- *Corresponding Author:
- Madhumita Roy
Department of Biotechnology, Techno India University, EM 4/1, Sector V, Kolkata-700 091, India
E-mail: roymadhumita@rediffmail.com
| Date of Submission | 12 September 2016 |
| Date of Revision | 05 April 2017 |
| Date of Acceptance | 08 November 2017 |
| Indian J Pharm Sci 2018;80(1):26-35 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In this study, antimicrobial property of Suaeda maritima, a mangrove associate species collected from lower Gangetic delta complex was evaluated. The antibacterial activity of the extract against a wide spectrum of Gram-negative and Gram-positive isolates and multiple antibiotic resistant strains suggested that it may be a potential antibacterial agent to control antibiotic resistant infections. Among the different solvent fractions examined, n-hexane extract of the shoot of S. maritima L (Dumort) was found to possess highest antibacterial activity against four Gram-positive (Bacillus subtilis, Staphylococcus haemolyticus, Staphylococcus aureus, Enterococcus faecalis) and six Gram-negative (Escherichia coli, Citrobacter sp., Klebsiella pneumoniae, KPC producing K. Pneumonia, Pseudomonas sp. and Stenotrophomonas maltophila) bacteria. Fungicidal activity was noted against Saccharomyces cerevisiae. Minimum inhibitory concentration and minimum bactericidal concentration values were determined in disc diffusion and macrodilution assays. The inhibition zones ranged between 13-20 mm. GC-MS analysis of the n-hexane fraction showed the presence of various fatty acid esters and essential oils. In addition, scytalone known for its antioxidant property, campesterol and ethanone were detected. Antioxidant activity was found having an IC50 value of 52.53 mg/ml.
Keywords
Suaeda maritima, mangrove associate, multiple drug resistant (MDR), antimicrobial activity, bioactivity assay, GC-MS
The problem of antibacterial resistance to frequently used antibiotics has led to a search for newer and alternative compounds for the treatment of drugresistant infections [1]. Although pharmaceutical companies have designed a number of new antibiotics in the last three decades, resistance against these drugs by microorganisms has also been observed. Drug resistant bacterial infections are causing immense mortality and morbidity worldwide. For example, in 2005, in the United States, 19 000 out of 95 000 patients affected from methicillin-resistant Staphylococcus aureus (MRSA) have died. Number of death was higher than number of deaths combined from HIV/ AIDS, Parkinson’s disease, emphysema, and homicides combined [2]. As many branches of treatments like postsurgical care, neonatal care, transplantation medicine, cancer chemotherapy, and care of the critically ill patients need effective antibiotic treatment, failure of conventional antibiotic treatment to combat multidrug resistant (MDR) pathogens, caused high rate of mortality. With this background, World Health Organization (WHO) identified MDR bacteria as one of the top three high priority threats to human health. Infectious Disease Society of America has addressed the biomedical community to declare a war against the MDR bacterial threat. Along with intensifying research on understanding the resistance pattern of MDR infection, they have stressed that the ultimate goal of scientist would be to identify appropriate and efficient antimicrobial drugs to the patients.
As for a long period of time, plants have been a valuable source of natural products for maintaining human health, certain plant extracts can be a cure for infections caused by MDR. Along with different herbs, seaweeds and higher plants, many workers have suggested the usefulness of mangroves in traditional medicines. Mangroves have been used as a source of timber, food, fuel, fodder, medicines and fish poison in tropical coastal zones. Recently scientists have found evidence that mangroves have medicinal properties that can cure many diseases like asthma, diabetes, cancer, ulcer, wounds and AIDS [3]. Local people use mangrove plant extracts for centuries as the folk medicine for curing many health disorders [4,5]. Suaeda maritima (L.) Dumort of the family Chenopodiaceae is a salt marsh mangrove annual herb that grows in very alkaline and saline moist soils. The plant is distributed throughout the east and west coast of India including Sundarbans in West Bengal. Traditionally, different parts of S. maritima have been used as an ethnomedicine for curing various ailments. The juice of the plant has been used for treating hepatitis and has been reported to have antiviral, hepatoprotective, antiinflammatory and antioxidant activities [6,7]. The leaves are known for curing liver, heart, and lipid disorders [8]. Ravikumar et al. [7] showed that the leaves of S. maritima significantly attenuated concanavalin (a hepatotoxin)- induced biochemical changes (serum aspartate aminotransferase, AST; alanine aminotransferase, ALT and bilirubin) and histopathological changes in liver.
The overall objective of the present study was to ascertain the antimicrobial activity of the S. maritima against selected MDR pathogens and other clinical Gram-positive and Gram-negative strains known to cause disease in humans and animals and identify the probable compounds present in the extract responsible for the activities. This study showed potency of the compounds against strong multidrug resistant pathogens.
Materials and Methods
S. maritima plant was collected from Bonnie camp of Sundarbans (21°49′53.581′′ 0 N latitude and 88°36′44.860′′ 0E longitude) on August, 2015. The plant was authenticated as S. maritima by Botanical Survey of India, Apex Research Organization under the Ministry of Environment and Forests, Govt. of India (Figure 1). The shoots were collected, cut into small pieces and air dried in the shade. The dried plant material was ground to a coarse powder by mortar and pestle. The dried powdered plant material was extracted with different solvents viz., hexane, benzene, chloroform, ethyl acetate, DMSO and methanol. The resultant extracts were concentrated in a rotary evaporator after filtration through Whatman filter paper. The crude extracts were kept in air tight containers for further analysis.
Microorganisms and their antibiotic susceptibility
The studied microorganisms included both antibiotic sensitive and MDR strains of Escherichia coli, Citrobacter sp., Staphylococcus haemolyticus, S. aureus subsp. aureus, Stenotrophomonas maltophila, Bacillus subtilis, Klebsiella pneumonia, Pseudomonas sp., and Enterococcus faecalis obtained either clinically or from the microbial type culture collection (Table 1). Among the strains used, the isolated clinical MDR strains Citrobacter sp., S. maltophila and Pseudomonas sp. were tested for antibiotic resistance by placing antibiotic discs (HiMedia Hexa G, rifampicin, polymixin B and vancomycin) on the agar plates previously inoculated with the respective strains. Their MAR index values (total number of antibiotics to which the strain is resistant divided by the total number of antibiotics tested) were calculated. Table 1 shows the antibiotics susceptibility nature of the isolates.
| Antibiotics | Concentration | S. maltophila | Citrobactersp. | Pseudomonassp. |
|---|---|---|---|---|
| Zone of inhibition (cm)* of the susceptible strains | ||||
| Ampicillin (ß-lactam) | 10 µg/ml | - | - | - |
| Chloramphenicol (phenicols) | 25 µg/ml | - | - | - |
| Penicillin G (ß-lactam) | 1 unit | - | - | - |
| Streptomycin (aminoglycosides) | 10 µg/ml | - | - | - |
| Sulphatriad | 300 µg/ml | 1.6 | - | - |
| Tetracycline (tetracyclines) | 25 µg/ml | 0.8 | 1.1 | - |
| Ceftazidime (ß-lactam) | 30 µg/ml | 1 | 1.3 | - |
| Ciprofloxacin (fluoroquinolones) | 5 µg/ml | 1.5 | - | - |
| Amikacin (aminoglycosides) | 30 µg/ml | - | - | - |
| Nitrofurantoin | 300 µg/ml | - | - | - |
| Netillin | 30 µg/ml | - | - | - |
| Nalidixic acid (fluoroquinolones) | 30 µg/ml | - | - | - |
| Co-trimoxazole | 25 µg/ml | - | - | - |
| Amoxyclav | 30 µg/ml | - | - | - |
| Gentamicin (aminoglycosides) | 10 µg/ml | - | 1.4 | - |
| Ofloxacin (fluoroquinolones) | 5 µg/ml | 1.15 | - | - |
| Cefuroxime (cephalosporins) | 30 µg/ml | - | - | - |
| Cefotaxime (cephalosporins) | 30 µg/ml | 0.8 | 1.2 | - |
| Levofloxacin (fluoroquinolones) | 5 µg/ml | 1.7 | 1.1 | - |
| Aztreonam (monobactams) | 30 µg/ml | 0.65 | 1.3 | - |
| Imipenem (ß-lactam) | 10 µg/ml | - | 1.7 | - |
| Rifampicin | 5 µg/ml | - | - | - |
| Polymixin B (polyeptides) | 300 units | - | - | 2.0 |
| Vancomycin (glycopeptides) | 50 µg/ml | - | - | - |
| Kenamycin (aminioglycosides) | 50 µg/ml | - | - | - |
| MAR index | 0.68 | 0.72 | 0.96 | |
Table 1: Antibiotic Susceptibility Test of Three Clinical MDR Isolates
Disc diffusion assay
The disc diffusion assay was carried out to detect the antimicrobial nature of the crude plant extract. The crude extract (0.1 g/ml) was dissolved in 100 % n-hexane, methanol, chloroform, benzene, ethyl acetate and DMSO and sterilized by filtration. Mueller Hinton Agar (MHA) medium (HiMedia) was used in the disc diffusion assay. For testing antifungal activity, potato dextrose agar media was used. A 50 μl sample of the filtration-sterilized plant extract was loaded onto a sterile paper disc of 6 mm diameter, which was then placed on the surface of the agar plate previously inoculated with the bacteria. For negative control, a disc was prepared with 50 μl of solvent. A similar disc was loaded with the reference antibiotic standard (ampicillin for Gram-positive bacteria and kanamycin for Gram-negative bacteria and nystatin for fungi) at a concentration of 50 μl/ml. All the plates were incubated at 37° for 24 h. Antibacterial and antifungal activities were determined by calculating the diameter of the growth inhibition zones (mm) around the discs. Each assay was performed in triplicate and the results were expressed as average values. The inhibition zones of the extracts prepared in different solvents were compared and n-hexane extract, which gave the best results for all the microbes tested were used in later experiments. The antimicrobial index was calculated by following formula, antimicrobial index=(inhibition zone of sample/inhibition zone of the standard)×100 [6]. Activity index or antimicrobial index gives an overview of the activity of the antimicrobial agent against a variety of microbes.
Minimum inhibitory concentration (MIC) determination using agar dilution assay (macrodilution)
The MIC of the extracts, which inhibited the growth of the microbes was determined by macrodilution assay carried out in MHA medium. Serial dilutions of the extracts were prepared in n-hexane. Commercial antibiotics were diluted in sterile water. One milliliter of the serially diluted extracts were added in 9 ml of molten MH agar, vortexed and dispensed into petri plates for solidification. Two microliters of each working bacterial suspension was then punctually placed on the solidified agar surface. Bacterial growth control was made in control plate by adding 1 ml of sterile water in the molten agar medium and placing the same amount of bacterial suspension at different places like before on the solidified agar plates. Bacterial growth was determined on each plate after 24 h at 37° by comparing the punctual growth zones with those in the controls.
Determination of minimum bactericidal concentration (MBC)
The MBC was determined by the method of Vila et al. [9] with slight modifications. Approximately, 2 μl of the sample from MIC assay was spread onto freshly prepared MH agar plates, incubated at 37° for 24 h and monitored for the presence of bacterial growth.
Thin layer chromatography (TLC) and bioautography analysis
The TLC assay was performed according to Quiroga et al. [10], with slight modifications. First, TLC was run on Silica Gel 60 plates in different solvent systems like ethyl acetate:formic acid:acetic acid:water (100:11:11:27 v/v), chloroform:methanol (85:15 v/v), and ethyl acetate:n-hexane (0.5:9.5 v/v). After developing the plates, the positions of the various compounds were determined by fluorescence under UV light (254 and 366 nm) and the Rf values were measured. After TLC, direct bio-autography was performed with direct agar overlay technique [11]. Ten millilitres of the extract was spotted on the chromatograph silica gel plates. For separation, the solvent systems as mentioned above were used. The developed chromatogram was kept in sterilized petri plate and the fresh overnight grown culture of test bacteria mixed with molten nutrient agar was poured over the chromatogram as a thin layer. The plates were kept at room temperature for 5 min before its final incubation at 37° for overnight. The zone of growth inhibition was recorded. S. maltophila was used for seeding.
Gas chromatography-mass spectrometry (GC-MS) analysis
GC analysis was conducted on FactorFour™ capillary column (VF-5 ms, 30 m, 0.25 mm id, 0.25 μm film thickness; Varian, Middelburg, Netherlands) with the following parameters: constant flow of helium, 0.8 ml/min; initial inlet temperature, 70° ramped to 280°at 200°/min after a 20 s delay and held for 5.0 min; injection volume, 8 μl (large volume injections) in the liner with an open purge valve (40:1 split ratio) for 18 s, closed until 4.0 min, and open again (30:1) until the end of the run; oven temperature program, 70° for 2 min, then 20°/min ramp to 180° followed by a 2°/min ramp to 220° and held for 30 s, again 100°/min ramp to 285° and held for 5 min, followed by 100o/min ramp to 295° and held for 2 min. The MS instrument transfer line temperature was 280°, with 220° ion trap and 120° manifold temperatures. Full-scan (40-650 m/z) EI (auto) mode with 20 μ a filament current was used for MS analysis from 9.5-35.00 min, which gave 0.92 s/scans. Target automatic gain control was 20 000, and the multiplier voltage was 1450 V. Baseline offset -5, peak find with S/N of the quantifier ion at least 3 and peak width 2 s was set as the parameters for processing the peaks in the chromatograms. Minimum similarity match with regards to the NIST library spectra was kept at 500 (reversed fit). Quantification was carried out on the basis of diagnostic ion and the peak assignments and integration were automatically done through software.
Free radical scavenging activity by 1,1-diphenyl-2- picrylhydrazyl (DPPH)
The free radical scavenging activity of the n-hexane extract was determined using the stable radical DPPH [12]. Aliquots (20-100 μl) of the test sample were placed in test tubes and 3.9 ml of freshly prepared DPPH solution (25 mg/l) in methanol was added in each of the test tubes and mixed. The absorbance was measured at 517 nm. Methanol and DPPH solution were used as blank sample and control sample, respectively and ascorbic acid was used as reference standard. The DPPH radical scavenging activity was calculated using following Eqn., DPPH scavenged (%)={(Ac– At)/Ac}×100, where, Ac defines the absorbance of the control reaction and At is the absorbance of the extract in the presence of the sample. The antioxidant activity of the extract was referred as IC50. The IC50 value is defined as the concentration of dry material (in mg) per milliliter that inhibits the formation of DPPH radicals by 50 %. Each value was determined from the regression equation [12].
Preliminary phytochemical screening
The n-hexane plant extract was used for phytochemical screening of different phytochemicals including proteins, terpenoids, resins, saponins, steroids, tannins, phenol and cardiac glycosides by standard protocols of Simlai and Roy [13]. Presence of proteins was detected by Biuret test. For this, 0.5 mg of extract was mixed with equal volume of 40 % NaOH solution and two drops of 1 % CuSO4 solution was added. The appearance of violet colour indicates presence of protein. Salkowski test is done for terpenoids. Here, 5 ml of extract was mixed in 2 ml of chloroform followed by the addition of 3 ml concentrated H2SO4. A layer of the reddish brown colouration at the interface indicates presence of terpenoids. For determination of resin, 1 ml of extract was treated with few drops of acetic anhydride solution followed by 1 ml of concentrated H2SO4. Presence of resin gives colouration ranging from orange to yellow.
About 0.5 mg of the extract was shaken with 5 ml of distilled water. Formation of frothing (appearance of creamy miss of small bubbles) indicates presence of saponins. For testing steroids, 0.5 g of the extract was mixed with 2 ml of acetic anhydride followed by 2 ml of H2SO4. The change of colour from violet to blue or green indicates presence of steroids. For identification of tannins, a small quantity of extract was mixed with water and heated on a water bath. The mixture was filtered and ferric chloride was added to the filtrate. Formation of a dark green colour indicates presence of tannins. Ferric chloride test was carried out for detection of phenol. Ten milligrams of extract was treated with few drops of FeCl3 solution. Formation of bluish black colour indicates presence of phenol. Keller-Killani test was done for detection of cardiac glycosides. Five millilitres of extract was mixed with 2 ml of glacial acetic acid containing one drop of FeCl3 solution. Then, 1 ml concentrated H2SO4 was added. Brown ring formation at the interface indicates the presence of glycosides.
Statistical analysis
Each assay was carried out three independent times in triplicate. In case where they were different, the MIC or MBC were taken as the most frequently occurring values. For calculation of the inhibition zones, the data were expressed as mean±standard deviation (SD) of the triplicate values. The half-maximal inhibitory concentrations (IC50) values were determined from the AA % versus concentration plots using a linear regression graph.
Results and Discussion
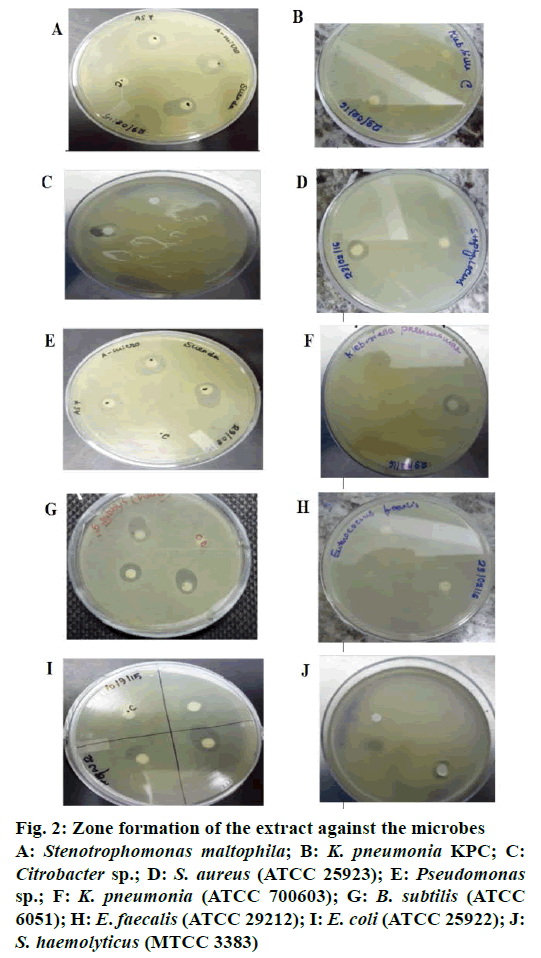
Crude extract of S. maritima shoot was prepared in different solvents like n-hexane, benzene, ethyl acetate, chloroform, DMSO and methanol. Among the different solvent extracts tested, extracts prepared in solvents other than n-hexane either gave no activity or lower activity than n-hexane. The n-hexane extract of the S. maritima shoot showed antibacterial activity against 4 Gram-positive (B. subtilis, S. haemolyticus, S. aureus, E. faecalis) and 6 Gram-negative (E. coli, Citrobacter sp., K. pneumoniae, K. pneumoniae carbapenemase (KPC), Pseudomonas sp. and S. maltophila) bacteria. The n-hexane extract possessed not only antibacterial property but antiyeast property also. The antimicrobial activity of the extract assessed by determining their MIC, MBC/MFC and inhibition zone values have been presented in Table 2. The antimicrobial index ranged from 72-125 %. Lowest activity was recorded against E. coli (72 %) and highest activity was recorded against KPC producing K. pneumonia (125 %). Figure 2 shows the antibacterial activity of the extract against the microbes specified in disc diffusion assay.
| Microbe tested | MIC* | MBC (MFC*) | IZ (mm) | AI | |||
|---|---|---|---|---|---|---|---|
| Shoot extract | Standard | Shoot extract |
Standard | Shoot extract | Standard | ||
| Gram-positive | |||||||
| B. subtilis(ATCC 6051) | 99 | 16a | 205 | 32a | 19±0.5 | 16±0.0a | 118.75±0.5 |
| S. haemolyticus(MTCC 3383) | 105 | 25a | 215 | 50a | 18±0.0 | 20±0.2a | 90±0.2 |
| S.aureus(ATCC 25923) | 100 | 50a | 200 | 100a | 17±0.0 | 20±0.0a | 85±0.0 |
| E. faecalis(ATCC 29212) | 125 | 75b | 250 | 180b | 13±0.0 | 18±0.5b | 72.22±0.5 |
| Gram-negative | |||||||
| Citrobactersp. | 155 | 300b | 330 | 600b | 15±1.0 | - b | - |
| E. coli(ATCC 25922) | 160 | 100b | 310 | 200b | 15±1.8 | 20±0.0b | 75±1.8 |
| K. pneumonia(ATCC 700603) | 160 | 100b | 150 | 100b | 19±0.0 | 20±0.0b | 95±0.0 |
| K. pneumoniaKPC ATCC BAA1705) |
180 | 100b | 200 | 100b | 20±1.5 | 16±0.0b | 125±1.5 |
| Pseudomonas sp. | 130 | 250b | 250 | 800b | 18±1.5 | -b | - |
| S. maltophila | 140 | 250b | 280 | 750b | 20±2.0 | -b | - |
| Fungi | |||||||
| S. cerevisiae | 280 | 20c | 320* | 40c | 20±0.5 | 19±0.5c | 105.26±0.5 |
Table 2: MIC, MBC and Inhibition Zone (IZ) value of S. maritime (l) shoot extract against the Microorganisms by Microdilution broth assay
Figure 2: Zone formation of the extract against the microbes
A: Stenotrophomonas maltophila; B: K. pneumonia KPC; C: Citrobacter sp.; D: S. aureus (ATCC 25923); E: Pseudomonas sp.; F: K. pneumonia (ATCC 700603); G: B. subtilis (ATCC 6051); H: E. faecalis(ATCC 29212); I: E. coli (ATCC 25922); J: S. haemolyticus (MTCC 3383)
TLC analysis of the n-hexane extract was done in different solvent systems. Best separation and highest number of bands was obtained with ethyl acetate and n-hexane run in 0.5:9.5 ratios. Bio-autographic assay of the TLC plate run on the above mentioned solvent system showed one zone of inhibition at the site of the topmost band (Rf value of 0.9).
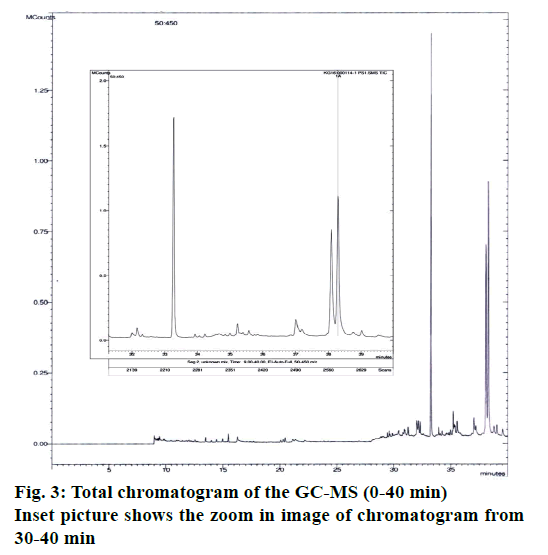
A number of different compounds were detected in the GC-MS analysis. Table 3 [14-28] shows the name of the compounds detected, their peak areas, type of metabolite and whether that type of metabolite had any previous record of antimicrobial activity. Figure 3 shows the total chromatogram of the GC-MS. Mainly different types of essential oils and fatty acids, which are well known for their role as antimicrobial compounds [29,30] have been detected in the extract. In addition to the various essential oils and fatty acid, campesterol (steroid) and ethanone (phenolic compound) were also found. Both of them have reports of having antibacterial activity. Among the compounds detected, highest peak area were observed for hexacontanoic acid (34.25 %), aciphyllene (22.195 %) and campesterol (22.615 %). Among the compounds detected having reports as antioxidant activity, most significant was scytalone, which is known to be a precursor of melanin, which is a powerful cation chelator and acts as a radical sink [31].
| Compound name | Retention time (min) |
Molecular formula | Molecular weight | Type of metabolite | Biological activity reported |
|---|---|---|---|---|---|
| Cirtonellyl propionate | 13.512 | C13H24O2 | 212 | Essential oil | Strong antimicrobial activity, pest and bird repellent activity (has use in food flavor) [14] |
| Cirtonellyl acetate | 14.434 | C12H22O2 | 198 | Essential oil | Strong antimicrobial activity, pest and bird repellent activity (has use in food flavor) [15] |
| Tetradecanoic acid (myristic acid) | 15.496 | C17H34O2 | 270 | Fatty acid | Good antimicrobial activity reported by many authors [16] |
| Scytalone | 16.249 | C10H10O4 | 194 | Precursor of melanine | Melanine has report as antioxidant [31] |
| Hexadecanoic acid, methyl ester | 17.25 | C17H34O2 | 270 | Fatty acid | Good antimicrobial activity reported by many authors [16] |
| Tritetracontane | 17.450 | C43H88 | 604 | Alkane hydrocarbon | Bioactivity reported against bacteria and mycobacteria [17] |
| 6,11 Eicosadienoic acid methyl ester | 20.089 | C21H38O2 | 322 | Fatty acid | Report not present |
| 9,12-Octadecadienoic acid (C18:2) | 20.097 | C18H32O2 | 280 | Fatty acid | Antifungal activity [18] |
| Linoleic acid ethyl ester | 20.110 | C20H36O2 | 308 | Fatty acid | Antimicrobial activity reported [19] |
| 2-hexyl-1-octanol | 20.346 | C14H30O | 214 | Essential oil | Antimicrobial activity reported [20] |
| Diisobutylene | 23.569 | C8H16 | 112 | hydrocarbon | No activity reported yet |
| n-Hexatriacontane | 27.062 | C36H74 | 506 | hydrocarbon | Report present as antioxidant [21] |
| Citronellal | 32.001 | C10H18O | 154 | Essential oil | Reports present [22,23] |
| Palmitic acid | 32.147 | C16H32O2 | 256 | Long chain fatty acid | Report present as antimicrobial [16,17] |
| Hexacontanoic acid | 33.286 | C60H12O2 | 872 | Long chain fatty acid | Not much report present as antimicrobial |
| n-Octadecane | 35.218 | C18H38 | 254 | Essential oil | Reports present [24] |
| 9-Eicosyne | 37.005 | C20H38 | 278 | Straight chain alkane | Bioactivity reported [25] |
| Campesterol | 38.076 | C28H48O | 400 | Steroid | Bioactivity reports present [26] |
| Aciphyllene | 38.291 | C15H24 | 204 | Plant essential oil | Has reports of antibacterial activity [27] |
| Ethanone | 38.294 | C16H14O4 | 210 | Phenolic | Report present as antimicrobial [28] |
Table 3: Phytochemicals identified by GC-MS in the N-hexane fraction of shoot of S. maritima
The evaluation of the antiradical property was performed by DPPH radical scavenging assay. The IC50 value was determined to be 82.528 mg of dry material/ml as calculated from the regression equation. Our value is in line with values obtained by others [6].
A preliminary qualitative estimation of the presence of different phytochemicals in the n-hexane extract showed the presence of proteins, xanthoproteins, steroids, cardiac glycosides, resins, acidic compounds, and phenols. However, saponins, terpenoids, alkaloids and tannins were not detected to be present.
The Sundarbans, a UNESCO World Heritage Site is the largest halophytic mangrove forest in the world. Mangroves grow in estuarine swamps; have unique adaptations to combat environmental stresses like high salinity, humidity, temperature, daily tidal cycles, low nutrient and excessive radiation. Because of the unique adaptation they have developed defensive stress responses by producing secondary metabolites of various types like alkaloids, flavonoids, phenolics, saponins, steroids, terpenoids and tannins, many of which play role as antioxidant, antibacterial, antilarval, antiviral, antifungal and antiinsecticidals [32]. An inevitable consequence of exposure to various environmental stresses results in the generation of ROS and accordingly plants produce various antioxidant molecules. Although many of these mangroves and mangrove associates have shown promising therapeutic applications as reported by various ethnomedicinal and experimental studies, the specific metabolites responsible for these bioactivities are remained to be elucidated. In our study, we found S. maritima extract to possess antimicrobial activity against multidrug resistant pathogens. We tested the extract on a wide range of Gram-positive and Gram-negative isolates that causes various types of diseases. S. aureus is known to cause serious diseases such as pneumonia and meningitis, where E. coli, P. aeruginosa, K.pneumonia cause the urinary tract infections, pulmonary tract infections, infection in wounds, dysentery-like diarrhoea and other blood infections. KPC-producing bacteria is a new group of highly drug-resistant Gramnegative bacilli whose incidence is rapidly increasing throughout the globe. Infections caused by KPCenzyme (responsible for carbapenem resistance) producing K. pneumoniae have been associated with frequent treatment failures and death. Production of KPC enzyme is an important mechanism of drug resistance and is no longer limited to K. pneumonia and is rapidly spreading among other Gram-negative bacteria. Effective antibiotics against KPC producing pneumonia are presently limited to polymyxins, tigecycline and occasionally aminoglycosides but scientists fear that in near future they may become familiar with the limited effective antibiotics and drug resistance against these limited spectrums of antibiotics may make them more imperishable [33]. So the activity of Suaeda extract against the KPC producing K. pneumonia is indeed significant and requires in depth research on its active pharmaceutical ingredients. The extract showed its antibacterial activity against another very important class of MDR strain S. maltophila whose MDR power has been the research topic for a decade [34,35]. By definition antimicrobial compounds are those phytochemicals that produce MICs of 100 to 1000 μg/ml during susceptibility tests [36]. Activity of crude extracts can be declared significant if MIC values are below 100 μg/ml, moderate when 100<MIC< 625 μg/ml or low when MIC<625 μg/ml [36]. Our extract was more effective on Gram-positive pathogens than Gram-negative pathogens. MIC ranged from 99-125 μg×ml-1 in Gram-positive pathogens while in Gram-negative pathogens it ranged from 130- 180 μg×ml-1. Therefore the antimicrobial activity of the extract recorded against the pathogens can be considered as significant to moderate. The reason for the less activity of the extract against Gram-negative strains may be due to the difference in the cell wall structure between the two groups. Previously many authors have found that many medicinal plant extracts are less active against Gram-negative strains [37]. Relatively large sized molecules may not gain good entrance by size selective porin channels of the outer membrane of the Gram-negative cell wall. This may have prevented the compounds from reaching the inner membrane and cytoplasm of Gram-negative species. With most strains the MBC values were almost double the MIC values, while for K. pneumonia, MBC values were almost the same as the MIC values. Almost same behaviour of the extract was noticed against antibiotic sensitive KPC producing K. pneumonia. Moreover, its activity against broad range of organisms certainly is significant and demands more research on the bactericidal or bacteriostatic nature and modes of operandi. When ratio of MBC/MIC≤4 then the sample is considered as bactericidal and when this ratio is >4 then it is considered as bacteriostatic [38]. It seems from Table 2 that the extract exhibited bactericidal effect on all the test strains. However, more studies need to be done for establishing the mode of action.
As it can be noticed from Table 3, the GC-MS analysis of the n-hexane fraction revealed 20 phytochemicals and most of them were essential oils, characterized by the presence of fatty acids including octadecanoic, hexadecanoic, and 9,12-octadecadienoic acids. Additionally, there were phenolic compounds such as ethanone. Previous studies have shown that some of the identified compounds in the shoot extract such as scytalone [31] and n-hexatriacontane [21] possess antioxidant activities. 9,12-Octadecadienoic acid (C18:2) was detected which have reports of being an antifungal agent [18]. The rest of the compounds that we detected have previous reports of antibacterial activity except 6,11 eicosadienoic acid methyl ester, hexacontanoic acid and diisobutylene for which no such reports were found. The different phytochemicals found here may explain its versatile antibacterial activity against such a broad range of Gram-positive and Gram-negative bacterial strains tested. Although we have not performed any toxicological analysis of the extract, reports are there on the non-toxic nature of the leave extract of the plant. The lethal dose of S. maritima leaf extract was identified as 3000 mg/kg/ body weight. Except for haematological parameters no toxicity was noted in other parameters like organ weights, and biochemical parameters such as serum glutamic-oxaloacetic transaminase (SGOT), Serum glutamic pyruvic transaminase (SGPT), alkaline phosphatase (ALP), sugar and urea [39].
To the best of our knowledge there is no report on the antimicrobial property of the n-hexane extract on multiple antibiotic resistant Gram-negative and Gram-positive pathogens. However, previously Patra et al. [6] carried out experiments on the antibacterial activity of shoot and root extract of S. maritima on 10 strains of bacteria (S. aureus, Shigella flexneri, B. licheniformis, B. brevis, Vibrio cholera, P. aeruginosa, Streptococcus, S. epidermidis, B. subtilis and E. coli), they observed activity only against few strains like B. brevis, S. flexneri, B. licheniformis, P. aeruginosa and S. aureus using solvents like acetone, ethanol and methanol extracts. However, none of the strains used were multidrug resistant and the probable compounds were not identified.
In summary, the n-hexane extract of the stem of S. maritima showed promise as a new source of antibacterial compounds. The study gave evidence for the ethnopharmacological use of this plant in the treatment of infections and wounds. Individual bioactive compounds should be isolated to evaluate cytotoxicity, in vivo potency; mechanism of action and pharmacokinetic properties before the plant or its extracts or compounds isolated from the extracts be regarded as new potential treatments for infections caused by MDR bacteria.
Acknowledgements
The authors thank Techno India University for providing required facilities to carry out this research work. They are grateful to KPC Medical College and Hospital for gifting us the MDR strains isolated from their patients.
Conflict of interest
The authors declare that there is no conflict of interests.
Financial support and sponsorship
Nil.
References
- Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science 1994;264:375-82.
- Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999-2005. Emerg Infect Dis 2007;13(12):1840-46.
- Premanathan M, Nakashima H, Kathiresan K, Rajendran N, Yamamoto N. In vitro antihuman immunodeficiency virus activity of mangrove plants. Indian J Med Res 1996;130:276-9.
- Banerjee D, Chakrabarti S, Hazra AK, Banerjee S, Ray J, Mukherjee B. Antioxidant activity and total phenolics of some mangroves in Sundarbans. Afr J Biotechnol 2008;7:805-10.
- Muthazhagan K, Thirunavukkarasu P, Ramanathan T, Kannan D. Studies on phytochemical screening, antimicrobial and antiradical scavenging effect coastal salt marsh plant of a Suaedamonoica. Res J Phytochemistry 2014;8:102-11.
- Patra JK, Dhal NK, Thatoi HN. In vitrobioactivity and phytochemical screening of Suaeda maritime (Dumort): A mangrove associate from Bhitarkanika, India. Asian Pac J Trop Med 2011:727-34.
- Ravikumar S, Gnanadesigan M, Inbaneson SJ, Kalaiarasi A, et al. Hepatoprotective and antioxidant properties of Suaedamaritima (L.) Dumortethanolic extract on concanavalin-A induced hepatotoxicity in rats. Indian J ExpBiol 2011;49:455-60.
- Bandaranayake WM. Traditional and medicinal uses of mangroves. Mangroves Salt Marshes 1998;2:133-48.
- Vila R, Santana AI, Peez-Roses R, Valderrama A, Castelli MV, Mendonca S, et al. Composition and biological activity of the essential oil from leaves of Pliniacerrocamanensis, a new source of a-bisabolol. BioresourTechnol 2010;101:2510-14.
- Quiroga EN, Sampietro AR, Vattuone MA. In vitro fungitoxic activity of Larreadivaricata Cav. Extracts. LettApplMicrobiol 2004;39:7-12.
- Slusarenko AJ, Longland AC, Whitehead IM. Convenient, sensitive and rapid assay for antibacterial activity of phytoalexins. Bot Helv 1998; 99:203-07.
- Venugopal S, Devarajan S. Estimation of total flavonoids, phenol and antioxidant activity of local and New Zealand manuka honey. J Pharm Res 2011;4:464-66.
- Simlai A, Roy A. Analysis of and correlation between phytochemical and antimicrobial constituents of Ceriopsdecandra, a medicinal mangrove plant, from Indian Sundarban estuary. J Med Plants Res 2012;6:4755-65.
- Bigos M, Wasiela M, Kalemba D, Sienkiewicz M. Antimicrobial activity of geranium oil against clinical strains of Staphylococcus aureus. Molecules 2012:17:10276-91.
- Nezhad FM, Zeigham H, Mota A, Sattari M, Yadegar A. Antibacterial activity of eucalyptus extracts on methicillin resistance Staphylococcus aureus. Res J BiolSci 2009;4:905-08.
- Huang CB, Alimova Y, Myers TM, Ebersole JL. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch Oral Biol 2011;56:650-4.
- Green E, Obi LC, Samie A, Bessong PO, Roland NND. Characterization of n-Hexane sub-fraction of Brideliamicrantha(Berth) and its antimycobacterium activity. BMC Complement Altern Med 2011;11:28.
- Mishra AK, Dubey NK. Fungitoxicity of essential oil of Amomumsubulatumagainst Aspergillusflavus. Econ Bot 1990:44:530-33.
- Gurnani N, Gupta M, Mehta D, Mehta BK. Chemical composition, total phenolic and flavonoid contents, and in vitro antimicrobial and antioxidant activities of crude extracts from red chilli seeds (Capsicum frutescensL.). J TaibahUnivSci 2016;10(4):462-70.
- Sarmada M, Mahalakshmipriyaa A, Senthil K. Chemical composition and in vitroantimicrobial activity of Barlerialupulinaessential oil. J Herbs Spices Med Plants 2012;18:101-09.
- Yassa N, Masoomi F, RohaniRankouhi SE, Hadjiakhoondi A. Chemical composition and antioxidant activity of the extract and essential oil of Rosa damascenafrom Iran, Population of Guilan. DARU J Pharm Sci 2009; 17:175-80.
- Hile AG. Avoidance of plant secondary compounds by European starlings: citronellyls. Crop Prot 2004;23:973-8.
- Lopez-Romero JC, González-Ríos H, Borges A, Simões M. Antibacterial effects and mode of action of selected essential oils components againstEscherichia coliand Staphylococcus aureus. Evidence-Based Complement Alternat Med 2015:795435.
- Boussaadaa O, Ammarb S, Saidanab D, Chriaac J, Chraifb I, Daamid M, et al. Chemical composition and antimicrobial activity of volatile components from capitula and aerial parts ofRhaponticumacauleDC growing wild in Tunisia. Microbiol Res 2008;163:87-95.
- Okoronkwo NE, Mbachu KA, Nnaji AB, Igboanugo OS. Evaluation of chemical compositions and antimicrobial activities of Acalyphaciliataleaves. Int J Plant Sci Ecology 2015:1:67-71.
- Delaportea RH,Sarragiottob MH, Takemuraa OS, Sánchezc GM, Filhod BPD,Nakamura CV. Evaluation of the antioedematogenic, free radical scavenging and antimicrobial activities of aerial parts of Tillandsiastreptocarpa Baker – Bromeliaceae. J Ethnopharmacol 2004;95:229-33.
- Hossain MA, Kabira MJ, Salehuddina SM, Rahmanb SM, Dasc AK, Singhac SK. Antibacterial properties of essential oils and methanol extracts of sweet basil Ocimumbasilicumoccurring in Bangladesh. Pharm Biol 2010;48:504-11.
- Al-Abd NM, Nor ZM, Mansor M, Azhar F, Hasan MS,Kassim M. Antioxidant, antibacterial activity, and phytochemical characterization of Melaleucacajuputi extract. BMC Complement Altern Med 2015;15:385.
- Dorman HJD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J ApplMicrobiol 2000;88:308-16.
- Huang CB, Alimova Y, Myers TM, Ebersole JL. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch Oral Biol 2011;56:650-4.
- Kumar CG, Mongolla P, Pombala S, Kamle AJ. Physicochemical characterization and antioxidant activity of melanin from a novel strain of AspergillusbridgeriICTF-201. LettApplMicrobiol 2011;53:350-58.
- Kokpal V, Miles DH, Payne AM, Chittarwong V. Chemical constituents and bioactive compounds from mangrove plants. Stud Nat Prod Chem 1990;7:175-99.
- Arnold RS, Thom KA, Sharma S, Phillips M, Johnson JK, Morgan DJ. Emergence of Klebsiellapneumoniaecarbapenemase (KPC)-producing bacteria. South Med J 2011;104:40-5.
- Ohene-Agyei T, Mowla R, Rahman T, Venter H. Phytochemicals increase the antibacterial activity of antibiotics by acting on a drug efflux pump. Microbiologyopen 2014;3:885-96.
- Nayak B, Roy S, Mitra A, Roy M. Isolation of Multiple Drug Resistant and Heavy Metal Resistant Stenotrophomonasmaltophilastrain BN1 a Plant Growth Promoting Rhizobacteria from Mangrove Associate Ipomoea pes-caprae of Indian Sundarbans. J Pure ApplMicrobio 2016;10(4):3131-39.
- Kuete V. Potential of Cameroonian plants and derived products against microbial infections: a review. Planta Med 2010;76:1479-91.
- Smith JE, Tucker D, Watson K, Jones GL. Identification of antibacterial constituents from the indigenous Australian medicinal plant EremophiladuttoniiF.Muell. (Myoporaceae). J Ethnopharmacol 2007;112:386-93.
- Noumedem J, Mihasan M, Lacmata S, Stefan M, Kuiate J, Kuete V. Antibacterial activities of the methanol extracts of ten Cameroonian vegetables against Gram-negative multidrug-resistant bacteria. BMC Complement Altern Med 2013;13:26.
- Banerjee MB, Ravikumar S, Gnanadesigan M, Rajakumar B, Anand M. Antiviral, antioxidant and toxicological evaluation of mangrove associate from South East coast of India. Asian Pac J Trop Biomed 2012;2(3):S1775-S79.