- Corresponding Author:
- S. Das
Sri Sai Aditya Institute of Pharmaceutical Sciences and Research, A. D. B. Road, Surampalem, Peddapuram-533 437
E-mail: sanjoydas90@gmail.com
| Date of Submission | 12 February 2014 |
| Date of Revision | 13 January 2015 |
| Date of Acceptance | 01 June 2015 |
| Indian J Pharm Sci 2015;77(3):352-356 |
This is an open access article distributed under the terms of the Creative Commons Attribution−NonCommercial−ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non−commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Phytochemical evaluation of the chloroform extract of roots of Polygonum viscosum has yielded six compounds, stigmasterol, 7,4-dimethylquercetin, kaempferol, quercetin, myricetin and scutellarein. Among the six compounds isolated and characterized by chemical and spectral (UV, NMR and Mass) analysis in the present phytochemical evaluation, stigmasterol was not reported earlier from P. viscosum. The compounds, 7,4ʹ-dimethylquercetin, quercetin and scutellarein were reported from P. hydropiper. Kaempferol from P. amphibium, P. aviculare, P. convolvulus, P. hydropiper, P. lapathifolium and P. persicaria and myricetin from P. aviculare and P. lapathifolium were also reported earlier. This appers to be the first report of the occurrence of all the six compounds from P. viscosum.
Keywords
Polygonum viscosum, Polygonaceae, phytochemical evaluation, stigmasterol, 7,4−dimethylquercetin,kaempferol, quercetin, myricetin, scutellarein
Polygonum viscosum Buch-Ham. (Polygonaceae), common Bengali name ″Bishkatali″ is an annual odoriferous herb (50-90 cm) indigenous to Nepal and is widely distributed in Bangladesh, north-east India, China and Japan. The genus Polygonum is well-known for producing pharmacologically active substances and also for its use in Oriental traditional medicine systems. Ethanol extract of Polygonum viscosum is known to have antibacterial properties [1]. A flavonoid glycoside, quercetin- 3-O-(6″-feruloyl)-β-D-galactopyranoside from aerial parts of the plant was reported to have antiHIV1, anticholinergic, analgesic and CNS depressant activities and significant cytotoxicity against the ovarian cancer cell line (OVCAR-3). The sesquiterpenes from aerial parts, viscosumic acid and viscozulenic acid for antiinflammatory, analgesic and CNS depressant activities, viscoazucine for analgesic and CNS depressant activities, viscoazulone for antiinflammatory, antiHIV1 and CNS depressant activities and viscozulenic acid methyl ester, viscoazucinic acid and polygosumic acid for antibacterial acitivity against penicillin-resistant Escherichia coli and methicillin-resistant Staphylococcus aureus were reported [2,3].
The sequiterpenes, viscoazusone, viscoazulone, viscozulenic acid, viscozulenic acid methyl ester, viscoazucine, viscoazucinic acid, viscosumic acid and polygosumic acid and flavonoids, 3′,5-dihydroxy- 3,4′,5′,7-tetramethoxyflavone, 3′,5,7−trihydoxy−3,4′,5′- trimethoxyflavone, quercetin−3−O−(6″-caffeoyl)-β- D-galactopyranoside, quercetin−3−O−(6″-feruloyl)- β-D-galactopyranoside and quercetin−3−O−(6″- galloyl)-β-D-galactopyranoside were reported so far from the species P. viscosum [1-6]. As a part of the ongoing phytochemical and bioactivity studies on the Polygonum genus, the authors have reinvestigated its roots for its bioactive phytoconstituents.
Silica gel (Merck, Mumbai, India) 100-200 mesh for column chromatography and silica gel (SDFCL, Mumbai. India) 350 mesh for preparative TLC were used. Successive gradient elution was accomplished by the solvents, n-hexane, chloroform and methanol (Merck, Mumbai, India). Melting points were recorded on Cipla I-28 digital apparatus (Cipla, Mumbai, India). 1H NMR using DMSO-d6 (2, 3, 4, 5, 6) and 13C NMR using CDCl3 (1) were run on Bruker 400 MHz spectrometer (Bruker, Ettlingen, Germany). All the mass spectra were taken accurately under API-ES conditions using Agilent 1100 series LC/MS (Agilent 1100 series, Agilent Technologies Deutschland GmbH, Waldbronn, Germany).
The plant material Polygonum viscosum was collected from forest Pilak, India. Authentication of the plant specimen (SD001) was done at the Botanical Survey of India, Deccan Regional Centre, Hyderabad. A voucher specimen (SD001) was deposited at the Herbarium of the University College of Pharmaceutical Sciences, Andhra University, Visakhapatnam, India.
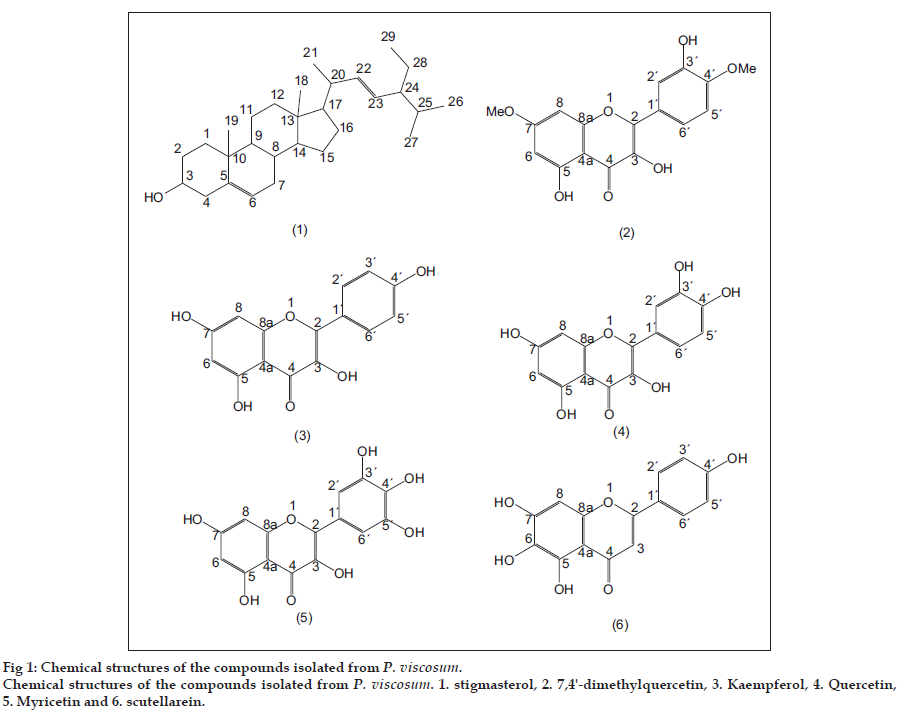
One kilogram of the dried root powder was extracted with CDCl3 (3×1.5 l) for 24 h at room temperature. TLC examination of the residue showed numbers of prominent spots (MeOH-CDCl3, 1:99). The pooled extract was concentrated under reduced pressure and yielded 14 g brown residue. The extract (12 g) was chromatographed over silica gel following gradient elution (each 200 ml fraction) technique successively using n-hexane, CDCl3 and MeOH. Fractions 43-51 (CDCl3-hexane, 25:75) obtained white amorphous powder which on repeated crystallisation from hexane afforded white needles of stigmasterol, mp 163-165°, identical with an authentic sample [7]. Fractions 124-127 (MeOH-CDCl3, 5:95) yielded dark yellow solids which on repeated crystallisation from a mixture of MeOH-CDCl3, 19:1 obtained 0.02 g of yellow crystals of 7,4′-dimethylquercetin, mp 238-240°, identical with an authentic sample [8]. Fractions 128-131 (MeOHCHCl 3, 5:95) yielded yellow solid which on repeated crystallisation from MeOH obtained 0.02 g yellow crystals of kaempferol, mp 275-277°, identical with an authentic sample [9]. Fractions 132-135 (MeOH-CDCl3, 10:90) yielded yellow amorphous mass which on recrystallisation from MeOH, afforded 0.02 g yellow needles of quercetin, mp 318-320°, identical with an authentic sample [9]. Fractions 139-142 (MeOH-CDCl3, 15:85) yielded another dark yellow solid which on repeated crystallisation from MeOH afforded 0.03 g yellow needles of myricetin, mp 357-359°, identical with an authentic sample [10]. Fractions 143-148 (MeOH-CDCl3, 20:80) yielded clump of yellow mass which on subsequent recrystallisation from MeOH, obtained 0.02 g pale yellow crystals of scutellarein, mp 327-329°, identical to an authentic sample [11].
Stigmasterol (1), mp 163-165°; molecular mass 412.3 requires positive API-ES, m/z (rel. int.): 413.3 [M+H]+ (15) (calcd. for C29H49O 413.37). Liebermann-Burchard?s test: A play of colours (pink to blue to green). The 13C NMR spectral data are summarized in Table 1. 7,4′-dimethylquercetin (2), mp 238-240°, molecular mass 330.07 requires positive API-ES, m/z (rel. int.): 331.1 [M+H]+ (100) (calcd. for C17H15O7 331.08) and 353.1 [M+Na]+ (20) (calcd. for C17H14O7Na 353.06). UV MeOH λmax nm: 250, 262 and 375; MeOH/AlCl3 264 and 432. The 1H NMR spectral data are represented in Table 1.
| C/H | 2c (δH) | 3c (δH) | 4c (δH ) | 5c (δH) | 6c (δH) | 1b (δC) | C | 1b (δC) |
|---|---|---|---|---|---|---|---|---|
| 1 | 37.30 | 9 | 50.21 | |||||
| 2 | 31.72 | 10 | 36.55 | |||||
| 3 | 6.73 (1H, s) | 71.84 | 11 | 19.80 | ||||
| 3-OH | 9.76 (1H, s) | 9.42 (1H, s) | 9.40 (1H, s) | 9.46 (1H, s) | 12 | 39.83 | ||
| 4 | 47.74 | 13 | 45.92 | |||||
| 5 | 140.81 | 14 | 56.82 | |||||
| 5-OH | 12.51 (1H, s) | 12.48 (1H, s) | 12.52 (1H, s) | 12.61 (1H, s) | 13.00 (1H, s) | 15 | 24.32 | |
| 6 | 6.32 (1H, d, 1.6) | 6.18 (1H, d, 2) | 6.19 (1H, d, 1.7) | 6.18 (1H, d, 1.6) | 121.71 | 16 | 29.25 | |
| 6-OH | 10.44 (1H, s) | 17 | 56.13 | |||||
| 7 | 36.16 | 18 | 11.98 | |||||
| 7-OH | 10.82 (1H, s) | 10.90 (1H, s) | 10.30 (1H, s) | 10.35 (1H, s) | 19 | 21.12 | ||
| 7-OMe | 3.90 (6H, s) | 20 | 42.36 | |||||
| 8 | 6.78 (1H, d, 1.6) | 6.44 (1H, d, 2) | 6.41 (1H, d, 1.7) | 6.38 (1H, d, 1.6) | 6.67 (1H, s) | 34.02 | 21 | 23.13 |
| 2' | 7.79 (1H, d, 1.6) | 8.04 (2H, d, 8.8) | 7.73 (1H, d, 2.1) | 7.29 (2H, s) | 7.90 (2H, d, 8.8) | 22 | 138.27 | |
| 3′ | 6.93 (2H, d, 8.8) | 6.91 (2H, d, 8.8) | 23 | 129.34 | ||||
| 3′-OH | 9.53 (1H, s) | 9.18 (1H, s) | 8.40 (3H, s) | 24 | 51.27 | |||
| 4′-OH | 10.13 (1H, s) | 9.61 (1H, s) | 8.40 (3H, s) | 8.69 s | 25 | 31.95 | ||
| 4′-OMe | 3.90 (6H, s) | 26 | 18.80 | |||||
| 5′ | 6.90 (1H, d, 8) | 6.93 (2H, d, 8.8) | 6.94 (1H, d, 8.4) | 6.91 (2H, d, 8.8) | 27 | 19.40 | ||
| 5′-OH | 8.40 (3H, s) | 28 | 25.40 | |||||
| 6′ | 7.76 (1H, dd, 8, 1.6) | 8.04 (2H, d, 8.8) | 7.62 (1H, dd, 8.4, 2.1) | 7.29 (2H, s) | 7.90 (2H, d, 8.8) | 29 | 11.87 |
Table 1: NMR spectral data of the compounds of P. viscosum RrootsA
Kaempferol (3), mp 275-277°, molecular mass 286.04 requires positive API-ES, m/z (rel. int.): 287.2 [M+H]+ (100) (calcd. for C15H11O6 287.05) and 309.2 [M+Na]+ (20) (calcd. for C15H10O6Na 309.03). Lead acetate test: Yellow precipitate, ferric chloride test: Olive green and Shinoda test: Magenta colour. UV MeOH λmax nm: 253 (sh), 265, 294 (sh), 322 (sh), 365; MeOH/AlCl3: 275, 420. The 1H NMR spectral data are shown in Table 1. The compound (20 mg) was treated with acetic anhydride (1 ml) and sodium acetate (100 mg) at 140° and refluxed for 4 h. On usual workup and recrystallisation from alcohol afforded crystalline needles (10 mg) of kaempferol tetraacetate, mp 186-188°, molecular mass 454.09 for the tetraacetate derivative requires positive API-ES, m/z (rel. int.): 455.2 [M+H]+ (100) (calcd. for C23H19O10 455.09). Similarly, the compound (20 mg) was treated with repeated quantities of diazomethane in ether, until it showed a negative ferric reaction. It was concentrated under reduced pressure, cooled and recrystallised from MeOH as yellow crystals (8 mg) of kaempferol tetramethyl ether, mp 163-164°, molecular mass 342.11 for the tetramethyl derivative requires positive API-ES, m/z (rel. int.): 343.2 [M+H]+ (100) (calcd. for C19H19O6 343.11).
Quercetin (4), mp 318-320°, molecular mass 302.04 requires positive API-ES, m/z (rel. int.): 303.3 [M+H]+ (100) (calcd. for C15H11O7 303.04). Ferric chloride test: Dense green and Shinoda test: Magenta colour. UV MeOH λ max (nm): 257, 267 (sh), 301 (sh) and 370; EtOH/AlCl3 265, 301 (sh), 359 and 425. The 1H NMR spectral data are displayed in Table 1. Myricetin (5), mp 357-359°, molecular mass 318.03 requires positive API-ES, m/z (rel. int.): 319.2 [M+H]+ (100) (calcd. for C15H11O8 319.04). Ferric chloride test: Olive green and Shinoda test: Magenta colour. UV MeOH λmax(nm): 293, 374; MeOH/AlCl3 310, 369, 452. The 1H NMR spectral data are given in Table 1. Scutellarein (6), mp 327-329°, molecular 286.04 requires positive API-ES, m/z (rel. int.): 287.2 [M+H]+ (100) (calcd. for C15H11O6 287.05). Ferric chloride test: Deep green colour. UV MeOH λmax (nm): 280, 335. The 1H NMR spectral data are specified in Table 1.
The first compound (1), mp 163-165°, was analysed for its molecular formula C29H48O based on LC/MS data (Positive API-ES: [M+H]+ 413.3, calcd. 413.37). It gave a positive Liebermann-Burchard?s test for sterols. NMR spectral data also supported the structural assignment. The 13C NMR spectrum displayed two signals, shielded highly by the surrounding methyl, methylene and methine groups at δ 11.87 (C-29) and 11.98 (C-18). The signals at δ 18.80 (C-26) and 19.40 (C-27) indicated the methyl groups of the isopropyl moiety. The chemical shifts at δ 21.12 and 23.13 represented the methyl carbons at 19- and 21-positions. Four olefinic carbons of the compound showed peaks at δ 140.81 (C-5), 121.71 (C-6), 138.27 (C-22) and 129.34 (C-23). The spectrum also displayed a characteristic signal at δ 71.84 signifying the attachment of the hydroxyl group at 3-position. All the spectral characteristics of the compound were in close agreement with those of stigmasterol. Identity of the compound was further confirmed by comparison with an authentic sample through mean melting point (mmp) and co-TLC.
The second compound (2), mp 238-240°, was analysed for its molecular formula C17H14O7 (Positive API-ES: [M+H]+ 331.1, calcd. 331.08 and [M+Na]+ 353.1, calcd. 353.06). NMR spectral data also supported the structural assignment. It is a flavonol containing 1′,3′,4′-trisubstituted phenyl nucleus and chelated hydroxyl group (highly deshielded sharp singlet at δ 12.51) as observed in the 1H NMR spectrum. The presence of chelated hydroxyl groups at 3- and 5-positions was revealed by a bathochromic shift of 57 nm in the UV spectrum with MeOH/AlCl3 [12]. The spectral characteristics of the compound were in close agreement with those of 7,4′-dimethylquercetin. Further identity was confirmed by comparison with an authentic sample through mmp and co-TLC.
The third compound (3), mp 275-277°, was analysed for its molecular formula C15H10O6 (Positive API-ES: [M+H]+ 287.2, calcd. 287.05 and [M+Na]+ 309.2, calcd. 309.03). The compound showed a positive lead acetate, ferric chloride and Shinoda test for flavonoids. The presence of chelated hydroxyl groups was revealed at 5- position by a highly dishielded sharp singlet at δ 12.48 and 3-position by a deshielded broad singlet at δ 9.42 in the 1H NMR spectrum and further supported by the large bathochromic shift of 55 nm in the UV spectrum with MeOH/AlCl3 [12]. The 1H NMR spectrum also disclosed the presence of p-disubstituted phenyl moiety by its signals of four aromatic protons at δ 8.04 and 6.93 and m-disubstituted pheny moiety by signals of two aromatic protons at δ 6.44 and 6.18 constituting two A2B2 systems. A broad singlet at δ 10.82 indicated the hydroxyl group at 7-position. A tetracetate derivative of the compound showed m.p. 186-188° and was analysed for the formula C23H18O10 (Positive API-ES: [M+H]+ 455.2, calcd. 455.09). A tetramethyl ether derivative of the compound showed m.p. 163-165° and was analysed for the formula C19H18O6 (Positive API-ES: [M+H]+ 343.2, calcd. 343.11). The properties of the compound and its derivatives closely approached to those of kaempferol and its corresponding tetraacetate and tetramethyl ether derivatives. Further identity was confirmed by comparison with an authentic sample through mmp and co-TLC.
The fourth compound (4), mp 318-320°, was analysed for the molecular formula C15H10O7 (Positive API-ES: [M+H]+ 303.3, calcd. 303.04). The compound exhibited positive ferric chloride and Shinoda test for flavonoids. It is a flavonol containing 1′,3′,4′- trisubstituted phenyl nucleus and chelated hydroxyl group (highly deshielded sharp singlet at δ 12.52) as evidenced in the 1H NMR spectrum. The presence of chelated hydroxyl groups at 3- and 5-positions was also revealed by a bathochromic shift of 58 nm in the UV spectrum with EtOH/AlCl3 [12]. Chemical evidence and spectral analysis of the compound closely approached to those of quercetin. Further identity of the compound was confirmed by mmp and co-TLC with an authentic sample.
The fifth compound (5), mp 357-359°, was analysed for its molecular formula C15H10O8 (Positive API-ES: [M+H]+ 319.2, calcd. 319.04). The compound showed positive ferric chloride and Shinoda test for flavonoids. The 1H NMR spectrum disclosed the presence of 1′,3′,4′,5′-tetrasubstituted phenyl nucleus by the signal (a singlet constituting A2 system) of two aromatic protons at δ 7.29 and chelated hydroxyl group by the highly deshielded sharp singlet at δ 12.61. A large bathochromic shift of 78 nm in the UV spectrum with MeOH/AlCl3 supported the presence of additional hydroxyl groups at 3′-,4′- and 5′-positions apart from those at 3- and 5-positions [12]. Chemical evidence and spectral characteristics of the compound closely agreed with those of myricetin. Further identity was confirmed by comparison with an authentic sample through mmp and co-TLC.
The sixth compound (6), mp 327-329° was analysed for the formula C15H10O6 (Positive API-ES: [M+H]+ 287.2, calcd. 287.05). The compound showed a positive ferric chloride test for flavonoids. It is a flavone with chelatyed hydroxyl group and was shown by absorption maxima at 280 and 335 nm in the UV spectrum and sharp singlet at δ 13.00 (chelated hydroxyl group) and 6.73 (characteristic H-3) in the 1H NMR spectrum. The 1H NMR spectrum also disclosed the presence of p-disubstituted phenyl moiety by its signals of four aromatic protons constituting A2B2 system. Chemical evidence and spectral analysis of the compound closely approached to those of scutellarein. Further identity was confirmed by comparison with an authentic sample through mmp and co-TLC.
Separation by conventional gradient chromatographic elution of the chloroform extract of Polygonum viscosum root afforded six compounds namely stigmasterol (1), 7,4′-dimethylquercetin (2), kaempferol (3), quercetin (4), myricetin (5) and scutellarein (6), the structures of which are shown in fig. 1. All the six compounds were identified by chemical and spectral analysis. A variety of bioactive compounds were recorded from Polygonum genus ranging from flavonoids, sesquiterpenes, anthraquinones, stilbene glycosides, terpenoids, coumarins to esters. Among the six compounds isolated and characterized in the present phytochemical evaluation, stigmasterol (1) was not reported earlier from P. viscosum. The compounds, 7,4′-dimethylquercetin (2) [8], quercetin (4) and scutellarein (6) [11] were reported from P. hydropiper. Kaempferol (3) from P. amphibium, P. aviculare, P. convolvulus, P. hydropiper, P. lapathifolium and P. persicaria and myricetin (5) from P. aviculare and P. lapathifolium were also reported earlier [13]. This is the occurrence of the six compounds for the first time from P. viscosum.
Acknowledgements
Authors are thankful to the Principal (SSAIPSR) and Vice Chairman of Aditya Educational Institutions for their support to carry out the work. Authors also acknowledge the support of Dr. P.V. Prasanna at BSI, Deccan Regional Centre, Hyderabad in authenticating the plant specimen.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Datta BK, Rahman MM, Gray AI, Nahar L, Hossein SA, Sarker SD, et al.Polygosumic acid, a new cadinanesesquiterpene from Polygonumviscosum, inhibits the growth of drug-resistant Escherichia coli and Staphylococcus aureus(MRSA)in vitro. J Nat Med 2007;61:391-6.
- Datta BK, Datta SK, Chowdhury MM, Khan TH, Kundu JK, Rushid MA, et al. Analgesic, antiinflammatory and CNS depressant activities of sesquiterpenes and a flavonoid glycoside from Polygonumviscosum. Pharmazie 2004;59:222-5.
- Datta B, Datta S, Khan T, Kundu J, Rashid M, Nahar L, et al. Anti-cholinergic, Cytotoxic and Anti-HIV-1 Activities of Sesquiterpenesand a Flavonoid Glycoside from the Aerial Parts of Polygonumviscosum. Pharm Biol 2004;42:18-23.
- Datta BK, Rashid MA, Kundu JK, Rouf AS, Sarker SD, Datta SK. Isolation and structure elucidation of viscoazucine, a novel sesquiterpene from Polygonumviscosum. Pharmazie 2001;56:578-9.
- Datta BK, Datta SK, Sarker SD. Quercetin 3- O-(6''-galloyl)-β-D-galactoside from Polygonumviscosum(Polygonaceae). Biochem SysEcol2000;28:805-7.
- Datta BK, Datta SK, Rashid MA, Nash RJ, Sarker SD. A sesquiterpeneacid and flavonoids from Polygonumviscosum.Phytochemistry 2000;54:201-5.
- Isah Y, Ndukwe IG, Amupitan JO. Isolation of stigmasterol from aerial plant part of SpillanthesAcmellaMurr. World J Life Sci Med Res2012;2:77-81.
- Haraguchi H, Hashimoto K, Yagi A. Antioxidative substances in leaves of Polygonumhydropiper. J Agric Food Chem 1992;40:1349-51.
- William BL, Wender SH. The isolation and identification of kaempferol and quercetin from Strawberries (Fragariachiloensis). J Am ChemSoc 1952;74:5919-20.
- David JM, Cruz FG, Guedes ML. Flavonol glycosides from Davillaflexuosa. J BrazChemSoc 1996;7:115-8.
- Peng JF, Strack D, Baumert A, Subramaniam R, Goh NK, Chia TF, et al. Antioxidant flavonoids from leaves ofPolygonumhydropiperL. Phytochemistry 2003;62:219-28.
- Jurd L. Aluminium complexes of phenolic flavones. Spectral and structural correlations. Phytochemistry 1969;8:445-62.
- Smolarz HD. Comparative study free flavonoid aglycones in herbs of different species of PolygonumL. Acta Pol Pharm 2002;59:145-8