- *Corresponding Author:
- Sangeeta Atul Godbole
Department of Botany,
Jai Hind College,
Churchgate,
Mumbai,
Maharashtra 400020,
India
E-mail: sangeeta.godbole@jaihindcollege.edu.in
| Date of Received | 04 December 2020 |
| Date of Revision | 02 November 2021 |
| Date of Acceptance | 14 May 2022 |
| Indian J Pharm Sci 2022;84(3):604-616 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present study deals with the extraction and quantification of phytochemicals, which play a key role in the pharmaceutical world. In this article, we focused on the study of plant secondary metabolites and analyzed ethanolic and methanolic extracts of bark and roots of two species of Ixora using the highperformance thin layer chromatography technique. Alcoholic extracts of bark and root of both the plants revealed the presence of alkaloids, terpenoids, glycosides, phenols, saponins, sterols, tannins, etc., but both extracts were devoid of flavonoids and coumarins. Analysis of alkaloid content revealed the highest amount of total alkaloid content in the methanolic extract of roots of Ixora coccinea (146 mg/ gm) followed by the methanolic extract of bark of Ixora coccinea (138 mg/g). The lowest total alkaloid content was observed in the methanolic extract of bark of Ixora barbata (64 mg/g). Both ethanolic, as well as methanolic extracts of Ixora coccinea, showed higher amounts of alkaloid content when compared to Ixora barbata in similar solvents. As per high-performance thin layer chromatography analysis, the highest amount of camptothecin was recorded in ethanolic extract of root of Ixora coccinea (117 μg/ml) followed by ethanolic extract of bark of Ixora barbata (64.43 μg/ml). The lowest amount of camptothecin was observed in methanolic extract of bark of Ixora coccinea (12.64 μg/ml). The results obtained from the present high-performance thin layer chromatography analysis also revealed that the above-mentioned selected plants are a source of a number of other phytoconstituents. This is the first reported study on the high-performance thin layer chromatography analysis of Ixora barbata. The study includes quantification of camptothecin compound in both methanolic and ethanolic extracts of Ixora barbata for which there are no previously published reports.
Keywords
Ixora species, phytochemicals, camptothecin, high-performance thin layer chromatography
Basic research is mainly done on plants, as they have a huge variety of chemical compounds stored in them. Plants play a vital role in each and every aspect of life such as food, clothing, shelter, medicines, etc. Medicines derived from plants help to serve against varied ailments. Developing countries depend on medicinal plants for maintaining the good health of their population. Scientists throughout the world are trying their best to explore more and more precious assets of medicinal plants to help humanity. More than 30 % of the pharmaceutical preparations, available all over the world are based on plants[1].
The genus Ixora belonging to the family Rubiaceae consists of about 400 species[2] of which 28 are cultivated species[3]. About 30 species occur in India, a large number of exotics are cultivated in gardens.
There exist several species of Ixora which have been used as traditional medicine to treat a wide range of diseases[1,4,5]. Almost all parts of the Ixora plant i.e. the roots, leaves, bark as well as flowers are shown to contain various active phytochemicals. Specific plant parts of various species of Ixora are used for some specific pharmacological actions[6]. Decoction of bark is used as a tonic in anaemia, general debility and treatment of sores. Dried, powdered flowers are used for whooping cough. In some species of Ixora , flowers are used in dysentery, dysmenorrhoea, leucorrhoea, haemoptysis and catarrhal bronchitis. Decoction of flowers is also useful in ophthalmic conditions as well as a source to treat ulcers. Fresh flowers of some Ixora species are considered beneficial in tuberculosis and hemorrhages[7]. Different species of Ixora exhibit different pharmacological actions and they were used widely for many different ailments. This suggests variations amongst different species of Ixora , in their phytochemical content, with respect to quality as well as quantity. Thus, work on different species of Ixora may reveal presence of some novel phytoconstituents and quantities of important phytoconstituents may be compared amongst all available species to select the best source of pharmacologically useful phytoconstituents. There is a present need to screen various species of Ixora in order to identify species with possibly larger amounts of pharmacologically active phytoconstituents as well as to check for presence of novel variations in phytochemical content.
Ixora coccinea Linn. (I. coccinea) is also known as Scarlet Ixora. It is a compact shrub or small glabrous tree. The plant flowers practically throughout the year, but it is at its best during the rainy season. It is native to the Western Peninsula, now widely cultivated throughout the tropics. This plant has been known in India since ancient times and the root has some repute in native medicine[8]. I. coccinea is known to contain wide varieties of phytochemicals. I. coccinea flowers show cytotoxicity and antitumor activity[9,10]. This activity is believed to be due to the presence of some quinoline alkaloid[11]. Leaves are used in diarrhoea, stomachache and febrifuge. Roots are used as sedative, stomachic, gonorrhea, loss of appetite and shown to stimulate gastric secretion of bile, relieve abdominal pain, chronic ulcers and in headache treatment[7,12,13]. Roots are known to act as a cholagogue and to give relief from pain to those suffering from dysentery[8]. I. coccinea is thus well studied with respect to the presence of various phytoconstituents. Some important phytoconstituents have been isolated and these have been proved to exert specific pharmacological effects, some of which are mentioned above. Although a large amount of literature is available on phytoconstituents present in I. coccinea, other species of Ixora still remain to be screened for the presence of similar or novel active phytoconstituents.
Ixora barbata (I. barbata) is a large glabrous shrub, introduced into the Kolkata Garden before Roxburgh’s time, but its native country was unknown till Kurz found it in the Andamans[4]. It is also known as Bearded Ixora because barbata in Latin means ‘bearded’ and refers to the woolly mouth of the corolla. Flowers are white and fragnant, and it blooms in the month of April to June. The plant is commonly cultivated in Kolkata and elsewhere in India[8]. As per literature survey, no report is available for the phytochemical constituents of the I. barbata, hence there is an urge to check presence and quantities of important phytochemical constituents as well as screen for presence of novel phytoconstituents or drugs present in this plant, comparable to its pharmacological properties. On the other hand, I. coccinea is well studied with respect to the presence of various phytoconstituents. Some important phytoconstituents have also been isolated and these have been proved to exert specific pharmacological effects. Thus a comparative study was initiated to investigate the presence as well as quantification of important phytoconstituents in the above two species.
The anticancer activity of the leaves of I. coccinea was found principally due to the known alkaloid, Camptothecin (CPT)[10]. CPT, is an isoquinoline alkaloid and is one of the most promising anti-cancer drugs of the 21st century[14]. The global demand for CPT in 2002 was valued at United States (US) $ 4045 million. Several water-soluble derivatives of CPT are currently being used for treating ovarian and colorectal cancer[15]. CPT was first discovered in the Chinese deciduous tree as Camptotheca acuminata, family Nyssaceae[16]. Later, the alkaloid has been reported from several plant species, Nothapodytes nimmoniana[17]. Presence of CPT has also been reported in the members of Icacinaceae, Olacaceae, Rubiaceae and Apocynaceae[18]. The worldwide market size for CPT derivatives (eg. topotecan and irinotecan) reached 1.5 billion US $ in 2002[19]. The main sources of the alkaloids are bark and roots of Camptotheca acuminata[20] and Nothapodytes nimmoniana[21].
Recent interest in the possible anti-cancer effects has focused the need for the extensive study of the bioactive compound CPT and also there is an urgent need to find the alternative sources of CPT from plants so as to cater to the demands of the pharma industry. Although I. coccinea shows leaves as the main source of this drug, the main sources of the alkaloids are bark and roots in case of Camptotheca acuminata[20] and Nothapodytes nimmoniana[21]. Thus, presence and quantity of alkaloid may vary in different parts of the plant body as per plant species or genus selected[18]. Preliminary phytochemical analysis revealed the presence of alkaloids only in the roots and bark of I. barbata and not in its flowers or leaves in the current study. Thus, roots and bark of I. barbata were selected for quantification and comparison of alkaloid content with root and bark of I. coccinea plant. The present study aims to be a comparative phytochemical analysis and High- Performance Thin Layer Chromatography (HPTLC) assay of I. coccinea and I. barbata growing in the natural habitat.
Materials and Methods
Collection and authentication of plant:
Plants were collected from Acharya Jagadish Chandra Bose Indian Botanic Garden, Botanical Survey of India, Central National Herbarium, Kolkata, India. Identification and authentication was done at the centre itself. A herbarium sheet of these plants have been kept in the Botany research laboratory of Jai Hind College, Mumbai. The reference No.: CNH/Tech.II/2016/27, Sp. No. DG-01 and DG-02.
Preparation of the extract:
The collected plant specimens of bark and root were separated, washed and dried in shade. The dried material was then powdered using a grinder and made into coarse powder which was stored in amber coloured bottles. 1 g of dried powder of the plant material i.e. the bark and root was then separately extracted in 10 ml of 60 % methanol and in 10 ml of 60 % ethanol of High-Performance Liquid Chromatography (HPLC) grade separately for 8 h in a shaker. Extracts were cooled at room temperature, then passed through Whatman’s filter paper No. 1 and centrifuged at 10 000 rpm for 15 min (Remi, India) [22]. The supernatant was then concentrated on a water bath until a semi solid mass was obtained. This semi solid mass was then used for further experimental analysis.
Phytochemical analysis of the extracts:
Both the root and bark extracts were tested for the presence of alkaloids, flavonoids, saponin, tannin, steroid, triterpenoid, coumarin, carbohydrate, glycosides, phenolic compounds so as to obtain its chemical composition profile. Following standard procedures were used[23,24].
Test for alkaloids: Dragendorff's test-In a test tube to 1 ml of the extract, add a few drops of Dragendorff’s reagent (solution of potassium bismuth iodide) and the colour obtained was noticed. Appearance of orange colour indicates the presence of alkaloids.
Wagner’s test-2 ml of Wagner’s reagent (iodine in potassium iodide) was added to the extract, the formation of the reddish brown precipitate indicates the presence of alkaloids.
Mayer’s test-To the extract, 2 ml of Mayer’s reagent (potassium mercuric iodide) was added, a dull white precipitate revealed the presence of alkaloids.
Hager’s test-3 ml of Hager’s reagent (saturated picric acid solution) was added to the extract, formation of the yellow precipitate confirms the presence of alkaloids.
Test for flavonoids: Ferric chloride test-To the extract when few drops of ferric chloride solution was added then the formation of blackish red colour indicates the presence of flavonoids.
Alkaline reagent test-When the extract was treated with Sodium hydroxide (NaOH) solution, it showed an increase in the intensity of yellow colour which would become colourless in addition to a few drops of dilute hydrochloric acid, indicating presence of flavonoids.
Lead acetate solution test-Few drops of lead acetate (10 %) solution when treated with the extract, formation of yellow precipitate indicates the presence of flavonoids.
Shinoda test-To the extract when few turnings of magnesium and 1-2 drops of concentrated Hydrochloric acid (HCl) were added, formation of red/pink colour indicates the presence of flavones.
Potassium hydroxide (KOH) (1 %)-To the test solution when KOH (1 %) were added then formation of yellow colour indicates the presence of flavonoid.
Test for flavanones-To the solution, 10 % NaOH was added; colour changes from yellow to orange shows the presence of flavanones.
To the solution, when concentrated sulphuric acid was added, orange to crimson red colour confirms the presence of flavanones.
Test for saponins: Foam test-The extract was mixed with 2 ml of water and shaken vigorously. Formation of foam persisting for 10 min indicates the presence of saponins.
Test for tannins: Ferric chloride test-To the test solution when ferric chloride was added, formation of a dark blue or greenish black colour indicates the presence of tannins.
Gelatin test-To the extract when 1 % gelatin solution containing sodium chloride was added; formation of white precipitates indicates the presence of tannins.
Test for steroids and triterpenoids: Liebermann Burchard test-The crude extract was mixed with a few drops of acetic anhydride, boiled and cooled. Concentrated sulphuric acid was then added from the sides of the test tube and observed for a brown ring at the junction of two layers. Green colouration of the upper layer and the formation of deep red colour in the lower layer would indicate a positive test for steroids and triterpenoids respectively.
Test for coumarin: 1 ml of 10 % NaOH was added to the 1 ml of extract. Formation of yellow indicates the presence of coumarins.
Test for glycosides: Keller Killiani Test-To the test solution, few drops of glacial acetic acid and ferric chloride solution was mixed. Concentrated sulphuric acid was added and observed for the formation of two layers. Lower reddish brown layer and upper acetic acid layer which turns bluish green would indicate a positive test for glycosides.
Bromine water test-Test solution was dissolved in bromine water and was observed for the formation of yellow precipitate to show a positive result for the presence of glycosides.
Test for carbohydrates: Benedict’s test-To the extract 5 ml of Benedict’s reagent was added and boiled for 2 min and cooled. Formation of a red precipitate showed the presence of carbohydrates.
Test for phenolic compounds: Ferric chloride test- Extracts were treated with 3-4 drops of ferric chloride solution; formation of bluish black colour indicates the presence of phenols.
Test for proteins: Biuret test-Test solution was treated with 10 % NaOH solution and two drops of 0.1 % of copper sulphate solution was added and observed. Formation of violet/pink colour indicates the presence of proteins.
Test for free amino acids: Ninhydrin test-Test solution when boiled with 0.25 ml solution of ninhydrin which results in the formation of purple colour suggesting the presence of free amino acids.
Determination of total alkaloid content by Bromocresol Green (BCG) and phosphate buffer test[25,26]:
Preparation of solutions: BCG solution (1×10-4) was prepared by heating 69.8 mg BCG with 3 ml of 2 N NaOH and 5 ml distilled water until it is completely dissolved, and the solution was diluted to 1000 ml with distilled water. Phosphate buffer solution (pH 4.7) was prepared by adjusting the pH of 2 M sodium phosphate (71.6 g Sodium dihydrogen phosphate (Na2HPO4) in 1 l distilled water) to 4.7 with 0.2 M citric acid (42.02 g citric acid in 1 l distilled water). Atropine standard solution was made by dissolving 10 mg pure atropine (Sigma Chemical, USA) in 10 ml of distilled water.
Preparation of standard curve:
Accurately measure aliquots (0.2, 0.4, 0.6, 0.8, 1, 1.2, 1.4, 1.6 and 1.8 ml) of atropine standard solution and transfer each to different test tubes. Then add 5 ml pH 4.7 phosphate buffer and 5 ml BCG solution and shake the mixture. The complex formed was extracted with 1, 2, 3 and 4 ml of chloroform by vigorous shaking. The extracts were collected in a 10 ml volumetric flask and then diluted to adjust volume with chloroform. The absorbance of the complex in chloroform was measured at 470 nm against blank prepared as above but without atropine.
Extraction:
Concentrated supernatant (1 g in 10 ml of ethanolic (60 %) and methanolic (60 %) solution) was taken and was dissolved with 2 N HCl and filtered. From this, 1 ml of the solution was transferred in a separatory funnel and washed with 10 ml of chloroform (3 times). The pH was adjusted to neutral with 0.1 N NaOH. Then 5 ml of BCG solution and 5 ml of phosphate buffer were added to this solution. The mixture was shaken and the complex formed was extracted with 1, 2, 3 and 4 ml of chloroform by vigorous shaking. The extracts were collected in a 10 ml volumetric flask and then diluted to adjust volume with chloroform. The absorbance of the complex in chloroform was measured at 470 nm.
Total alkaloid content was expressed as mg of atropine equivalents/g of extract.
Separation of alkaloid by HPTLC method[27,28]:
Preparation of plant extract and chemicals used: 1 g powder in 10 ml ethanolic and methanolic (60 %) concentrated supernatant was taken for the analysis. From obtained extract 20 mg of extract was dissolved in 1 ml of HPLC grade ethanol and methanol and sonicated for 5 min in Sonics-Vibra Cell, VCX-130, an instrument was used. The standard compound CPT was procured from Sigma Aldrich and the mobile phases, ethyl acetate, methanol (SD-Fine) and HPLC grade water was used for the present analysis.
Preparation of standard solution and linearity: The standard stock solution of CPT was prepared by dissolving 5 mg standard compound powder in 5 ml of ethyl acetate:chloroform in the ratio 1:1 v/v and sonicated for 5 min. From this stock (1 mg/ml), seven different concentrations (100-700 μg/ml) of each standard were prepared. The linearity of each standard compound was determined by applying standard solutions of different concentrations ranging from 0.5-3.0 μg/spot. All the solvents used in the analysis were of HPLC grade.
Chromatographic conditions: Chromatography was performed on pre-activated (at 1100°) silica gel 60 F254 HPTLC plates (20×10 cm). Both, sample and standard (10 μl each) compounds were applied to the layer as 6.0 mm wide bands, positioned 8.0 mm from the bottom of the plate, using an automated CAMAG Linomat 5, Thin Layer Chromatography (TLC) applicator instrument with nitrogen flow providing the delivery by 100 μl Hamilton syringe.
Detection and quantification of compound: TLC was performed on 20×10 cm HPTLC plates using a sample applicator. The response for CPT was measured for each band at 254 nm and 366 nm wavelengths, using CAMAG TLC scanner and WinCat software. The compounds were investigated according to their Retention factor (Rf) values with the corresponding standards. Calculation of percentage was done considering standard and sample Rf, Area Under the Curve (AUC) and dilution factor. For validation of the method, the calibration curve was obtained by plotting the peak area against the concentration of CPT, the spectrum obtained from the samples was correlated to the standard compound used. The percentage of CPT present in ethanolic and methanolic extract was calculated by comparison of the areas measured for the standard solution. The peak area of CPT was obtained by plotting a graph of peak vs. applied concentrations of studied constituents.
Chromatogram development: The sample loaded on TLC plate was placed in glass-twin trough developing chambers (10 mm ×10 mm, with metal lid) previously saturated with solvent vapor with mobile phase ethyl acetate:methanol:water (100:11:10 v/v/ v/v), for 30 min, at room temperature (24°±1°).
Photo-documentation: The developed plate was dried to evaporate the solvents from the plate by hot air dryer. The plate was kept in the photodocumentation chamber (CAMAG Reprostar-3) and the images were captured at Ultraviolet (UV) 254 nm and 366 nm at daylight mode.
Derivatization: To derivatize, the plate was sprayed with spraying reagent i.e. 20 ml of concentrated sulfuric acid in 180 ml of cold methanol and it was dried at 110° for 15 min on the hot plate. Immediately after drying the plate was photo-documented in UV- 254 nm and UV-366 nm in daylight mode using CAMAG-TLC equipment.
Statistical analysis:
All the experiments were performed in triplicate and the data represented as the mean±standard deviation.
Results and Discussion
The curative properties of medicinal plants are perhaps due to the presence of various secondary metabolites such as alkaloids, flavonoids, terpenoids, glycosides, phenols, saponins, sterols etc. Table 1 and Table 2, shows the result of phytochemical screening of I. coccinea and I. barbata root and bark extracts in ethanolic and methanolic solvents. These plant extracts are rich in phytochemical compounds such as alkaloids, terpenoids, glycosides, phenols, saponins, sterols, tannins, etc., but devoid of flavonoids and coumarin. Thus, making it useful in the detection of the bioactive principles and subsequently it may lead to the drug discovery and development and further these tests facilitate their quantitative and qualitative separation of pharmacologically active chemical compounds.
| S. No | Chemical tests | I. coccinea | I. barbata | ||
|---|---|---|---|---|---|
| Ethanolic extract | Methanolic extract | Ethanolic extract | Methanolic extract | ||
| 1 | Test for alkaloids | ||||
| Dragendorff’s test | +++ | +++ | ++ | +++ | |
| Wagner’s test | +++ | +++ | +++ | ++ | |
| Mayer’s test | - | +++ | ++ | + | |
| Hager’s test | +++ | - | ++ | + | |
| 2 | Test for flavonoids | ||||
| Ferric chloride test | - | - | - | - | |
| Alkaline reagent test | - | - | - | - | |
| Lead acetate solution test | - | - | - | - | |
| Shinoda test | - | - | - | - | |
| KOH (1 %) test | - | - | - | - | |
| Test for flavanones | - | - | - | - | |
| 3 | Test for saponin | ||||
| Foam test | - | +++ | ++ | +++ | |
| 4 | Test for tannins | ||||
| Ferric chloride test | +++ | +++ | ++ | ++ | |
| Gelatin test | ++ | ++ | ++ | ++ | |
| 5 | Test for steroids and triterpenoids | ||||
| Liebermann-Burchard test | ++ | ++ | +++ | ++ | |
| 6 | Test for coumarin | - | - | - | - |
| 7 | Test for glycosides | ||||
| Keller-Killiani test | - | ++ | + | + | |
| Bromine water test | - | +++ | + | ++ | |
| 8 | Test for carbohydrates | ||||
| Benedict’s test | - | +++ | - | + | |
| 9 | Test for phenolic compounds | ||||
| Ferric chloride test | +++ | - | + | - | |
| 10 | Test for proteins | ||||
| Biuret test | + | - | - | - | |
| 11 | Test for free amino acids | ||||
| Ninhydrin test | ++ | - | + | - | |
Note: (+): Less; (++): Moderate; (+++): High positive reactions and (-): Negative reactions
Table 1: Phytochemical Screening of the Bark Extracts of I. coccinea and I. barbata.
| S. No | Chemical tests | I. coccinea | I. barbata | ||
|---|---|---|---|---|---|
| Ethanolic extract | Methanolic extract | Ethanolic extract | Methanolic extract | ||
| 1 | Test for alkaloids | ||||
| Dragendorff’s test | ++ | +++ | +++ | +++ | |
| Wagner’s test | +++ | +++ | ++ | +++ | |
| Mayer’s test | + | +++ | ++ | ++ | |
| Hager’s test | ++ | ++ | ++ | ++ | |
| 2 | Test for flavonoids | ||||
| Ferric chloride test | - | - | - | - | |
| Alkaline reagent test | - | - | - | - | |
| Lead acetate solution test | - | - | - | - | |
| Shinoda test | - | - | - | - | |
| KOH (1 %) test | - | - | - | - | |
| Test for flavanones | - | - | - | - | |
| 3 | Test for saponin | ||||
| Foam test | - | +++ | +++ | +++ | |
| 4 | Test for tannins | ||||
| Ferric chloride test | ++ | ++ | +++ | ++ | |
| Gelatin test | ++ | +++ | ++ | ++ | |
| 5 | Test for steroids and triterpenoids | ||||
| Liebermann-Burchard test | ++ | - | ++ | ++ | |
| 6 | Test for coumarin | - | - | - | - |
| 7 | Test for glycosides | ||||
| Keller-Killiani test | ++ | +++ | + | - | |
| Bromine water test | +++ | +++ | + | - | |
| 8 | Test for carbohydrates | ||||
| Benedict’s test | +++ | +++ | - | - | |
| 9 | Test for phenolic compounds | ||||
| Ferric chloride test | + | - | + | + | |
| 10 | Test for proteins | ||||
| Biuret test | + | - | - | - | |
| 11 | Test for free amino acids | ||||
| Ninhydrin test | + | - | - | - | |
Note: (+): Less; (++): Moderate; (+++): High positive reactions and (-): Negative reactions
Table 2: Phytochemical Screening of the Root Extracts of I. coccinea and I. barbata.
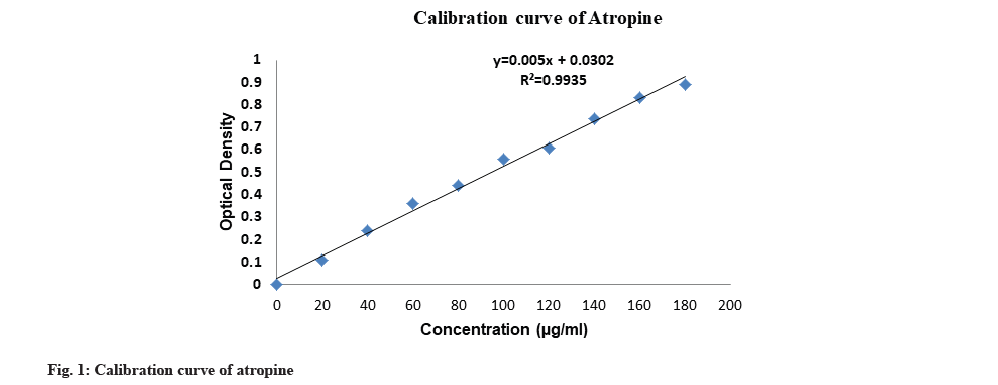
In the present study, the total alkaloid content of the plant extracts were determined by BCG and phosphate buffer test. A calibration curve was plotted for various concentrations of atropine (fig. 1). It was observed that methanol extracts gave better results for I. coccinea than that of ethanol extracts and ethanol extracts gave better results for I. barbata than that of methanol extracts.
The highest amount of alkaloid content was found in the root extract of I. coccinea in methanolic solvent 146 mg/g followed by the bark extraction in methanolic solvent 138 mg/g whereas the least amount was observed in the bark extract of I. barbata in methanolic solvent 64 mg/g (Table 3). I. coccinea gave a higher amount of alkaloid content as compared to I. barbata.
| S. No | Parts used | I. coccinea | I. barbata | ||
|---|---|---|---|---|---|
| Ethanolic extract (mg/g) | Methanolic extract (mg/g) | Ethanolic extract (mg/g) | Methanolic extract (mg/g) | ||
| 1 | Bark | 114 | 138 | 88 | 64 |
| 2 | Root | 124 | 146 | 96 | 80 |
Table 3: Determination of Alkaloid Content of the Total Extracts of I. coccinea and I. barbata.
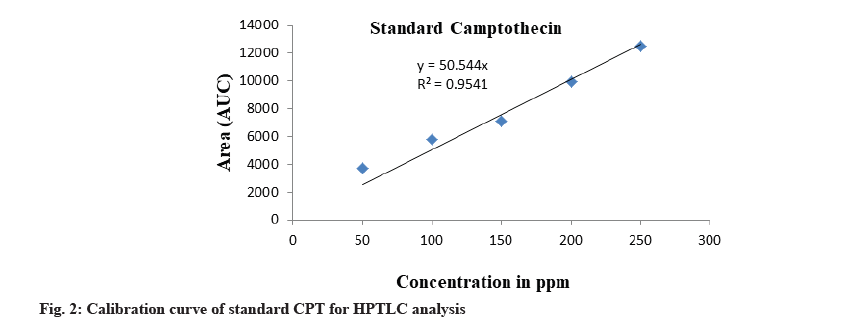
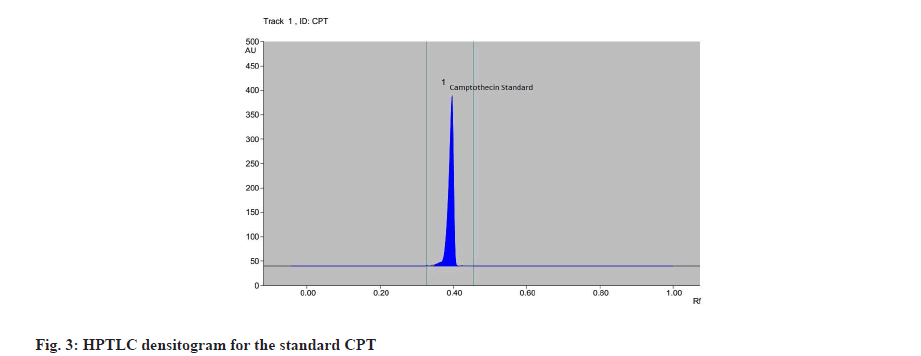
The calibration curve was prepared by plotting the concentration of CPT vs. average area of the peak and its linearity was in the range of 50-250 μg/ml for CPT (fig. 2). The correlation coefficient was found to be 0.954. The calibration curve of CPT was obtained by spotting CPT on HPTLC plate. After derivatization the plate was scanned densitometrically at 366 nm, CPT showed a single peak in HPTLC chromatogram at 0.41 Rf (fig. 3). The experiment was performed in triplicate for reproducibility and accuracy, and was found to be correct. The obtained data was analysed statistically. The results obtained of CPT content in plant extract of bark and root of I. barbata and I. coccinea in ethanolic and methanolic solvents are as discussed below.
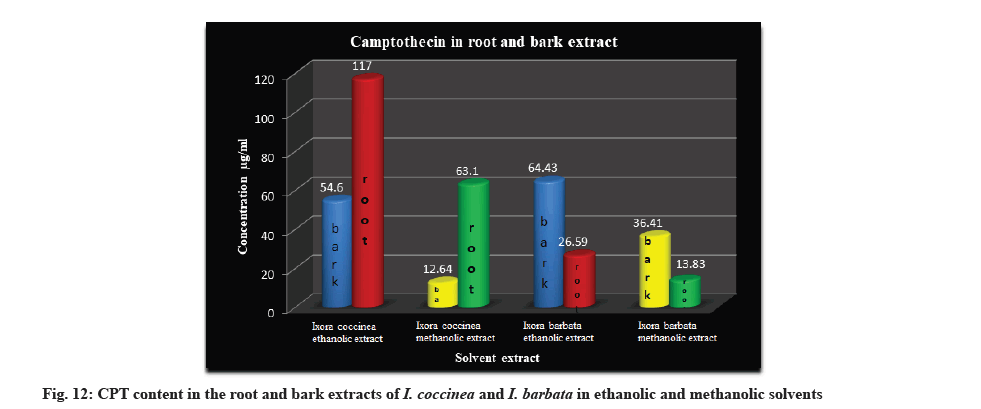
HPTLC analysis in the root and bark extracts of I. barbata and I. coccinea in ethanolic and methanolic solvents gave better indication of occurrence of the CPT constituent in the plant. The highest amount of CPT was recorded in ethanolic extract of root of I. coccinea (117 μg/ml) followed by bark of I. barbata (64.43 μg/ ml) and the lowest were observed in methanolic extract of bark of I. coccinea (12.64 μg/ml) (Table 4).
| S. No | Parts used | I. coccinea | I. barbata | ||
|---|---|---|---|---|---|
| Ethanolic extract (µg/ml) | Methanolic extract (µg/ml) | Ethanolic extract (µg/ml) | Methanolic extract (µg/ml) | ||
| 1 | Bark | 54.60 | 12.64 | 64.43 | 36.41 |
| 2 | Root | 117 | 63.10 | 26.59 | 13.83 |
Table 4: Determination of CPT Content in the Extracts of I. coccinea and I. barbata by HPTLC Analysis (µg/ml).
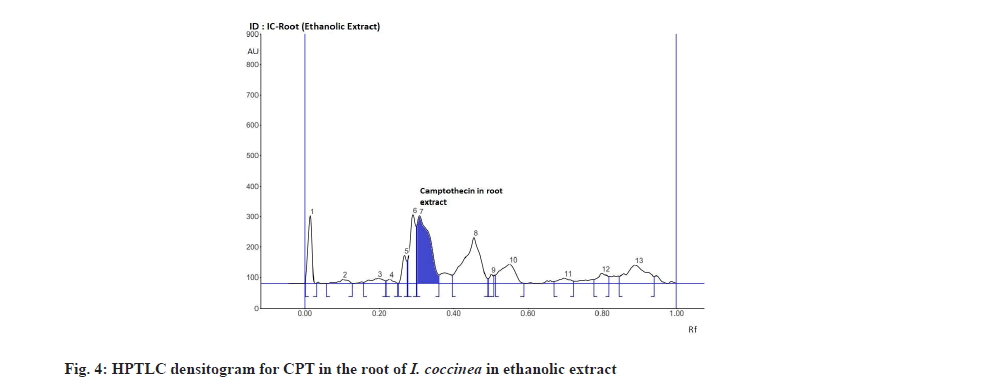
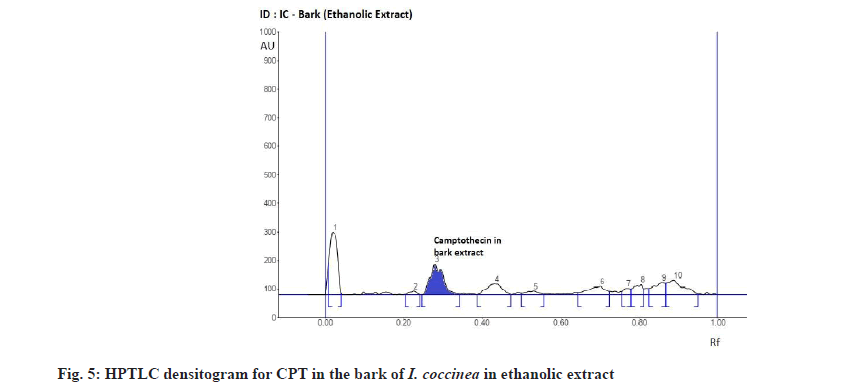
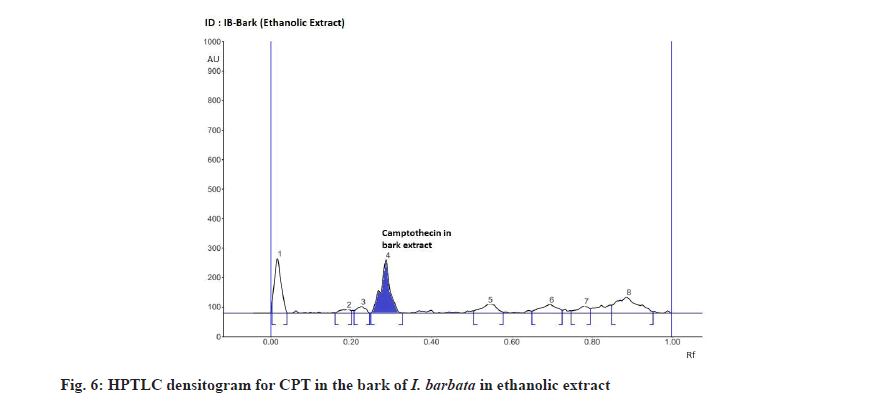
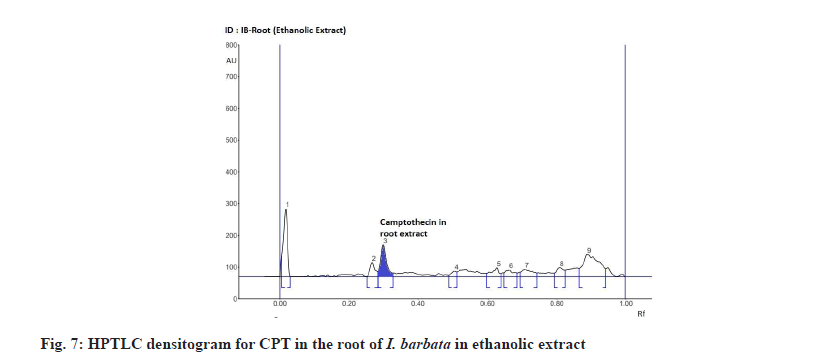
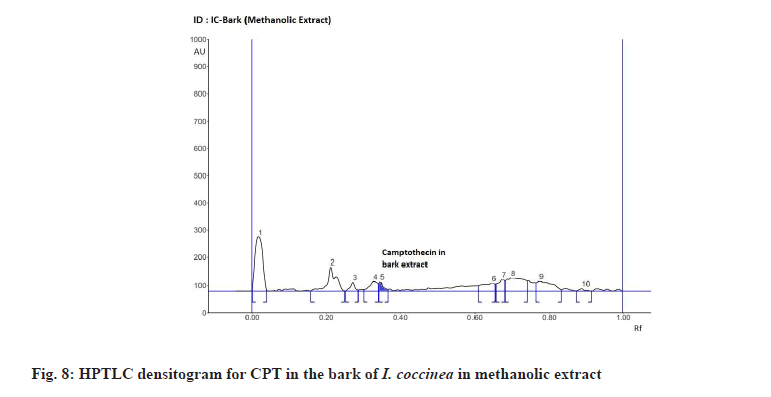
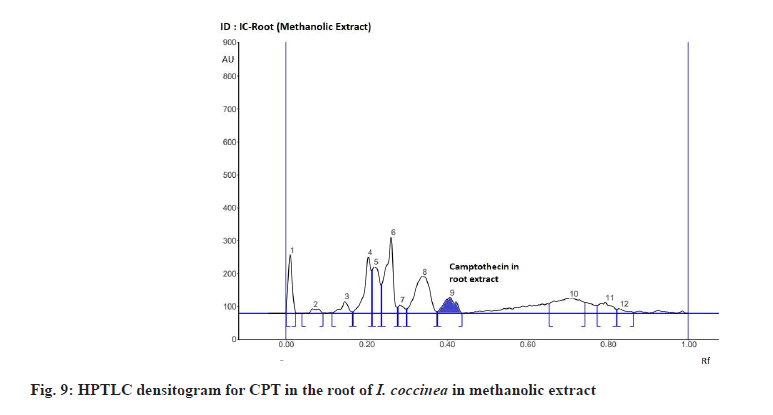
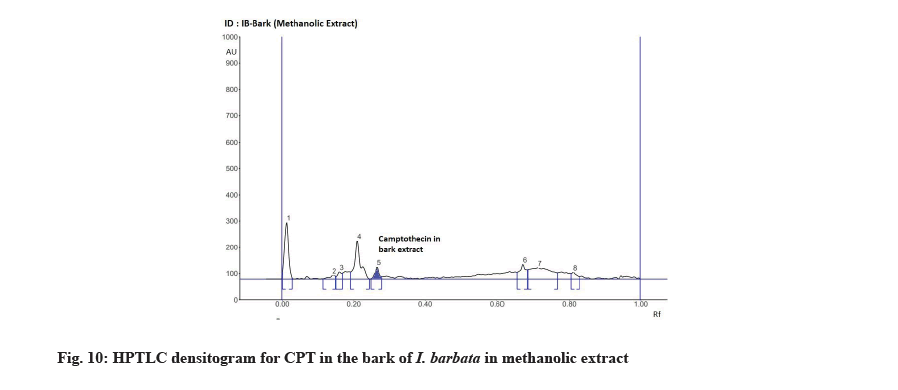
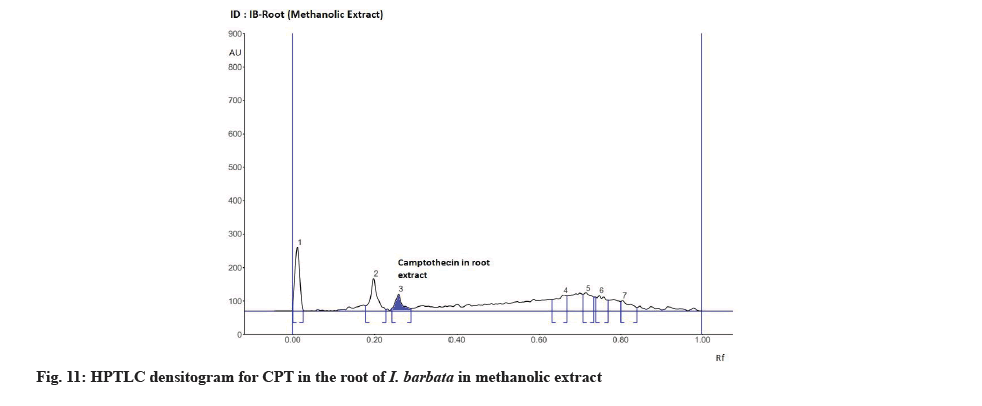
The Rf value and retention area of CPT was found to be 0.41 and area 5821 (fig. 3). The ethanolic extract of the I. coccinea root showed 13 peaks and the 7th peak with Rf value 0.36 and retention area 5913.7 (fig. 4) was homologous to the standard CPT. The ethanolic extract of the I. coccinea bark showed 10 peaks and the 3rd peak with Rf value 0.37 and retention area 2760.2 (fig. 5) was coinciding with the standard CPT. The ethanolic extract of the I. barbata bark showed 8 peaks and the 4th peak with Rf value 0.38 and retention area 3256.8 (fig. 6) was homologous to the standard CPT. The ethanolic extract of the I. barbata root showed 9 peaks and the 3rd peak with Rf value 0.36 and retention area 1344.2 (fig. 7) showed homology to the standard CPT. Whereas the methanolic extract of the I. coccinea bark showed 10 peaks and the 5th peak with Rf value 0.39 and retention area 639.2 (fig. 8) was coinciding with the standard CPT. The methanolic extract of the I. coccinea root showed 12 peaks and the 9th peak with Rf value 0.40 and retention area 3189.8 (fig. 9) was homologous to the standard CPT. The methanolic extract of the I. barbata bark showed 8 peaks and the 5th peak with Rf value 0.36 and retention area 1840.7 (fig. 10) was homologous to the standard CPT. The methanolic extract of the I. barbata root showed 7 peaks and the 3rd peak with Rf value 0.35 and retention area 698.7 (fig. 11) was homologous to the standard CPT. HPTLC densitogram of all plant extract exhibited the presence of total 13 types of phytoconstituents when scanned at 366 nm with Rf values ranging 0.00 to 0.87 (fig. 4-fig. 11). There is marked variation observed in the presence of CPT content in the alcoholic extracts of root and bark of I. coccinea and I. barbata (fig. 12). There is marked variation observed in the presence of CPT content as observed and compared in the alcoholic extracts of root and bark of I. coccinea and I. barbata. Although I. barbata has lower CPT content, it still remains a very important potential candidate for extraction of CPT. The variation, whether can be related to any observed pharmacological effects of these plants, especially when used for particular ailments, needs to be accessed. At times, higher or lower amounts of various phytoconstituents or change in proportions of various phytoconstituents to each other, may also relate to better pharmacological properties. As these drugs are used in traditional medicine as a composite preparation without purifying the active medicinal constituent, the medicinal effect probably lies in the holistic composition i.e. variation in the ratio of various phytoconstituents to each other in different species of the same genera. Various genera of Ixora need to be evaluated to determine the effect, if any, of variability in proportion of phytoconstituents on their respective reported pharmacological actions. The presence and quantities of important medicinal phytoconstituents like CPT need to be evaluated in various species of Ixora. This will help to find many better species as sources, for extraction of important medicinal alkaloids like CPT.
In the present study, phytochemical investigation of root and bark extracts of I. barbata and I. coccinea revealed the presence of bioactive compounds such as alkaloids, terpenoids, saponins, etc. This study also leads us to identify and isolate CPT from the extracts of these plants using HPTLC assay. The alkaloid CPT was found in large quantities in the root extract of I. coccinea in ethanolic solvent followed by in the bark extract of I. barbata in ethanolic solvent whereas the least quantity was observed in the bark extract of I. coccinea in methanolic solvent. This also suggests that the ethanolic solvent gave better yield than that of methanolic solvent. This paper is also the first to quote for the quantification of CPT compound in the extracts of I. barbata. CPT is known to be used as an anti-cancer compound and hence these plant extracts can be further studied as a potential source of anticancer drugs.
Acknowledgements:
The authors are thankful to the Department of Botany, Jai Hind College for the practical lab facilities provided for this research work. Authors are also thankful to the Institute of Science, Mumbai, for analytical (HPTLC) facilities provided for carrying out this research work.
Conflict of interests:
The authors declared no conflict of interest.
References
- Bachheti RK, Pandey DP, Joshi A, Rana V, Rai I. Phytochemical analysis of aerial parts of Ixora parviflora. Int J Chem Tech Res 2011;3(3):1028-32.
- Willis JC. A dictionary of the flowering plants and ferns. 7th ed. London: Cambridge University Press; 1966.
- Huxley A. The new royal horticultural society dictionary of gardening. 2nd ed. London: Macmillan Press; 1992.
- Hooker JD. The Flora of British India. 3rd ed. London: Biodiversity Heritage Library; 1882. p. 137-9.
- Rajaseger GH, Tan HT, Turner IM, Kumar PP. Analysis of genetic diversity among Ixora cultivars (Rubiaceae) using random amplified polymorphic DNA. Ann Bot 1997;80(3):355-61.
- Satyavati GV, Gupta AK, Tandon N. Medicinal plants of India. 2nd ed. New Delhi: Indian Council of Medical Research; 1987. p. 92-5.
- Sastri BN. The Wealth of India-Raw materials. 5th ed. New Delhi: Council of Scientific and Industrial Research (CSIR); 1959. p. 333-54.
- Bombay natural history society. Some beautiful Indian climbers and shrubs. J Bombay Nat Hist Soc; 1941. p. 456-7.
- Latha PG, Panikkar KR. Cytotoxic and antitumour principles from Ixora coccinea flowers. Cancer Lett 1998;130(1):197-202.
- Saravanan P, Boopalan E. Occurrence of camptothecin an anticancer drug from Ixora coccinea Linn. Int J Appl Biol 2011;2(2):30-4.
- Ni X, Wen S, Wang W, Wang X, Xu H, Kai G. Enhancement of camptothecin production in Camptotheca acuminata hairy roots by overexpressing ORCA3 gene. J Appl Pharm Sci 2011;1(8):85-8.
- Kirtikar KR, Basu BD. Indian Medicinal Plants. 2nd Allahabad: Lalit Mohan Basu; 1975. p. 2440-1.
- Nadkarni KM, Nadkarni AK. Indian Materia Medica. Popular Prakashan; 1994;2:293.
- Lorence A, Nessler CL. Camptothecin, over four decades of surprising findings. Phytochemistry 2004;65(20):2735-49.
- Romanelli S, Perego P, Pratesi G, Carenini N, Tortoreto M, Zunino F. In vitro and in vivo interaction between cisplatin and topotecan in ovarian carcinoma systems. Cancer Chemother Pharmacol 1998;41(5):385-90.
- Wani MC, Wall ME. Plant antitumor agents. II. Structure of two new alkaloids from Camptotheca acuminata. J Org Chem 1969;34(5):1364-7.
- Govindachari TR, Viswanathan N. Alkaloids of Mappia foetida. Phytochemistry 1972;11(12):3529-31.
- Malik SS, Laura JS. Distribution of camptothecin through the plant kingdom. Int J Curr Res 2014;6(5):6497-507.
- Lorence A, Medina-Bolivar F, Nessler CL. Camptothecin and 10-hydroxycamptothecin from Camptotheca acuminata hairy roots. Plant Cell Rep 2004;22(6):437-41.
- Vincent RM, Lopez-Meyer M, McKnight TD, Nessler CL. Sustained harvest of camptothecin from the leaves of Camptotheca acuminata. J Nat Prod 1997;60(6):618-9.
- Aiyama R, Nagai H, Nokata K, Shinohara C, Sawada S. A camptothecin derivative from Nothapodytes foetida. Phytochemistry 1988;27(11):3663-4.
- Singh I, Kumaravadivel N, Gnanam R, Vellaikumar S. RP-HPLC analysis for camptothecin content in Nothapodytes nimmoniana, an endangered medicinal plant. J Med Plant Res 2010;4(3):255-9.
- Raamen N. Phytochemical technique. New Indian Publishing Agencies: New Delhi; 2006. p. 19.
- Harborne JB. Phytochemical methods. New Delhi: Springer (India) Pvt. Ltd.; 2005. p. 17.
- Shamsa F, Monsef H, Ghamooshi R, Verdian-rizi M. Spectrophotometric determination of total alkaloids in some Iranian medicinal plants. Thai J Pharm Sci 2008;32:17-20.
- Tambe VD, Bhambar RS. Estimation of total phenol, tannin, alkaloid and flavonoid in Hibiscus tiliaceus Linn. wood extracts. J Pharmacogn Phytochem 2014;2(4):41-7.
- Kharat SN, Ansari N, Mendhulkar VD. HPTLC screening for flavonoids content in leaf extracts of Syzygium cumini (Linn.) and its antimicrobial activity. Res J Pharm Technol 2020;13(6):2720-6.
- Kharat SN, Singh R, Mendhulkar VD. Quantitative analysis for “Diosgenin” content in Elephantopus scaber (Linn.) by HPTLC using successive solvent extraction method. Pharm Lett 2015;7(5):236-44.