- *Corresponding Author:
- A. El-Shabasy Department of Biology, College of Science, Jazan University, Jazan 45142, Kingdom of Saudi Arabia E-mail: ael-shabasy@jazanu.edu.sa
| Date of Received | 09 December 2023 |
| Date of Revision | 22 November 2024 |
| Date of Acceptance | 04 December 2024 |
| Indian J Pharm Sci 2024;86(6):1968-1978 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The current study evaluated the different drying methods used for Canarium schweinfurthii Engl. fruit pulp whether traditional like sun dried and oven-dried as well as modern dried method and expressed their influences on the phytochemical by products. Canarium schweinfurthii is a species of rainforest of Nigeria whose fruits are intensively gathered and eaten due to its medicinal values. It is critically endangered due to poor seed germinability and limited usage. Phytochemical compositions were measured as protein, fat, ash, fiber and carbohydrate contents. Moisture content was determined in presence of other moisture parameters that influenced on chemical composition of fruit pulp like relative humidity and moisture isotherm for each primary metabolite. Moisture isotherm have also been applied to understand the physiological processes occurring in viable seeds and how medicinal metabolite properties differ. Correlation and analysis of variance analysis calculated the degree of significant and computability based on simple linear regressions. Thermal phytochemical denaturation curve was established against anti-sickling analgesic markers of Canarium schweinfurthii Engl. fruit pulp; epielemadienolic acid, 3 beta-fluorotirucalla-7, 24-dien-21-oic acid and 3α-hydroxytirucalla-8, 24-dien-21-oic acid. There was a degree of improvement in measuring chemical composition of fruit pulp as carbohydrates and fats were moderate in content besides increasing in conductivity and capacitance by using modern drying methods that reflected the enhancing of active molecules with the medicinal values. This investigation confirmed on the important modern technique that should be followed for drying fruits and seeds to keep their medicinal values and viability especially for endangered plant species and much more consumed ones.
Keywords
Metabolite, isotherm, anti-sickling, analgesic markers, active molecule
Drying is the process of dehydrating food content to be able to store without any microbial activity. This process is used for preserving food for storage, trade exchange, or, nowadays, as a gene bank. Gene bank is prepared for conserving plant resources all over the world as gene bulk used as weapons facing all modern challenges as endemic diseases, exploring barren areas, climatic changes, environmental pollution etc. It is also used to protect such endangered medicinal plants from extinction. To ensure the storage of fruit or seeds with high quality, maximum longevity, and full viability, the drying process should be carried out to high standards. The main problem of drying process is temperature that influences on physical and chemical properties of fruits and seeds. Phytochemical materials are natural products from medicinal plants which have many vital roles in relieve or healing such diseases whether pathogenic or not. The Moisture Content (MC) is the sensor of keeping phyto-natural products with the intact structures without any denaturation. The much less water content inside the fruits has the negative impact on nature of chemical composition[1,2].
The medicinal values of phytochemical ingredients for fresh and dried fruits depend on thermal efficiency to obtain the most configurations of chemical structures. Shading is an example of natural drying used as traditional methods for plant parts. It inhibits the acceleration of water vitalization besides it saves the capacity of volatile compounds. High temperature may or may not fulfill medical quality requirements. Killing Enzymes and decrease the antioxidant activities in the fruits are the most impact figures of temperature effects. Modern techniques like microwave and far-infrared drying methods possess different effects with appropriate results. They are applied on Phytolaccae radix that decline the toxicity of extracted chemical ingredients like Salvianolic acid B (Sal-B) to be more effective against brain, heart, liver, kidney and liver[3,4].
Canarium schweinfurthii (C. schweinfurthii) Engl. is called African olive belonging to Burseraceae family. It situated in West and central Africa as native plants. The active secondary phytochemicals are present in the fruit pulp. It contains saturated, non-saturated fatty acids, vitamin C, phenolic compounds, flavonoids, sterols, glycosides, alkaloids etc. This plant was reported to have antimicrobial activities against different pathogenic microbes with gram positive or gram negative. Minimum Inhibitory Concentration (MIC) values range from 64-1024 μg/ ml. It has antimalarial and antiplasmodial activities against many mosquitos’ parasites like Plasmodium falciparum. Kingsley et al.[5] stated that there is anticancer activity of C. schweinfurthii. Studies proved its effects on leukemia CCRF-CEM cells by oxidative toxicity to damage cancer cells. The plant can be used as a cure against renal injuries, eliminate inflammation of kidney cells and improve infiltration and discharge of tubular casts and necrosis[6,7].
The aim of this study is to illustrate the effect of temperature on different dry methods of C. schweinfurthii fruit pulps and the gradually influencing on medicinal quality and effectiveness especially for active components for sickle cell disease treatment.
Materials and Methods
Study area:
The study was conducted over the three summer months, from June to August 2022, under bright sunshine in the Pankshin Local Government Area of Plateau State, Nigeria, with its headquarters in the town of Pankshin. It has an area of 1524 km² and a population of 191 685 at the 2006 census (National Population Commission, 200). The geographical coordinates of Pankshin are latitude 9.3279° N and longitude 9.4312° E, and an altitude of 1371 meters elevation above sea level. Pankshin enjoys a more temperate climate than much of the rest of local government areas in Plateau state. Average monthly temperatures range from 20°- 24° (70°F-79°F) and the annual rainfall is at an average of 1150 mm (45.28 inches) of rainfall per year, or 95.8 mm (3.77 inches) per month. The economic activities of people in Pankshin mainly farming with predominant crops such as maize and guinea corn. There are also a few civil servants and dry season farmers (irrigation vegetable farming). Pankshin is dominated by the Ngas people. The Igbos, the Hausas, the Yorubas, and a few other ethnic minorities in Nigeria are in Pankshin.
Sample collection and preparation:
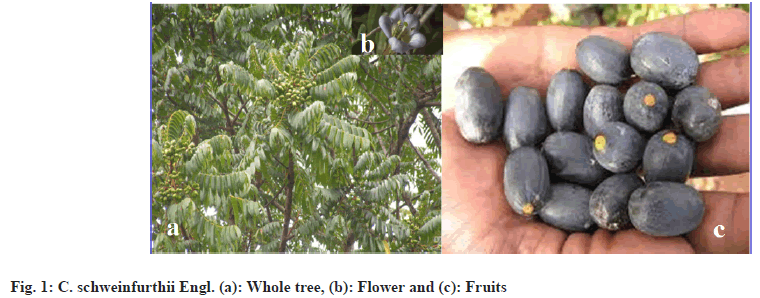
1 kg of freshly harvested ripe fruits of African olive with thick mesocarp was purchased in Daily Market, Pankshin Local Government Area. The fruit was then washed properly with clean deionized water to remove dirt and was divided into three for sun drying, ovendrying and modern drying. The mesocarp was removed leaving the fluted-stone seed for easy drying (fig. 1a-fig. 1c). For each parameter analysis, 5 replicas were used.
Sun drying:
500 g of each sample was used for the experiment. The sliced Canarium mesocarp was evenly spread on a tray and left to dry in the sunshine for at least 7 h/d for a period of 4 d until the Canarium mesocarp was brittle and considered to be dry.
Oven drying:
For oven drying, 500 g of sliced mesocarp of Canarium were evenly spread on an oven tray to allow for moisture escape and placed in a gas oven. The tray containing the African olive mesocarp to be dried was placed in the oven and allowed to dry at a temperature of 65o-75o for 4 h. The oven was opened at intervals of 30 min to allow moisture to escape. After brittle hard drying had been achieved, the Canarium was placed on shelves to cool.
Modern drying:
It was the same method of oven drying but the temperature of 50°-55° for a period of 2 h. Then, fruits were dried again by using anhydrous calcium chloride granules in a desiccator keeping at 20o. After 24 h, remove the fruits and immerse them in self-indicating blue silica gel. Regenerate the silica gel daily by heating it at 100° until it turns deep blue again. Allow it to cool in an airtight container before reusing[8].
Determination of nutrient content:
The determination of various nutrient parameters was conducted at the National Veterinary Research Institute (NVRI Lab.) food/biochemistry laboratory in Jos. The following nutrients were analyzed
Determination of ash content: Ignition was carried out in a muffle furnace by using a porcelain crucible with a lid for 1 min, then directly transferred into a desiccator to obtain constant weight (W1). Nearly 4 g of Canarium fruit pulp powder was weighed again in the crucible (W2). After that, it was heated gently by using Bunsen burner in a fume hood till smoking is stopped then it was transferred into the muffle heated at a range 550°-570° to burn all organic matter and the carbon chars left in the form of CO2 and a white ash remain. Finally, the crucible was transferred into the desiccator to obtain (W3)[9]
Percentage of ash=weight of ash/weight of sample×100
= w3-w1/w2-w1×100
Determination of crude fat: A soxhlet extractor is used for crude fat determination. 5 g of fruit pulp powder was put in a fat-free extraction thimble to dry and obtain (W1), then plugged with cotton pieces and (W2) measured. It was replaced again in the fat free extraction thimble with solvent. The temperature was adjusted to 250° for 6 h till it had siphoned. After that, the thimble was dried in a fat-free clean beaker at 50° to constant weight. It was transferred into a desiccator to obtain (W3). The solvent was concentrated through a rotary evaporator then dried, cooled and weighed[10]:
Lipid (%)=weight gain of flask/weight of sample×100
Determination of protein content: 0.1 g of dried fruit pulp powder was transferred into Kjeldahl digestion flask. Few crystals of both Kjeldahl digestion mixture and granule crystals of anti-bumping were added. Digestion of sample was occurred by adding 10 ml concentrated H2SO4 for 3 h for complete oxidation. The residue was cooled then diluted with 100 ml distilled water. Steam distillation was applied in Markham’s distillation apparatus by using 40 % NaOH. The colour of the sample is changed as a result of ammonia liberation from protein content. Finally the distillate was titrated against 0.11 M Na2CO3 to obtain the total crude protein[11].
Determination of crude fiber: 2 g of dried fruit pulp powder was digested with 100 ml of 0.25 N H2SO4 for 4 min with continuous shaking. The residue was filtered by ashless filter paper through gentle suction. Then, washed with 100 ml distilled water followed by addition of 50 ml ethanol and 60 %-80 % 50 ml petroleum ether gradually. The sample was ashed at 600° for 4 h till constant weight then cool and reweighted. The loss in weight was referred to total crude fiber[12]:
Crude fiber (%)=b-c/a×100
Where a=weight of sample digested, b=weight of sample after digestion and c=weight of ash
Determination of carbohydrate content: Carbohydrate content of the mesocarp fruit of C. schweinfurthii was determined[13] by subtracting the total ash content, crude fat plus crude protein and crude fiber from the total dry matter.
Determination of moisture parameters:
MC can be expressed as percentage of water loss per dry weight as the following:
MC (%)=Wet weight-dry weight/dry weight×100
Relative Humidity (RH) is the degree of water evaporation that occurs from fruit to the surrounding air.
It can be calculated by adding sufficient fruit pulps in a closed covered container fill up the half till night then psychrometer measured RH[14]. Electrical Conductivity (ECo) is a measure of the electrical resistance of fruit material while Electrical Capacitance (ECa) is a measure of the ability of fruits to store electrical charge. They can be determined by standard calibration curve assigned by respectively[15,16]. Equilibrium MC (EMC) is the conventional MC intersecting between MC and RH which can be estimated by the following equation:
Me=∫_1^n((1-D0)×√(-440×In (1-R)))/(1.1+(T/90))
Where, (D0) is fruit oil content and R is RH at a given temperature (T in °C) (17).
Moisture isotherm for each primary metabolite is the moisture regime capturing for each main primary metabolite in the fruit[18].
Investigation of predicted effects on specific phytochemicals:
After evaluation of some potential triterpenoic acids from C. schweinfurthii against sickle cell disease through Thin Layer Chromatography (TLC) and High Performance Liquid Chromatography (HPLC) X-rays crystallography was performed by[19], the predicted effective curve of temperature on degree of analogue chemical structure decomposition was established[20].
Statistical analysis:
Pearson correlation coefficient among oven, sun and drying parameters achieved p values for significance tests based on degrees of freedom. The parameters were subjected to statistical analysis to work out two-way Analysis of Variance (ANOVA). F-test was used to test the difference between them on basis standard error >0.5 p. The representation of correlated data was done by the linear regression approaches which explored the extent effect for both parameters[21] (fig. 1).
Results and Discussion
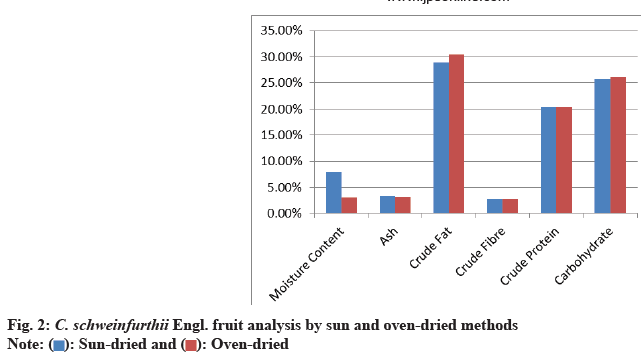
Analysis of C. schweinfurthii fruit dried using sun drying was shown in (Table 1). The results showed that the percentage moisture, ash, crude fat, crude fiber, crude protein and carbohydrate content of the sun dried fruit were 8.05 %, 3.25 %, 28.96 %, 2.76 %, 20.43 % and 25.79 % respectively while (Table 2) shows the result of analysis of oven-dried Canarium fruits. The results showed that the percentage moisture, ash, crude fat, crude fiber, crude protein and carbohydrate content of the oven-dried fruit are 3.00 %, 3.16 %, 30.42 %, 2.76 %, 20.43 % and 26.14 % respectively. Both tables were represented by char bar graph (fig. 2).
| S. No. | Composition | Sun-dried (%) | Raw (%) |
|---|---|---|---|
| 1 | MC | 8.05±0.02 | 32.25 |
| 2 | Ash | 3.25±0.02 | 3.41 |
| 3 | Crude fat | 28.96±0.09 | 23 |
| 4 | Crude fiber | 2.76±0.03 | 2.92 |
| 5 | Crude protein | 20.43±0.02 | 20.43 |
| 6 | Carbohydrate | 25.79±0.02 | 20.1 |
Table 1: C. Schweinfurthii Engl. Fruit Analysis by Sun-Dried Method
| S. No. | Composition | Oven-dried (%) | Raw (%) |
|---|---|---|---|
| 1 | MC | 3.00±0.02 | 32.25 |
| 2 | Ash | 3.16±0.02 | 3.41 |
| 3 | Crude fat | 30.42±0.09 | 23 |
| 4 | Crude fiber | 2.76±0.03 | 2.92 |
| 5 | Crude protein | 20.43±0.02 | 20.43 |
| 6 | Carbohydrate | 26.14±0.02 | 20.1 |
Table 2: C. schweinfurthii Engl. Fruit Analysis by Oven-Dried Method
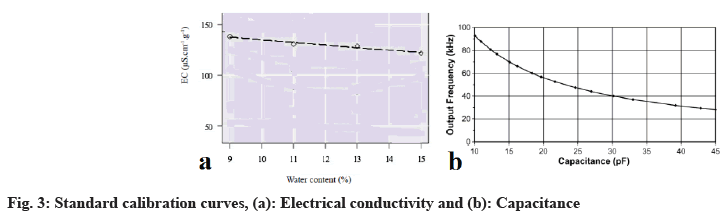
Electrical conductivity of sun dried fruits recorded the highest value while oven dried ones recorded the lowest one. The electrical capacitance was nearly the same for sun-dried and modern dried but RH scored the highest value at modern dried fruits. Moreover, oven dried fruits showed the lowest values in both RH and Me (Table 3). The standard calibration curves were represented in (fig. 3a and fig. 3b).
| Parameter | Sun-dried | Oven-dried | Modern-dried |
|---|---|---|---|
| Conductivity (μS.cm-1.g-1) | 133.3 | 120.5 | 148 |
| Capacitance (pF) | 23.2 | 22.5 | 23.9 |
| RH (%) | 44.30 % | 10.20 % | 60.50 % |
| Equilibrium MC (%) | 10.35 | 8.09 | 10.34 |
| F test | sun#oven 1.062 | sun#modern 1.247 | oven#modern 1.324 |
| P value | sun#oven 0.962 | sun#modern 0.860 | oven#modern 0.822 |
Table 3: C. schweinfurthii Engl. Fruit Water Analysis
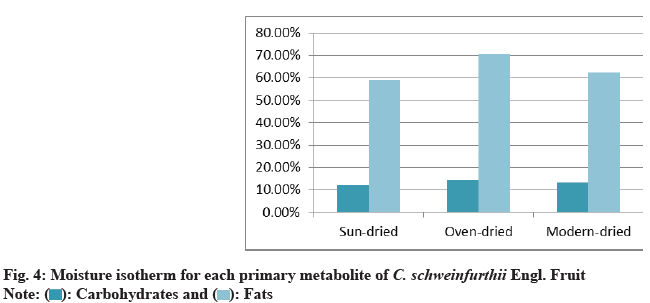
Moisture isotherm for each primary metabolite can easily be constructed from standard calibration curve by allowing fruits to reach equilibrium in environments with known RH maintained by saturated salt solutions at a given temperature. The following saturated salt solutions provide a series of RHs at 25° (Table 4, Table 5, fig. 4, and fig.5).
| Parameter | Sun-dried | Oven-dried | Modern-dried |
|---|---|---|---|
| Carbohydrates | 12.20 % | 14.40 % | 13.50 % |
| Fats | 58.90 % | 70.80 % | 62.30 % |
| F test | sun#oven 1.459 | sun#modern 1.092 | oven#modern 1.336 |
| P value | sun#oven 0.881 | sun#modern 0.972 | oven#modern 0.908 |
Table 4: Moisture Isotherm for each Primary Metabolite of C. schweinfurthii Engl. Fruit
| Salt | Corresponding RH (%) |
|---|---|
| Sodium hydroxide | 7.5 |
| Lithium chloride | 13 |
| Magnesium chloride | 32 |
| Magnesium nitrate | 54 |
| Ammonium nitrate | 65 |
| Sodium chloride | 75 |
| Potassium chloride | 85 |
Table 5: Saturated Salt Solutions Provide a Series of RHS at 25°
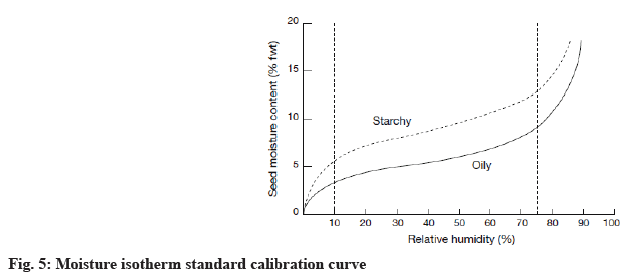
According to statistical analysis, the two way ANOVA test was designed to establish the relation between two independent variables on one dependent variable. It was clear that F test of water analysis recorded the highest value (1.324) between oven and modern dried methods against sun-dried method moreover; sun and oven-dried methods against modern method obtained the highest F value (1.459) for moisture isotherms. Finally correlation analysis confirmed that sun-dried and modern-dried methods were high correlated that represented by regression scatter graph as highly positive regressed (Table 6 and fig. 6a-fig. 6c).
| Sun-dried | Oven-dried | Modern-dried | |
|---|---|---|---|
| Sun-dried | 0.94 | 0.995 | |
| Oven-dried | 0.94 | 0.902 | |
| Modern-dried | 0.995 | 0.902 |
Table 6: Correlation of all Dried Methods of C. schweinfurthii Engl. Fruit
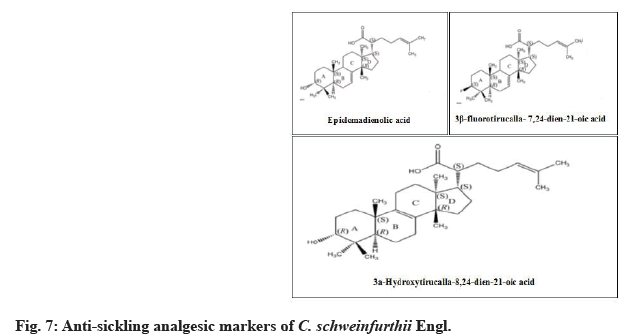
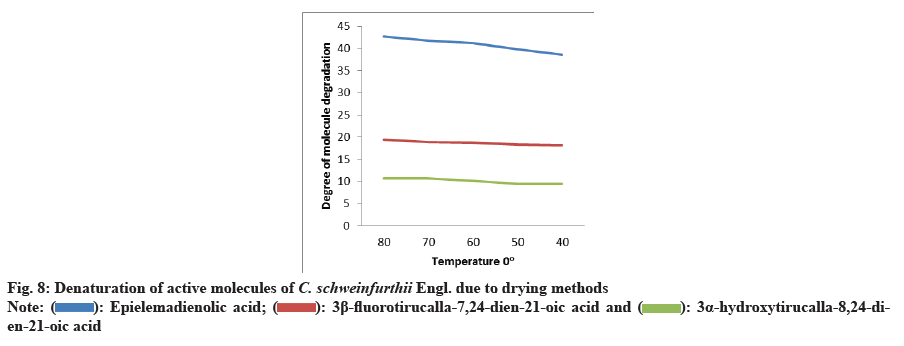
Chemical screening of C. schweinfurthii Engl. phenolic compounds were deduced to be anti-sickling analgesic markers. They were Epielemadienolic acid, 3 Beta (β)-fluorotirucalla-7,24-dien-21-oic acid and 3α-Hydroxytirucalla-8,24-dien-21-oic acid (fig. 7). Thermal phytochemical denaturation curve was established through other analogue chemical structure in number of carbon atoms, number of unsaturated bonds, type of active functional groups, polarity index and stereo-isometrcal properties. This current study had undergone representation between the antisickling analgesic markers of C. schweinfurthii against ambient degradation temperature induced from drying processes[22] (fig. 8).
Epielemadienolic acid possesses analgesia and inflammatory action on induction of oedema. 3β-fluorotirucalla-7,24-dien-21-oic acid is able to control the polymerization of Hemoglobin S (HbS) and reduce the degree of bleeding besides manage the biological indicators affect sickle cell disease. 3a-Hydroxytirucalla-8, 24-dien-21-oic acid relieves the pain from harsh denatured blood cells by inhibiting acetic acid elevator with anti-inflammatory properties[23].
From previous studies, there were different isomeric analogues for 3 anti-sickling markers of C. schweinfurthii Engl. that possessed medicinal values for various diseases. These chemical structures were summarized in Table 7. Alpha (α)-linolenic acid is multipurposed medicinal metabolite as cardioprotective, neuroprotective, anticancer, anti-inflammatory, antidiabetic, antioxidant and hepatoprotective agent[30]. Anti- Alzheimer’s agent was found at 3-hydroxytirucallic acid[31]. Suppression of T lymphocyte proliferation could be performed by applying 3α-hydroxytirucalla- 8,24-dien-21-oic acid[32].
| S. No. | Core analogous structure for epielemadienolic acid | Treating disease | Core analogous structure for 3β-fluorotirucalla-7,24-dien-21-oic acid | Treating disease | Core analogous structure for 3α-Hydroxytirucalla-8,24-dien-21-oic acid | Treating disease |
|---|---|---|---|---|---|---|
| 1 | α-Linolenic acid | Tumor disease | 3-oxotirucalla-7,24-dien-21-oic acid | Cancer breast disease | α-Elemolic acid | Rheumatoid arthritis |
| 2 | 3-Hydroxytirucallic acid | Alzheimer’s disease | (2S)-6-methyl-2-((5R,9R,10R,13S,14S,17S)-4,4,10,13,14-pentamethyl-3-oxo-1,2,5,6,9,11,12,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl)hept-5-enoic acid and lanosta-7,24-dien-21-oic acid | Parkinson’s disease | 2-(3-hydroxy-4,4,10,13,14-pentamethyl-2,3,5,6,7,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-17-yl)-6-methylhept-5-enoic acid | Bulimia nervosa |
| 3 | 3α-Hydroxytirucalla-8,24-dien-21-oic acid | Immunological disease | (2S)-6-methyl-2-((5R,9R,10R,13S,14S,17S)-4,4,10,13,14-pentamethyl-3-oxo-1,2,5,6,9,11,12,15,16,17-decahydrocyclopenta(a)phenanthren-17-yl)hept-5-enoic acid | Leukemia disease | - | - |
| 4 | Decahydro-1H-cyclopenta[a]phenanthren-17-yl]-6-methylhept-5-enoic acid | Diabetic disease | - | - | - | - |
Table 7: core analogous structures for studied anti-sickling markers of c. Schweinfurthii engl. Fruit
Decahydro-1H cyclopenta(a)phenanthren-17-yl-6- methylhept-5-enoic acid was tested by several clinical trials to confirm its ability to loss body weight by alteration of binding mechanism for target orexigenic receptors including the neuropeptide Y1 receptor[33]. 3-oxotirucalla-7,24-dien-21-oic acid had been succeeded to execute as cytotoxic potential against cell line (MCF-7) of human breast female adenocarcinoma disease[34].
(2S)-6-methyl-2-((5R,9R,10R,13S,14S,17S)- 4 , 4 , 1 0 , 1 3 , 1 4 - p e n t a m e t h y l - 3 - o x o - 1,2,5,6,9,11,12,15,16,17-decahydrocyclopenta[a] phenanthren-17-yl)hept-5-enoic acid had been approved to be neurotrophin for brain-derived factor and nerve growth factor that acquired beneficial role for central nervous system against neurodegenerative diseases like Parkinson’s disease[35].
Furthermore, studies included (2S)-6-methyl- 2 - ( ( 5 R , 9 R , 1 0 R , 1 3 S , 1 4 S , 1 7 S ) - 4 , 4 , 1 0 , 1 3 , 1 4 - pentamethyl-3-oxo-1,2,5,6,9,11,12,15,16,17- decahydrocyclopenta(a)phenanthren-17-yl-hept-5- enoic acid that played a vital medicinal role at breast diseases on A549 and MCF7 cell line due to binding Vascular Endothelial Growth Factor Receptor (VEGFR) on an exelekto kinase[36]. α-elemolic acid was an enzymatic inhibitor against Phospholipase A2 (PLA2) that reduce much more the degree of inflammatory reactions against internal inflammatory diseases[37].
2 - ( 3 - h y d r o x y - 4 , 4 , 1 0 , 1 3 , 1 4 - p e n t a m e t h y l - 2,3,5,6,7,11,12,15,16,17-decahydro-1H-cyclopenta(a) phenanthren-17-yl)-6-methylhept-5-enoic acid was the main human activator serotonin receptor that mediated the activity of nuclear factor and cyclooxygenases[38].
The MC of 8.05 % in sun-dried sample is quite high compared to that of the oven-dried fruit which is 3.00 %. The lower MC of the oven-dried sample is due to higher heat load of oven at 65o-75o compared to sun-drying temperature of 35°-45°. The MC in both sun-dried and oven-dried fruits are very low compared to that of the fresh fruit which is 32.25 %. Low MC implies that the shelf life (storage) of sun-dried and oven Canarium fruits have been greatly improved by drying. Moisture or water content is an important parameter that determines the quality of dried fruits for storage. Amount of water in C. schweinfurthii fruit allow hydrolysis reaction of the oil present in the fruit and contributes to the formation of free fatty acids. In addition, high level water content causes bacterial contamination that is able to hydrolyze fat molecules[24,25]. The best way of drying is able to control the moisture gradient and migration of molecules within inter and intracellular spaces besides inner capillaries. The critical moisture which fruit loses its main physical and chemical properties is prohibited via modern drying methods. Understanding the relationship between equilibrium fruit MC and RH is important in determining the appropriate drying regime for fruits.
The electrical conductivity is based on the amount of MC and associated with the fruit cell membrane integrity and structure. The reduced number of electrical conductivity refers to the reinforced mechanism leading to more cell membrane strain as losing much more electrolytes. Electrical capacitance values presumably correlated with electrical conductivity as it relies how the fruit pulp can imbibe and capture charges, be more vital and resist the damage. The ash content is regarded as the most important analytical biological material which determines the inorganic content after removal of organic one in the form of CO2. Ash content can reflect the degree of percentage of moisture and the effect of temperature on it. Hence, as the drying temperature increases, the moisture decreases, this increases the chances of complete burning. This explains why there was a difference in ash content when using various drying methods. The presence of active phytochemicals comes from the determination of inorganic and organic matter. Therefore, ash content indicates the amount of mineral elements, while protein, fat, carbohydrates, and fibers refer to organic matter[26].
The crude fiber is not affected much more from various drying methods because it is a transition equilibrium state of matter which induces the balance and combination between organic and inorganic content inside the intact whole structure like fruit pulp or seed. It helps the maintenance of MC and keeps active phytochemical structures more stable[27].
On the other hand, crude protein content was found to be the same. This implies that protein structure is the less affected from temperature change due to nature of folded chains of amino acids that can repair and replace the active sites in globular materials so as to reduce the relative impact of enzymatic behavior for such specific protein.
Moisture isotherms depend on the chemical composition of fruits. It measures the degree of water harvesting from the total MC of the entire fruit. It reflects on the viability of the main primary metabolites mainly fats and carbohydrates. The crude fat of raw Canarium fruit was 23 % that of sundried Canarium fruit was 28.96 % while that of oven dried is 30.42 %. This shows temperature has a small effect on fat. This could be attributed to reduction in water content that increased the concentration of oil and lipids present in the samples. Fat helps to increase the feel of food in the mouth. It is also important in food because it promotes fat-soluble vitamin absorption; it is a highenergy nutrient and does not add to the bulk of diet[28]. Carbohydrate content of raw Canarium fruit was 20.10 % that of sundried fruit was 25.79 % while that of oven-dried was 26.14 %. These figures show that the carbohydrate content of Canarium fruit is only slightly affected by the drying method. The mean carbohydrate content ranges from 20.10 %-26.14 %, indicating that Canarium fruit is a rich source of energy and capable of supplying the body's daily energy requirements[29].
Three natural triterpenoic acids prove to be the best anti-sickling analgesic markers with more antiinflammatory properties and antioxidants than other artificial used drugs like 3,5-dimethoxy-4-hydroxy benzoic acid and 2,3,4-trihydroxyacetophenone. They can enhance reshape of red blood cells in addition to increase blood factors to improve the stability of blood cells inside intact blood capillaries[23].
Statistical analysis expressed the actual status of fruit pulp against different parameters. F test was considered the monitor of the most convenient variables interacted with high significant versus other variables. It reflected the degree of affinity between the modern method and the less modern one; the oven method, although, was far more inter-compatible with other methods for moisture isotherms. It means that modern method maintains the water properties inside living plant tissues furthermore it possess the best standard level for optimum configuration for phytochemicals. As whole correlated data, the integrated results indicated that modern method can reach to optimized standardization for pharmacological and medicinal values as they appeared in intact fruit pulp before drying due to high positively simple linear curve between modern vs. sun method. From molecular degradation curve, this study deduced that the high temperature has a negative impact on integrity of the chemical composition especially in the active functional groups that have the main pharmaceutical action on the target disorder of disease. The extent of chemical denaturation differs from one species to another according to the quantity of moisture enclosure. This investigation gives an alarm notification on the importance of drying methods to keep our fruits and seeds more effective with medicinal useful roles.
The drying methods for fruits and seeds should be accomplished with scientific procedures to keep different genetic accessions with their medicinal values and protect the endangered or much more exhausted plants in the world without loss of any vital characterizations. Hence, they will be conserved for more generations to face all global challenges on our planet like environmental disasters, economic shortages, endemic or pandemic disease or climatic changes. Gene bank, seed multiplication and Deoxyribonucleic Acid (DNA) conservation are the most suggested international tools for keeping fruits and seeds with highest viability and longevity besides most effective standard levels for active phytochemical ingredients.
Conflict of interest:
The authors declared no conflict of interests.
References
- Thamkaew G, Sjöholm I, Galindo FG. A review of drying methods for improving the quality of dried herbs. Crit Rev Food Sci Nutr 2021;61(11):1763-86.
[Crossref] [Google Scholar] [PubMed]
- Irondi AE, Anokam KK, Ndidi US. Effect of drying methods on the phytochemicals composition and antioxidant activities of Carica papaya seed.2013;3(11):1-7.
- Yang J, Miao M. Analysis of the influence of different drying methods on the active ingredients of fresh medicine. Chin Med Nat Prod 2022;2(01):e1-4.
- Amarjeet S., Thounaojam, Jenish P. Drying Techniques in Medicinal and Aromatic Plants and its Impact on Quality. Futuristic Trends in Agriculture Engineering and Food Sciences Publisher: Iterative International Publishers, Karnataka 2024; pp. 186-209.
- Omeje KO, Ezema BO, Ozioko JN, Omeje HC, Ossai EC, Eze SO, et al. Biochemical characterization of Soxhlet-extracted pulp oil of Canarium schweinfurthii Engl. fruit in Nigeria. Sci Rep 2022;12(1):10291.
[Crossref] [Google Scholar] [PubMed]
- Milivojevic M, Ripka Z, Petrovic T. ISTA rules changes in seed germination testing at the beginning of the 21st century. J Process Energy Agric 2018;22(1):40-5.
- Karimov K, Akhmedov A, Kushimov B, Yuldoshev B. Justification, development of new technology and design for drying seeds of desert fodder plants. InIOP Conference Series: Materials Science and Engineering 2020;883(1):012107.
- Curry HA. The history of seed banking and the hazards of backup. Soc Stud Sci 2022;52(5):664-88.
[Crossref] [Google Scholar] [PubMed]
- Nyam MA, Makut MD, Itelima JU, Daniel AM. Nutritional potential of the fruits of black olive (Canarium schweinfurthii Linn) from Plateau State, Nigeria. Pak J Nutr 2014; 13:335-9.
- Abayeh OJ, Abdulrazaq AK, Olaogun R. Quality characteristics of Canarium schweinfurthii Engl. oil. Plant Foods Hum Nutr 1999;54:43-8.
[Crossref] [Google Scholar] [PubMed]
- Anyalogbu EA, Onyeike EN, Monanu MO. Amino acid profile of heat-processed Canarium schweinfurthii pulp. J Sci Res Rep 2014;3(14):1973-85.
- Cohen JS, Yang TC. Progress in food dehydration. Trends Food Sci 1995;6(1):20-5.
- Horwitz W, Latimer GW. Association of official analytical chemists. Gaithersburg, MD, USA. 2000;1(4):442-56.
- Ehiem JC, Ndirika VI, Onwuka UN, Gariepy Y, Raghavan V. Water absorption characteristics of Canarium Schweinfurthii fruits. Inf Process Agric 2019;6(3):386-95.
- da Silva Ferreira LB, Alves Fernandes N, Costa de Aquino L, Rodrigo da Silva A, Marcos Nascimento W, Leão-Araújo ÉF. Temperature and seed moisture content affect electrical conductivity test in pea seeds. J Seed Sci 2017;39(4).
- McIntosh RB, Casada ME. Fringing field capacitance sensor for measuring the moisture content of agricultural commodities. IEEE Sens J 2008;8(3):240-7.
- Ehiem JC, Ndirika VI, Onwuka UN. Effect of moisture content on some physical properties of Canarium schweinfurthii Engl. fruits. Res Agr Eng 2016;62(4):162-9.
- Corbineau F. The effects of storage conditions on seed deterioration and ageing: How to improve seed longevity. Seeds 2024;3(1):56-75.
- Kitadi JM, Mazasa PP, Sha-Tshibey Tshibangu D, Kasali FM, Tshilanda DD, Ngbolua KT, et al. Ethnopharmacological survey and antisickling activity of plants used in the management of sickle cell disease in Kikwit city, DR Congo. Evid Based Complement Alternat Med 2020;8(1):1346493.
[Crossref] [Google Scholar] [PubMed]
- Tcheghebe OT, Seukep AJ, Tatong FN. A review on traditional uses, phytochemical composition and pharmacological profile of Canarium schweinfurthii Eng. Nat Sci 2016;14(11):17-22.
- Areshi S, Mashlawi AM, El-Shabasy A, Daim ZA, Mohsen A, Salama SA. Larvicidal, pupalicidal and adulticidal effects of Artemisia absinthium L. against dengue vector Aedes aegypti (Diptera: Culicidae) in Jazan region, KSA. Saudi J Biol Sci 2023;30(12):103853.
[Crossref] [Google Scholar] [PubMed]
- Rocha RP, Melo EC, Radünz LL. Influence of drying process on the quality of medicinal plants: A review. J Med Plant Res 2011;5(33):7076-84.
- Gamaniel K, Samuel BB, Kapu DS, Samson A, Wagner H, Okogun JI, et al. Anti-sickling, analgesic and anti-inflammatory properties of 3, 5-dimethoxy-4-hydroxy benzoic acid and 2, 3, 4-trihydroxyacetophenone. Phytomedicine 2000;7(2):105-10.
[Crossref] [Google Scholar] [PubMed]
- Sharma A, Chen CR, Lan NV. Solar-energy drying systems: A review. Renew Sustain Energy Rev 2009;13(6-7):1185-210.
- Asiah N, Astuti RM, Cempaka L, Setiani R. Physical and chemical characteristic of virgin coconut oil under mix culture fermentation technique. J Phys Conf Ser 2019;1364(1):012009.
- Matlala ME, Ndhlovu PT, Mokgehle SN, Otang-Mbeng W. Ethnobotanical investigation of Mimusops zeyheri, an underutilized indigenous fruit tree in Gauteng Province, South Africa. Sustainability 2024;16(4):1410.
- Karel M, Lund D. Physical principles of food preservation: Revised and expanded. CRC Press; 2003.
- Erbay Z, Icier F. A review of thin layer drying of foods: theory, modeling, and experimental results. Crit Rev Food Sci Nutr 2010;50(5):441-64.
[Crossref] [Google Scholar] [PubMed]
- Boudhrioua N, Djendoubi N, Bellagha S, Kechaou N. Study of moisture and salt transfers during salting of sardine fillets. J Food Eng 2009;94(1):83-9.
- Srivastava D, Singh V, Kumar U, Venkatesh KR. Alpha-linolenic acid: A pharmacologically active ingredient from nature. Indian J Nutr Diet 2021;58(4):534-53.
- Siddiqui A, Shah Z, Jahan RN, Othman I, Kumari Y. Mechanistic role of boswellic acids in Alzheimer’s disease: Emphasis on anti-inflammatory properties. Biomed Pharmacother 2021;144:112250.
[Crossref] [Google Scholar] [PubMed]
- Zimmermann-Klemd AM, Reinhardt JK, Nilsu T, Morath A, Falanga CM, Schamel WW, et al. Boswellia carteri extract and 3-O-acetyl-alpha-boswellic acid suppress T cell function. Fitoterapia 2020;146:104694.
[Crossref] [Google Scholar] [PubMed]
- Wong AR, Hung A, Yang AW, Gill H, Lenon GB. Poria cocos compounds targeting neuropeptide Y1 receptor (Y1R) for weight management: A computational ligand and structure-based study with molecular dynamics simulations identified beta-amyrin acetate as a putative Y1R inhibitor. Plos One 2023;18(6):e0277873.
[Crossref] [Google Scholar] [PubMed]
- Al-Zikri PN, Taher M, Susanti D, Rezali MF, Read RW, Sohrab MH, et al. Cytotoxic tirucallane triterpenes from the stem of Luvunga scandens. Rev Bras Farmacogn 2014;24(5):561-4.
- Hassan K, Matio Kemkuignou B, Kirchenwitz M, Wittstein K, Rascher-Albaghdadi M, Chepkirui C, et al. Neurotrophic and immunomodulatory lanostane triterpenoids from wood-inhabiting Basidiomycota. Int J Mol Sci 2022;23(21):13593.
[Crossref] [Google Scholar] [PubMed]
- Salunke M, Mane P, Kumbhar S, Wakure B. An evaluation of Sargassum cinctum anticancer properties utilizing in vitro testing and molecular docking, with assistance from GC-HRMS and FTIR. J Appl Pharm Sci 2024;14(9):169-81.
- Giresha AS, Urs D, Manjunatha JG, Sophiya P, Supreetha BH, Jayarama S, et al. Group IIA secreted phospholipase A2 inhibition by elemolic acid as a function of anti-inflammatory activity. Sci Rep 2022;12(1):7649.
[Crossref] [Google Scholar] [PubMed]
- Uddin MZ, Paul A, Rakib A, Sami SA, Mahmud S, Rana MS, et al. Chemical profiles and pharmacological properties with in silico studies on Elatostema papillosum wedd. Molecules 2021;26(4):809.
[Crossref] [Google Scholar] [PubMed]
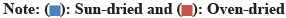
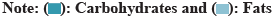
 3α-hydroxytirucalla-8,24-dien-21-oic acid
3α-hydroxytirucalla-8,24-dien-21-oic acid