- Corresponding Author:
- M. Ali
Department of Pharmacognosy and Phytochemistry, Faculty of Pharmacy, Jamia Hamdard, New Delhi-110 062, India
E-mail: maliphyto@gmail.com
| Date of Submission | 21 September 2010 |
| Date of Revision | 12 July 2011 |
| Date of Acceptance | 22 July 2011 |
| Indian J Pharm Sci, 2011, 73 (4): 447-451 |
Abstract
Phytochemical investigation of the ethanolic extracts of the seeds of Carica papaya L. (Caricaceae) led to the isolation of 2,3,4-trihydroxytoluene (caricaphenyl triol) and glyceryl-1-(2’,3’,4’-trihydroxybenzoyl)-2,3-dioleate (papayaglyceride) as the new phytoconstituents along with the known components glyceryl-1-oleiyl-2,3-dilinoleiate, glyceryl-1-oleiyl-2,3-diarachidate, glyceryl-1-linoleiyl-2,3-distearate, carpaine, glyceryl-1,2-dipalmitate, glyceryl trimyristate, glyceryl tristearate, glyceryl-1,2-dipalmityl-3-myristate, glyceryl-1-oleiyl-2,3-dimyristate, b-sitosterol glucoside, glyceryl-1-oleiyl-3-phosphate, glyceryl-1-oleiyl-2-lauryl-3-phosphate and glyceryl-1,2-distearyl-3- phosphate. The structures of all these compounds have been elucidated by spectral data analysis and chemical reactions. The methanolic extract of the seeds and 2,3,4-trihydroxytoluene (200 μg/ml) showed antifungal activity against Aspergillus flavus, Candida albicans and Penicillium citrinium.
Keywords
Antifungal activity, Carica papya L., seeds, glyceryl-1-(2’’3’4’-trihydroxybenzoyl)-2,3-dioleate, glycerides,2,3,4-trihydroxytoluene
Carica papaya L (family Caricaceae) is a fast growing, short-lived, single-stemmed, small tree, 2-10 m in height with straight, cylindrical, soft, hollow, grey trunk roughened by the presence of large leaf- and inflorescence scars. It is probably originated in southern Mexico and Costa Rica, now cultivated in tropical countries mainly in Australia, Hawai, India, Sri Lanka, The Philippines, South Africa and Nigeria. Its seeds are black, tuberculous and enclosed in a transparent aril [1]. The seeds are considered as carminative, emmenagogue, abortifacient, vermifuge and counter-irritant. A seed extract is used to treat bleeding piles and enlarged liver and spleen. A seed paste with glycerine is applied to cure ringworm and psoriasis [1]. The seeds are beneficial as carminative and thirst quencher [2]. The ripe seeds are taken with rice and useful to treat diarrhoea [3]. The seeds are effective to control diabetes mellitus, hypertension and hypercholesterolemia [4]. A seed decoction is beneficial to cure liver and renal disorders [5]. The seeds contained a fixed oil composed of myristic, palmitic, stearic, arachidic, behenic and unsaturated fatty acids [1,6], phospholipids, carpaine, benzylisothiocyanate, benzyl glucosinolate, glucopaeolin [7], hentriaontane, b-sitosterol[8], caricin (sinigrin) and myosine [1]. The papaya seed extracts showed antifertility effect [9-13], inhibited jejunal contraction [14] and suppressed cauda epididymal sperm motility [15]. The present paper describes isolation and characterization of phytoconstituents and antifertility of papaya seeds collected from Delhi.
Melting points were determined on a PeRfit melting point apparatus (Ambala, India) and are uncorrected. IR spectra were recorded on KBr discs, using a Bio-Rad FT-IR 5000 spectrometer (FTS 135, Hong Kong). UV spectra were measured with a Lambda Bio 20 spectrophotometer (Perkin Elmer, Switzerland) in methanol. 1H and 13C NMR spectra were scanned using Bruker Advance DRX 400 spectrospin and Bruker Advance DRX 400 spectrospin instruments (Germany), respectively, in CDCl3 and TMS as an internal standard. MS spectra were obtained using JEOL-JMS-DX 303 spectrometer. Column chromatography was performed on silica gel (Qualigens, Mumbai, India) 60-120 mesh. TLC was run on silica gel G (Qualigens, Mumbai, India). Spots were visualized by exposure to iodine vapours, UV radiation and by spraying reagents.
C. papaya seeds were procured from the Khari Baoli market of Delhi and identified at the Department of Botany, Jamia Hamdard. New Delhi. A voucher specimen No. PRL/JH/08/35 is deposited in the herbarium of the Department of Pharmacognosy and Phytochemistry, Faculty of Pharmacy, Jamia Hamdard, New Delhi.
The dried seeds (2 kg) was coarsely powdered and then exhaustively extracted with ethanol (95%). The combined extracts were then concentrated on a water bath and dried under reduced pressure to get 75 g (3.75% yield) of dark brown mass. The viscous dark brown mass was dissolved in little quantity of methanol and adsorbed on silica gel (60-120 mesh) for the preparation of slurry. It was dried, powdered and chromatographed over silica gel column packed in petroleum ether. The column was eluted with petroleum ether, chloroform and methanol successively in order of increasing polarity to isolate following compounds:
Elution of column with petroleum ether-CHCl3 (1:3) afforded pale yellow waxy mass of glyceryl-1-oleiyl- 2,3-dilinoleiate (1), recrystallized from CHCl3-MeOH (1:1), 125 mg (0.00625% yield). Rf: 0.80 (CHCl3). Mp 43-45°; +ve ion ESI MS m/z: 852 [M]+ (C55H96O6).
Elution of column with CHCl3 gave colourless waxy mass of glyceryl-1-oleiyl-2,3-diarachidate (2), recrystallized from CHCl3-MeOH (1:1), 210 mg (0.0105% yield). Rf: 0.75 (CHCl3); mp 50-51°; ESI MS m/z: 944 [M]+ (C61H116O6).
Elution of column with CHCl3-MeOH (49:1) furnished pale yellow sticky mass of glyceryl-1-linoleiyl-2,3- distearate (3), recrystallised from CHCl3-MeOH (1:1), 390 mg (0.0195% yield).; Rf: 0.70 (CHCl3: MeOH; 9.5:0.5); mp 53-55°; +ve ESI MS m/z: 886 (C57H106O6).
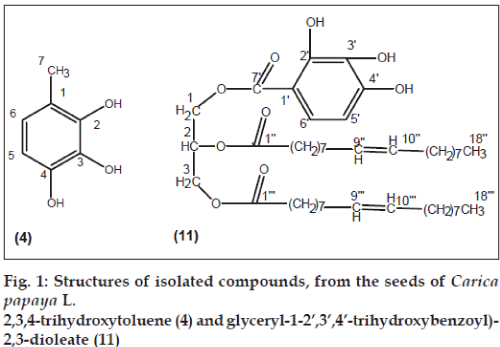
Further elution of column with CHCl3-MeOH (49:1) yielded cream coloured amorphous powder of caricaphenyl triol (4), recrystallized from chloroform, 350 mg (0.0175% yield). Rf: 0.70 (toloune:ethyl acetate:formic acid; 5:4.5:0.5); m.p.: 218-220°; UV lmax (MeOH): 281 nm (log e 6.2); IR nmax (KBr): 3415, 2940, 2824, 1640, 1513, 1210, 950, 792 cm−1; 1H NMR (DMSO-d6): d 10.37 (1H, brs, D2O exchangeable, OH), 7.79 (1H, d, J=8.4 Hz, H-5), 6.82 (1H, d, J=8.4 Hz, H-6), 2.26 (3H, brs, Me-7); 13C NMR (DMSO-d6): d 131.56 (C-1), 161.64 (C-2), 167.24 (C-3), 161.64 (C-4), 121.44 (C-5), 115.16 (C- 6), 29.04 (C-7); +ve ESI MS m/z: 140 [M]+ (C7H8O3).
Elution of column with CHCl3-MeOH (24:1) afforded colourless mass of carpaine (5), recrystallized from methanol, 450 mg (0.0225% yield); Rf: 0.41 (toluene:ethyl acetate:acetic acid; 5:4:1); m.p.: 118- 120°; 13C NMR (CDCl3): d 169.23 (C-15, C-15’), 77.41 (C-3’), 76.99 (C-5), 71.23 (C-2), 63.13 (C-6’), 61.17 (C-2’, C-6), 31.90 (CH2), 29.67 (25×CH2), 25.50 (CH2), 22.67 (CH2), 14.09 (Me-7,Me-7’); +ve ESI MS m/z: 478 [M]+ (C28H50N2O4).
Further elution of column with CHCl3-MeOH (24:1) furnished buff white amorphous powder of glyceryl- 1,2-dipalmitate (6), recrystallized from methanol, 450 mg (0.0225% yield); Rf: 0.55 (toluene:ethyl acetate:acetic acid; 5:4:1); m.p.: 70-72°; 13C NMR (CDCl3): d 173.41 (C-1’), 171.99 (C-1”), 71.23 (C-2), 63.13 (C-1), 61.17 (C-3), 31.90 (CH2), 29.67 (25×CH2), 25.50 (CH2), 22.67 (CH2), 14.09 (Me-16, Me-16″); +ve ESI MS m/z (rel. int.): 568 [M]+ C35H68O5 (3.5), 328 [M-CO(CH2)14CH3]+ (16.4).
Elution of column with CHCl3-MeOH (93:7) afforded brown amorphous powder of glyceryl trimyristate (7), recrystallized from methanol, 310 mg (0.0155% yield); Rf: 0.70 (CHCl3:EtOAc:MeOH; 8:1:1); m.p.: 61-62°; +ve ESI MS m/z: 722 [M]+ (C45H86O6).
Elution of column with CHCl3-MeOH (17:3) gave colourless amorphous powder of glyceryl tristearate (8), recrystallized from chloroform– methanol (1:1); 250 mg (0.0125% yield); Rf: 0.85 (CHCl3:EtOAc:MeOH; 8:2:2); m.p.: 66-67°; +ve ESI MS m/z: 890 [M]+ (C57H110O6).
Elution of column with CHCl3-MeOH (4:1) yielded colourless amorphous powder of glyceryl- 1,2-dipalmityl-3-myristate (9), recrystallized from chloroform-methanol (1:1); 460 mg (0.023% yield); Rf: 0.75 (CHCl3:MeOH:NH3; 2:1:0.5); m.p.: 68-69°; +ve ESI MS m/z: 778 [M]+ (C49H94O6).
Elution of column with CHCl3-MeOH (3:1) produced colourless sticky mass of glyceryl-1-oleiyl-2,3- dimyristate (10), recrystallised from chloroform– methanol (1:1); 250 mg (0.0125% yield); Rf: 0.70 (chloroform–methanol, 3:1); m.p.: 74-75°; +ive ESI MS m/z: 776 [M]+ (C49H94O6).
Elution of column with CHCl3-MeOH (4:1) afforded light brown amorphous powder of glyceryl-1-(2’,3’,4’- trihydroxybenzoyl)-2,3-dioleate (11), recrystallized from methanol, 320 mg (0.016% yield); Rf: 0.65 (CHCl3:EtOAc:MeOH; 6:2:2); m.p.: 86-88°; UV lmax (MeOH): 266 nm (log e 5.7); IR nmax (KBr): 3410, 3210, 2959, 2855, 1730, 1725, 1640, 1542, 1135, 1025, 773 cm−1; 1H NMR (DMSO-d6): d 7.17 (1H, d, J=8.4 Hz, H-5′), 6.95 (1H, d, J=8.4 Hz, H-6′) 5.32 (2H, m, H-9″, H-10″), 5.18 (2H, m, H-9″′, H-10″′), 4.81 (1H, m, H-2), 4.65 (2H, brs, H2-1), 3.86 (2H, brs, H2-3), 2.50 (4H, brs, H2-2″, H2-2″′), 2.27 (4H, brs, H2-8″, H2-11″), 1.98 (4H, brs, H2-8″′, H2-11″′), 1.53 (4H, brs, 2xCH2), 1.23 (36H, brs, 18×CH2), 0.85 (3H, t, J=6.1 Hz, Me-18″), 0.83 (3H, t, J=6.1 Hz, Me-18″′); +ve ESI MS m/z: 772 [M]+ (C46H76O9).
Elution of the column with chloroform-methanol (19:1) furnished colourless amorphous powder of b-sitosterol glucoside (12), recrystallized from methanol, 260 mg (0.013% yield); m.p.: 270-272°; Rf: 0.53 (benzene:chloroform:methanol; 5:4:1). IR nmax (KBr): 3450, 2917, 2849, 2383, 1636, 1460, 1074, 795 cm−1; +ve ESI MS m/z (rel. int.): 576 [M]+ (C35H60O6).
Elution of column with CHCl3-MeOH (97:3) afforded brown waxy mass of glyceryl-1-oleiyl-3-phosphate (13), recrystallised from methanol, 670 mg (0.0335% yield); Rf: 0.60 (toluene:ethyl acetate:formic acid; 5:4:1); m.p. 301-303°; UV lmax (MeOH): 249 nm (log e 4.1); IR nmax (KBr): 3424, 2924, 2853, 1725, 1640, 1442, 1380, 1261, 1075, 795, 773 cm−1; 13C NMR (CDCl3): d 132.11 (C-9′), 129.23 (C-10′), 128.26 (C-1′), 66.77 (C-2), 61.55 (C-1), 60.01 (C-3), 43.48 (CH2), 42.83 (CH2), 40.82 (CH2), 39.86 (CH2), 39.59 (CH2), 37.25 (CH2), 33.68 (CH2), 31.35 (CH2), 29.12 (CH2), 26.63 (CH2), 25.01 (2×CH2), 24.39 (CH2), 22.11 (CH2), 13.63 (me-18′); +ve ESI MS m/z: 436 [M]+ (C21H41O7P).
Elution of column with CHCl3-MeOH (19:1) yielded light brown amorphous powder of glyceryl-1-oleiyl-2- lauryl-3-phosphate (14), recrystallised from methanol, 425 mg (0.021% yield); Rf: 0.60 (toluene:ethyl acetate:acetic acid; 5:3:2); m.p.: 226-228°; +ve ESI MS m/z: 618 [M]+ (C33H63O8P).
Elution of column with MeOH gave brown sticky mass of glyceryl-1,2-distearyl-3-phosphate (15), recrystallized from methanol, 440 mg (0.022% yield); Rf: 0.60 (cyclohexane:diethyl ether:MeOH; 8:1:1).,m.p.: 220°; +ve ESI MS m/z: 704 [M]+ (C39H77O8P).
Fungal cultures of Apergillus flavus, Candida albicans and Penicillium citrinum were obtained from the culture collection centre, IGIB (CSIR), New Delhi. The fungal strains were maintained on malt extract agar media containing malt extract (2%), agar (1.5%) and distilled water (100 ml) at pH 5.5 at 28°.
For agar well diffusion bioassay, a fungal suspension in sterile normal saline was prepared. An aliquot of 1.5 ml was uniformly seeded on the malt extract media (15 ml, 4 cm thickness) in Petri dishes, left aside for 15 min and excess was then drained and discarded properly. Wells of 6 mm in diameter and about 2 cm apart were punctured into culture media using sterile cork borer (6 mm). Concentration of 1, 5, 10 and 20 mg/ml of each of the plant methanol extract and 25, 50, 100 and 200 mg/ml test compounds was prepared in dimethyl sulphoxide (DMSO). The plates were then incubated at 30° for 48 h. After incubation, bioactivity was determined by measuring the diameter of inhibition zones (DIZ) in mm. All samples were tested in triplicate. Controls included solvent without plant extracts/tested compounds, although no antifungal activity was noted in the solvent used for the test. Fluconazole (32 mg/ml) was taken as a positive control.
Compound 4 was obtained as a cream coloured amorphous mass from CHCl3-MeOH (49:1) eluents (fig. 1). It responded positively to ferric chloride indicating phenolic nature of the molecule. Its IR exhibited characteristic absorption bands for hydroxyl groups (3415 cm−1) and aromatic nucleus (1640, 1513 and 950 cm−1). The ESI MS spectrum of 4 showed a molecular ion peak at m/z 140 corresponding to molecular formula of a phenol, C7H8O3. The 1H NMR of 4 exhibited a deshielded D2O exchangeable oneproton signal at δ 10.37 for the hydroxyl proton. Two one-proton ortho-coupled doublets at δ 7.79 (J=8.4 Hz) and 6.82 (J=8.4 Hz) were assigned to H-5 and H-6 aromatic protons, respectively. A three-proton broad signal at δ 2.26 was ascribed to C-7 methyl protons attached to aromatic ring. Further evidence in support of the structure of 4 was provided by its 13C NMR spectral data which showed the existence of seven carbon atoms in the molecule. The carbon signals at δ 167.24 (C-3) and 161.64 (C-2, C-4) were due to hydroxyl aromatic carbons. The remaining aromatic carbons resonated in the range δ 131.57- 115.16. The C-7 methyl carbon appeared at δ 29.04. The HMBC spectrum of 4 exhibited correlations of C-1 with H-6 and Me-7; and C-4 with H-5. On the basis of foregoing discussion the structure of 4 has been elucidated as 2,3,4-trihydroxytoluene. This is a new aromatic compound.
Compound 11, named papayaglyceride, was obtained as a light brown amorphous powder from chloroformmethanol (4:1) eluents (fig. 1). It responded positively to FeCl3 test for phenolic moiety and with bromine water for unsaturation. Its IR spectrum showed characteristic absorption bands for hydroxyl groups (3410, 3210 cm−1), ester groups (1730, 1725 cm−1), unsaturation (1640 cm−1) and benzene ring (1542.1025 cm−1). The ESI MS of 11 displayed a molecular ion peak at m/z 772 corresponding to a molecular formula of benzoyldiglyceride C46H76O9. The 1H NMR of 11 exhibited two, one-proton, doublets at d 7.17 (J=8.4 Hz) and 6.95 (J=8.4 HZ) assignable to ortho-coupled aromatic protons H-5′ and H-6′, respectively. Two, two-proton, multiplets at d 5.32 (H-9′′ and H-10′′) and 5.18 (H-9′′′ and H-10′′′′) were due to vinyl carbons whereas the methylene protons adjacent to the vinylic linkage at H2-8′′ and H2-11′′ and H2-8′′′ and H2-11′′′ appeared as broad signals at d 2.27 and 1.98, four-proton each, respectively. Two, two-proton, broad signals at d 4.65 and 3.86 were ascribed to oxygenated methylene H2-1 and H2-3 protons whereas the oxygenated H-2 methine proton appeared as a one-proton multiplet at d 4.81, respectively. The remaining methylene protons appeared as broad singlets at d 1.53 (2×CH2) and 1.23 (18×CH2). Two primary methyl proton triplets, threeproton each, appeared at d 0.85 (J=6.1 Hz) and 0.83 (J=6.1 Hz) which were due to Me-18′′ and Me-18′′′, respectively. Alkaline hydrolysis of 11 yielded oleic acid, TLC comparable. On the basis of the above discussion, the structure of 11 has been elucidated as glyceryl-1-(2’,3’,4’-trihydroxybenzoyl)-2,3-dioleate, which is a new glyceride obtained.
The methanolic extract of the seeds of C. papaya was found to be ineffective at 1 mg/ml. However, the methanolic extract at 5, 10 and 20 μg/ml was found effective against A. flavus, C. albicans and P. citrinium. 2,3,4-Trihydroxytoluene was effective at 50 and 100 μg/ml against A. flavus and C. albicans and at 200 μg/ ml against all the antifungal strains (Table 1).
| Material | Concentration | Mean zone of inhibition | ||
|---|---|---|---|---|
| Aspergillus flavus |
Candida albicans |
Penicillium citrinium |
||
| Methanol | 1 mg/ml | Nil | Nil | Nil |
| extract | 5 mg/ml | 14 | 12 | 09 |
| 10 mg/ml | 18 | 12 | 10 | |
| 20 mg/ml | 19 | 13 | 14 | |
| 2,3,4-Trihydroxy- | 50 mg/ml | 10 | 12 | – |
| toluene | 100 mg/ml | 10 | 16 | – |
| 200 mg/ml | 12 | 16 | 10 | |
| Fluconazole | 32 mg/ml | 32 | 19 | 18 |
Table 1: Antifungal activity of methanolic extract of the seeds of carica papaya and 2,3,4-trihydroxytoluene
Acknowledgements
The Authors are thankful to the Head, SAIF, Central Drug Research Institute, Lucknow for recording mass spectra of the compounds and Prof. M. P. Sharma, Department of Botany, Jamia Hamdard for authenticating the plant material.
References
- Anonymous, The Wealth of India, Raw Materials, Vol. 3. New Delhi: Publication and Information Directorate, CSIR; 1992. p. 276-9.
- Mhaskar KS, Blatter E, Caius JF, editors. Kirtikar and Basu’s Illustrated Indian Medicinal Plants. Vol. 5. Delhi: Sri Satguru Publications; 2000. p. 1526-9.
- Parrotta JA, editor. Healing Plants of Peninsular India, Wallingford, UK: CABI Publishing; 2001. p. 202-3.
- Gill LS. editor. Carica papaya L. In: Ethnomedicinal uses of plants in Nigeria. Benin City: Uniben Press; 1992. p. 57-8.
- Adeneye AA, Olagunju JA, Banjo AAF, Abdul SF, Sanusi OA, Sanni OO, et al. The aqueous seed extract of Carica papaya Linn. Prevents carbon Tetrachloride induced hepatotoxicity in rats. Int J Appl Res Nat Prod 2009;2:19-32.
- Puangsri T, Abdulkarim SM, Ghazali HM. Properties of Caricapapaya L. (Papaya) seed oil following extractions using solvent andaqueous enzymatic methods. J Food Lipids 2005;12:62-76.
- Rossetto MR, Oliveira do Nascimento JR, Purgatto E, Fabi JP, Lajolo FM, Cordenunsi BR. Benzyl glucosinolate, benzylisothiocyanate and myrosinase activity in papaya Fruit during development and ripening. J Agric Food Chem 2008;56:9592-9.
- Rastogi RP, Mehrotra BN. editors. Compendium of Indian Medicinal Plants. New Delhi: Central Drug Research Institute and Publication and Information Directorate; 1993; p. 135.
- Lohiya NK, Goyal RB, Jayaprakash D, Ansari AS, Sharma S. Antifertility effects of aqueous extract of Carica papaya seeds in male rats. Planta Med 1994;60:400-4.
- Chinoy NJ, D’Souza JM, Padman P. Effects of crude extract of Caricapapaya seeds in male albino mice. ReprodToxicol 1994;8:75-9.
- Adebiyi A, Ganesan AP, Prasad RN. Tocolytic and toxic activity of papaya seed extract on isolated rat uterus. Life Sci 2003;74:581-92.
- Udoh P, Essien I, Udoh F. Effect of Carica papaya (paw paw) seeds extract on the morphology of pituitary-gonadal axis of male Wistar rats.
- Phytother Res 2005;19:1065-8. Manivannam B, Mittal, Goyal S, Ansari AS, Lohiya NK. Sperm characteristis and ultra structure of testes of rats after long-term treatment with the methanol subfraction of Carica papaya seeds. Asian J Androl 2009;11:583-99.
- Adebiyi A, Adaikan PG. Modulation of jejunal contractions by extract of Carica papaya L. seeds. Phytother Res 2005;19:628-32.
- Verma RJ, Nambiar D, Chinoy NJ. Toxicological effects of Caricapapaya seeds extract on spermatozoa of mice. J ApplToxicol 2006;26:533-5.