until 60 % of the distillate (Arka) was obtained. The pH and alcohol content of the Arka preparation were determined, with results confirmed in duplicates. Further, the preparation was subjected to phytochemical analysis.
Phytochemical analysis of the Dhathaki drug extract and Arka:
Qualitative tests were performed to examine the presence and absence of phytochemical compounds in the Dhathaki drug extract and Arka, as described elsewhere[32-34]. The phytochemical compounds that were screened from both preparations include; alkaloids through Dragendorff’s, Mayer’s, and Wagner’s tests; carbohydrates using Benedict’s and Fehling’s tests; tannins using bromine and lead acetate tests; glycosides using Borntrager’s test; steroids using the Salkowski reaction; saponins using the foam test; flavonoids using the sulfuric acid test; and phenols using Folin- Ciocalteu's reagent test.
Determination of effective antimicrobial concentration of Dhathaki cold maceration extracts preparation on clinical isolates:
To determine the antimicrobial activity of Dhathaki extract preparation, both bacterial and fungal strains were carefully selected. Clinical isolates of Escherichia coli, Klebsiella pneumoniae, Pseudomonas sp., Staphylococcus aureus, and Candida albicans were sourced from the SDMAH culture repository. The conventional method of cup diffusion in Muller- Hinton Agar (MHA) medium was used to assess the antimicrobial activity and to determine the effective antimicrobial concentration of the Dhathaki extract. Briefly, a total of 8 cups (5 mm diameter) were made in MHA plates using a sterile cork borer, and pure cultures of each selected strain were spread-plated during the exponential growth phase (105 CFU/ml). Then, 50 µl of the extract preparation at concentrations of 3000 µg/ ml, 2000 µg/ml, 1000 µg/ml, 900 µg/ml, 800 µg/ml, 700 µg/ml, and 600 µg/ml were placed in each well. The eighth cup received 50 µl of sterile distilled water, serving as the negative control. Each culture plate was maintained in duplicates. All bacteria-inoculated plates were incubated at 37° for 24 h, while the Candida albicans-inoculated plates were incubated at 30° for 48 h. After the completion of the incubation period, the plates were observed for the presence or absence of a zone of inhibition around the cups. If a zone was present, its size was measured and recorded in mm. The lowest concentration of the Dhathaki extract preparation that demonstrated inhibition against each microbial strain was considered the effective inhibitory concentration for that respective strain. The data were analyzed statistically using Statistical Package for Social Sciences (SPSS) software.
Intervention study:
Assessment of microbial load Reduction factor (Rf) induced by Dhathaki Arka preparation: In this study, the aim was to determine the efficacy of the Dhathaki Arka preparation as a hand sanitizer. To assess this, 2 interventional products were included; a conventional alcohol-based hand sanitizer (Sterillium® containing propan-2-ol and propan-1-ol) and Dhathaki Arka (diluted 1:3). Sterillium® was used as the reference standard. Before application, the volunteers were instructed to thoroughly rub their palms to ensure the uniform spread of microflora. Each volunteer was subjected to the application of Sterillium® (10 ml) on the left palm and Dhathaki Arka (10 ml) on the right palm. The applied hand sanitizers were left on the palms for 30 s, after which the palms were allowed to dry for 2 min. Before and after treatment, a sterile cotton swab was used to collect a sample from each palm separately, and the swabs were transferred to appropriately labeled sterile tubes. Serial dilutions of the collected swab samples were performed, and the microbial load (CFU/ ml) from each dilution was recorded.
The microbial load before treatment served as the baseline count. Both the baseline and post-treatment microbial counts were then used to determine the Rf. The resulting Rf (calculated using the formula provided below) directly indicated the efficacy of Dhathaki Arka.
Rf= log10 (baseline microbial count)-log10 (post treatment microbial count)
Results and Discussion
The phytochemical profiling of the aqueous drug extract prepared from dried Dhathaki flowers indicated the presence of secondary metabolites, including alkaloids, flavonoids, glycosides, and tannins. The presence of carbohydrates was also confirmed through qualitative tests (Table 2). These results are partially in agreement with earlier reports. For instance, Syed et al.[27] also reported the presence of saponins, along with alkaloids, flavonoids, glycosides, tannins, and carbohydrates, in the aqueous extract of W. fruticosa flowers[27].
| S. No. | Phytochemical component | Test method | Dhathaki drug extract | DhathakiArka preparation |
|---|---|---|---|---|
| 1 | Alkaloids | Dragendorff’s | - | - |
| Mayer’s | - | ND | ||
| Wagner’s | + | ND | ||
| 2 | Flavonoids | Sulphuric acid | + | - |
| 3 | Glycosides | Borntrager’s | + | - |
| 4 | Phenols | Folin-ciocalteu's | ND | - |
| 5 | Saponins | Foam | - | + |
| 6 | Tannins | Bromine | - | ND |
| Lead acetate | + | - | ||
| 7 | Carbohydrates | Molisch’s | ND | + |
| Fehling’s | + | ND | ||
| Benedict’s | + | ND | ||
| 8 | Steroids | Salkowski | - | - |
Note: (ND): Not Done; (+): Positive and (-): Negative
Table 2: Class of Phytochemical Compounds Present in the Aqueous Drug Extract of Dhathaki and the Arka (Aqueous Distillate) Preparation of Dhathaki that was prepared for Use as a Hand Sanitizer
However, in the present study, saponins were not detected. This difference in the phytochemical profile could be attributed to slight variations in the extraction process. Unlike the current study, Syed et al.[27] used a soxhlet extractor to prepare the aqueous extract, which may have influenced the yield of secondary metabolites. This underscores the impact of extraction methods on the final composition of the extract, even when using the same extraction medium, i.e., water. Additionally, the secondary metabolites identified in the Dhathaki extract are all hydrolysable, as they were effectively extracted into the aqueous solution. It is noteworthy that both studies used qualitative tests to detect the presence or absence of phytochemical compounds. However, detailed quantitative tests and specific chemical profiling using sophisticated techniques would provide more critical information regarding the specific components and their concentrations in the final extract.
The presence of flavonoids and tannins in the aqueous drug extract suggests that the predominant phenolic compounds of W. fruticosa are easily extractable into an aqueous format[24]. Although previous studies strongly support the presence of steroid compounds, such as hecogenin and meso-inositol, in the flowers of W. fruticosa, the drug extraction method used in this study failed to extract steroids into the aqueous solution[35]. In contrast to the aqueous drug extract, the Arka preparation made from dried flowers of W. fruticosa did not contain a diverse range of secondary metabolites. The only phytochemical compounds found in the Arka preparation were saponins and carbohydrates. Although the same aqueous medium was used for both preparations, the longer distillation duration may explain the absence of alkaloids, flavonoids, glycosides, and tannins in the Arka preparation. These compounds may have degraded during the extended distillation process. Interestingly, saponins were present in the Arka preparation, whereas they were absent in the aqueous extract. This suggests that the saponins in the Arka preparation are more stable than other compounds and could withstand the distillation conditions. Given that the primary goal of this study was to develop a promising, skin-friendly, and stable hand sanitizer, the Arka preparation was chosen over the aqueous drug extract. Compared to the aqueous extract, the Arka preparation is sterile, contains stable phytochemical compounds, and has a longer shelf life. Therefore, it was selected for further critical evaluation, including antimicrobial activity and its efficacy as a hand sanitizer.
Testing the antimicrobial activity of an Ayurvedic sanitizer is a fundamental and essential approach to evaluate its efficacy as a hand sanitizer, particularly for hospital settings[15,19-21,36]. In this context, the antimicrobial activity of the Dhathaki extract preparation was tested against gram-negative, gram-positive, and fungal models. Clinical isolates of Escherichia coli, Klebsiella pneumoniae, and Pseudomonas sp. were selected as representative strains for the gram- negative bacterial model. Staphylococcus aureus was used as the representative strain for the gram-positive bacterial model. Finally, a clinical isolate of Candida albicans was used to represent the fungal model. The selection of microorganisms was made to include those commonly associated with skin, oral, and gastrointestinal infections, which are frequent types of infections transmitted through contact.
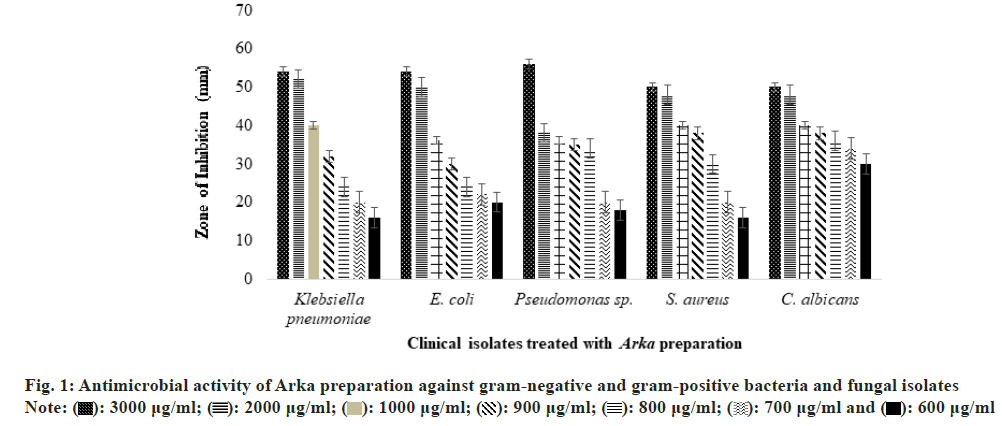
Fig. 1 shows that the inhibition effect on microbes increases with the concentration of the extract preparation, regardless of the microorganism tested. This general observation indicates that the Dhathaki extract preparation is effective and inhibitory against all three microbial models examined. As evidenced by the zone of inhibition data, the antimicrobial activity of the Dhathaki extract preparation was relatively consistent across higher concentration ranges (3000-900 µg/ml), with inhibition ranging from 50-56 mm at 3000 µg/ml, 38-52 mm at 2000 µg/ml, 36-40 mm at 1000 µg/ml, and 30-38 mm at 900 µg/ml.
However, for Candida albicans, only a minimal difference in inhibition was observed below the 900 µg/ml concentration of the Dhathaki extract preparation. A clear dose-response relationship was noted in the antimicrobial activity against the tested microorganisms below 900 µg/ml, with the inhibition remaining proportional to the concentration at lower concentrations (600 and 700 µg/ml), particularly for bacteria. In further elaboration, the zone of inhibition ranged from 16-20 mm against gram-negative bacteria, 16 mm against gram-positive bacteria, and 30 mm against Candida albicans at a concentration of 600 µg/ ml of the extract. At a concentration of 700 µg/ml, the inhibition zone increased to 20-22 mm against gram- negative bacteria, 20 mm against gram-positive bacteria, and 34 mm against Candida albicanS. In conclusion, the Dhathaki extract preparation demonstrated satisfactory antimicrobial activity against both gram-negative and gram-positive bacteria, as well as fungal isolates, at concentrations as low as 600 µg/ml. The antimicrobial activity observed at various concentrations in this study strongly supports previous findings, highlighting the plant’s potential as a potent antimicrobial agent, particularly against human pathogens[28].
To evaluate the efficacy of the Arka preparation as a hand sanitizer, a comparative study was designed using the commercial alcohol-based hand sanitizer, Sterillium®. The study was carefully designed as a randomized clinical trial, based on the observation that such an approach is essential for establishing the clinical application of a novel formulation in hospital settings, as suggested by previous studies[19,20,37,38].
The Arka preparation had a pH of 3.92 and an alcohol content of 0 % (data not shown). Based on this, it was categorized as a NAHS, contrasting with the composition of the commercial hand sanitizer. For the efficacy study, a total of 30 HCPs were enrolled. The right hand of each participant received the non-alcohol based Arka preparation treatment, while the left hand received Sterillium®. Swab samples were collected from each palm, both before and after hand sanitization. Microbial colonies, including both bacteria and fungi, were counted manually using a colony counter. Results from palm swabs taken before and after treatment with Sterillium® and the Arka preparation showed a reduction in colony numbers following hand sanitization.
The reduction in microbial load for both treatments was statistically significant, as confirmed by the Wilcoxon matched pairs test (p<0.05). To compare the effectiveness of Sterillium® and the Arka preparation, the microbial Rf was calculated for both products before and after hand sanitization. Table 3 presents a comparison of Rf across sample dilutions ranging from 10-1 to 10-7. Beyond the 10-7 dilution, no further microbial load reduction was observed. The significance of microbial load reduction for both Sterillium® and Arka preparation is evident from the median values, which show minimal differences across all dilutions. At 10-6 and 10-7 dilutions, both sanitizers exhibited identical median values, indicating comparable microbicidal activity and equivalent performance.
| Swab dilution (N=30) | Rf post treatment with hand sanitizer | |||
|---|---|---|---|---|
| Sterillium® | Arka preparation | |||
| Mean±Standard Deviation (SD) | Median | Mean±SD | Median | |
| 10-1 | 44.98±10.37 | 43.6 | 50.05±18.95 | 57.7 |
| 10-2 | 52.31±22.65 | 59.75 | 51.71±16.99 | 58.2 |
| 10-3 | 60.69±22.97 | 60.1 | 63.03±17.4 | 61.4 |
| 10-4 | 71.5±19.62 | 70.8 | 70.13±20.1 | 70.42 |
| 10-5 | 81.34±23.24 | 96.95 | 80.73±15.24 | 80.6 |
| 10-6 | 97.22±8.79 | 100 | 91.5±12.42 | 100 |
| 10-7 | 100 | 100 | 98.75±3.95 | 100 |
Table 3: Descriptive Rf Results of Hand Sanitization using Sterillium® and Arka Preparation in 30 HCPS
In conclusion, the results of this study suggest that Arka preparation is an effective alternative to ABHS, demonstrating equivalent efficacy in reducing germ load on the palms of HCPs. Although the preparation contained primarily carbohydrates and saponins, further investigation into the specific active compounds within these classes is recommended to identify those responsible for its microbicidal effects. The broad spectrum efficacy of Arka preparation against both bacterial and fungal strains supports its potential for hand hygiene in a skin friendly manner, thereby aiding in the prevention of contact-based disease transmission.
Acknowledgement:
We extend our sincere gratitude to our Principal, Dr. Prasana Narasimha Rao, for his unwavering support and valuable insights, which greatly contributed to the success of this study.
Conflict of interests:
The authors declare no conflict of interests.
References
- Curtis DE, Hlady CS, Kanade G, Pemmaraju SV, Polgreen PM, Segre AM. Healthcare worker contact networks and the prevention of hospital-acquired infections. PloS One 2013;8(12):e79906.
[Crossref] [Google Scholar] [PubMed]
- Bayleyegn B, Mehari A, Damtie D, Negash M. Knowledge, attitude and practice on hospital-acquired infection prevention and associated factors among healthcare workers at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. Infect Drug Resist 2021:259-66.
[Crossref] [Google Scholar] [PubMed]
- Khatrawi EM, Prajjwal P, Farhan M, Inban P, Gurha S, Al‐ezzi SM, et al. Evaluating the knowledge, attitudes, and practices of healthcare workers regarding high‐risk nosocomial infections: A global cross‐sectional study. Health Sci Rep 2023;6(9):e1559.
[Crossref] [Google Scholar] [PubMed]
- Gezie H, Leta E, Admasu F, Gedamu S, Dires A, Goshiye D. Health care workers knowledge, attitude and practice towards hospital acquired infection prevention at Dessie referral hospital. Clin J Nurs Care Pract. 2019;3(1):059-63.
- Joshi M, Kaur S, Kaur HP, Mishra T. Nosocomial infection: Source and prevention. Int J Pharm Sci Res 2019;10(4):1613-24.
- Samuel SO, Kayode OO, Musa OI, Nwigwe GC, Aboderin AO, Salami TA, et al. Nosocomial infections and the challenges of control in developing countries. Afr. J. Clin. Exp. Microbiol 2010;11(2).
- Lemiech-Mirowska E, Kiersnowska ZM, Michałkiewicz M, Depta A, Marczak M. Nosocomial infections as one of the most important problems of healthcare system. Ann Agric Environ Med 2021:28(3):16-19.
[Crossref] [Google Scholar] [PubMed]
- Haas JP, Larson EL. Measurement of compliance with hand hygiene. J Hosp Infect 2007;66(1):6-14.
[Crossref] [Google Scholar] [PubMed]
- Kampf G, Löffler H, Gastmeier P. Hand hygiene for the prevention of nosocomial infections. Dtsch Arztebl Int 2009;106(40):649.
[Crossref] [Google Scholar] [PubMed]
- Pittet D, Hugonnet S, Harbarth S, Mourouga P, Sauvan V, Touveneau S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. The Lancet. 2000;356(9238):1307-12.
[Crossref] [Google Scholar] [PubMed]
- Saloojee H, Steenhoff A. The health professional’s role in preventing nosocomial infections. Postgrad Med J 2001;77(903):16-9.
[Crossref] [Google Scholar] [PubMed]
- Suchitra JB, Devi NL. Impact of education on knowledge, attitudes and practices among various categories of health care workers on nosocomial infections. Indian J Med Microbiol 2007;25(3):181-7.
[Crossref] [Google Scholar] [PubMed]
- Tavolacci MP, Ladner J, Bailly L, Merle V, Pitrou I, Czernichow P. Prevention of nosocomial infection and standard precautions: Knowledge and source of information among healthcare students. Infect Control Hosp Epidemiol 2008;29(7):642-7.
[Crossref] [Google Scholar] [PubMed]
- Danzmann L, Gastmeier P, Schwab F, Vonberg RP. Health care workers causing large nosocomial outbreaks: A systematic review. BMC Infect Dis 2013;13:1-8.
[Crossref] [Google Scholar] [PubMed]
- Harsha MR, Mishra B, Chaithra CS, Ramana V. Evaluation of fungicidal activity of herbal hand sanitizer. J Res Tradit Med 2016;2(3):70-4.
- McDonnell G, Russell AD. Antiseptics and disinfectants: Activity, action, and resistance. Clin Microbiol Rev 1999;12(1):147-79.
[Crossref] [Google Scholar] [PubMed]
- Jing JL, Pei Yi T, Bose RJ, McCarthy JR, Tharmalingam N, Madheswaran T. Hand sanitizers: A review on formulation aspects, adverse effects, and regulations. Int J Environ Res Public Health 2020;17(9):3326.
[Crossref] [Google Scholar] [PubMed]
- Balkrishna A, Singh K, Singh H, Haldar S, Varshney A. GermiX: A skin friendly hand sanitizer with prolonged effectivity against pathogenic bacteria. AMB Express 2020;10(1):210.
[Crossref] [Google Scholar] [PubMed]
- Malabadi RB, Kolkar KP, Meti NT, Chalannavar RK. Role of plant based hand sanitizers during the recent outbreak of coronavirus (SARS-CoV-2) disease (COVID-19). Bioeng Biosci 2021;5(1):458-6.
- Wal P, Wal A, Pal RS, Pal Y, Saraswat N. An ayurvedic based dermal treatment for skin sanitization. Open Dermatol J 2021;15(1):59-65.
- Kulkarni SA, Sharma J, Bobade A. Vaidic care hand sanitizer. Medicon Pharm Sci 2022;2:24-27.
- Mahish PK, Fule U, Pandaw M, Rakshit A. The use of medicinal plant extract in hand sanitizer and spray to combat against COVID-19. Biosci Biotechnol Res Asia 2022;19(1):183-9.
- Das PK, Goswami S, Chinniah A, Panda N, Banerjee S, Sahu NP, et al. Woodfordia fruticosa: Traditional uses and recent findings. J Ethnopharmacol 2007;110(2):189-99.
[Crossref] [Google Scholar] [PubMed]
- Kumar D, Sharma M, Sorout A, Saroha K, Verma S. Woodfordia fruticosa Kurz.: A review on its botany, chemistry and biological activities. J Pharmacogn Phytochem 2016;5(3):293-8.
- Oudhia P. Interaction with the herb collectors of Gandai region. Chhatisgarh, MP, India. 2003.
- Khushalani H, Tatke P, Singh K. Antifertility activity of dried flowers of Woodfordia fruticosa kurz. Indian J Pharmaceut Sci 2006;68(4):528-9.
- Syed YH, Khan M, Bhuvaneshwari J, Ansari JA. Phytochemical investigation and standardization of extracts of flowers of Woodfordia fruticosa; A preliminary study. J Pharm Biosci 2013;1:134-40.
- Kumaraswamy MV, Kavitha HU, Satish S. Antibacterial potential of extracts of Woodfordia fruticosa Kurz. on human pathogens. World J Med Sci 2008;3(2):93-6.
- Burkill IH. A dictionary of the economic products of the Malay Peninsula. Ministry of Agriculture and Cooperatives, Kualalampur, 1966;2305.
- Dey KL. The indigenous drugs of India. Thacker, Spink; 1896;311.
- Du ZX, Wu HE, Li FY. Chemical constituents of volatile oil from Porella setigera (steph.) Hatt. Lishizhen Med Mater Med Res 2010;21:336-8.
- Fathima S, Jambiga PC, Thumma R, Ahmadi S, Askani S, Mohammed BS, et al. Phytochemical screening and antimicrobial activity of the plant extracts Andrographis paniculata against selected microbes. J Phytopharmacol 2023;12(5):305-310.
- dos Santos CV, de Souza MM, Feitosa AJ, Bandeira DM, Canton AG, Mallmann AP, et al. Phytochemical determination and antimicrobial activity of plant extracts from Cinnamomum amoenum (ness) Kosterm.(lauraceae) against salmonella spp. of poultry importance. Contrib Cienc Soc 2023;16(12):29308-22.
- Camadan Y, Begen HA, Ceylan S, Saral A, Eminagaoglu O. Investigating in vitro antioxidant and antimicrobial activity of different sorbus species in artvin province of Turkiye. J Inst Sci Technol 2023;13(4):2818-28.
- Chauhan JS, Srivastava SK, Srivastava SD. Chemical constituents of Woodfordia fruticosa Linn. J Indian Chem Soc 1979;56:1041.
- Hayati Z, Syahrizal D, Nurhikmah N, Husna F, Mahdani W, Oktiviyari A, et al. The effectiveness of patchouli oil as hand sanitizer: A comparative study between two antiseptic brands. Eur J Clin Microbiol Infect Dis 2022;2(1):11-5.
- Deshpande A, Fox J, Wong KK, Cadnum JL, Sankar T, Jencson A, et al. Comparative antimicrobial efficacy of two hand sanitizers in intensive care units common areas: A randomized, controlled trial. Infect Control Hosp Epidemiol 2018;39(3):267-71.
[Crossref] [Google Scholar] [PubMed]
- Tan Jr AK, Olivo J. Assessing healthcare associated infections and hand hygiene perceptions amongst healthcare professionals. Int J Caring Sci 2015;8(1).
 900 μg/ml;
900 μg/ml;  600 μg/ml
600 μg/ml



