- *Corresponding Author:
- Sahar J. Melebary
Department of Biology, College of Science, University of Jeddah, Jeddah 21493, Saudi Arabia
E-mail: sjmelebary@uj.edu.sa
| This article was originally published in a special issue, “Advanced Targeted Therapies in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(1) Spl Issue “1-14” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The male reproductive system is vulnerable to oxidative stress, which is linked to several disorders. Silibinin, a natural polyphenolic flavonoid, possesses antioxidant, anti-inflammation and anticancer properties. Considering silibinin's wide range of biological and metabolic roles (silibinin) plays, the current study was conducted to evaluate the biochemical, histological, immunohistochemical and morphometric changes that may develop in the rat testis exposed to oxidative stress and the possible protective role of silibinin. Thirty adult rats were used in the current study. Rats were equally divided into three groups. G1 negative control group, G2 was given thioacetamide (200 mg/kg body weight, G3 received silibinin at (100 mg/kg body weight) for 4 w concurrently with thioacetamide. Serum levels of sexual hormones and testicular oxidative stress markers were measured. Testicular sections were stained with haematoxylin and eosin, and immunohistochemical stains for Vimentin and p53 subjected to morphometric and statistical analysis. Testicular histological architecture and biochemical and morphometric markers all declined in the thioacetamide group. When rats were given silibinin, however, the normal biochemical and morphometric parameters were restored and the histological structure of their testicles was preserved. This investigation showed that silibinin might increase testosterone and gonadotropin levels and enhance several testicular indices (luteinizing hormone and follicle-stimulating hormone). The protective effects of silibinin on reproductive toxicity and oxidative stress were evidenced by the antioxidant activities and improvement of the histological structures of both Leydig and Sertoli cells. As a result, it can be used as a supplement to improve male reproductive function, particularly spermatogenesis.
Keywords
Silibinin, antioxidant, oxidative stress, Sertoli and Leydig cells, histological, testes, rats
Free radical-induced oxidative stress has been linked to several reproductive health issues, notably those affecting testicular function. 30 % to 80 % of infertile patients have significant levels of oxidative stress due to Reactive Oxygen Species (ROS)[1]. Environmental contaminants, chemotherapeutic agents and other medications, tobacco, poisons, radiation and diseases, including many types of cancer, all share a common trait called oxidative stress, which can have a devastating effect on fertility[2,3]. Potentially harming free radical attacks can impede spermatogenesis and lead to vascular blockage and severe damage to reproductive system cells[4]. Suppose the testicular biological system cannot detoxify or repair the detrimental effects of free radicals. In that case, cells and tissue are severely harmed, making it imperative to strike a balance between the production and metabolism of free radicals for proper testicular cell function[5,6]. Because they neutralize free radicals and inhibit their production in testicular cells, antioxidants help protect against this type of injury.
Since a natural antioxidant can inactivate damaging ROS, much interest has been in measuring their antioxidant activity in recent years[7-9]. Furthermore, natural antioxidants are considered healthy, helpful and safe[10-12].
One of the structural isomers of the flavonoid silymarin, Silibinin is isolated from milk thistle and is the most physiologically active form (Silybum marianum L. Gaert., Asteraceae)[13]. It has been traditionally used as a folk medicine for hepatic ailments[14]. Silibinin has metal-chelating and antioxidant properties[15]. Silibinin has gained popularity in the last decade as a potential herbal treatment for many illnesses. It has therapeutic advantages against liver problems, different malignancies and gynecological ailments due to its anti-inflammatory, antioxidant and anticancer qualities[16-18]. Silibinin’s antioxidant and anti-inflammatory capabilities change cell cycle regulators and trigger apoptosis in cancer cells[19]. It has been shown that Silibinin has a hypoglycemic effect and increases insulin sensitivity[20]. Silibinin strongly protects the testes because of the sequelae of antioxidative processes and modulation of the expression of effector proapoptotic caspase 3 activity[21,22]. Silibinin is also effective against arsenic[23] and lead[24] toxicity.
Considering Silibinin's wide range of biological and metabolic roles (SBN) plays, the current study was conducted to evaluate the biochemical, histological, immunohistochemical and morphometric changes that may develop in the rat testis exposed to oxidative stress and the possible protective role of SBN.
Materials and Methods
Drugs and chemicals:
Thioacetamide (TAA) and SBN were obtained from Sigma ChemicalCo., Missouri, United States of America (USA). Enzyme-Linked Immunosorbent Assay (ELISA) kits for (Cat.: MBS738685, MBS724319, MBS036924, MBS701908, MBS702057, MBS2018978 and MBS2021901; respectively) the determination of oxidative stress biomarkers (Malondialdehyde (MDA), Reduced Glutathione (GSH), Superoxide Dismutase (SOD) and Catalase (CAT)) and sexual hormones (Testosterone Hormone (TH), Luteinizing Hormone (LH) and Follicle-Stimulating Hormone (FSH))levels were obtained from MyBio Source, San Diego, California, US. A polyclonal rabbit anti-goat antibody to Vimentin, a marker for Sertoli cells, from Santa Cruz Biotechnology in Dallas, Texas, USA. P53, a marker for apoptosis Leydig cells, is a rabbit polyclonal antibody (Cat: ab131442, Abcam, Cambridge, UK).
Experimental animals:
A total of 30 adult male Wistar albino rats weighing between 180 and 250 g were purchased from the King Fahd animal house in Jeddah, Saudi Arabia. The animals were housed in plastic cages (10 animals for each) at a temperature of 25° and 50 %-70 % humidity, with 12 h light/dark cycles, and were fed a freely standard rodent diet and water. The experimental procedures were performed following the standard guidelines for the care and use of experimental animals by the committee for control and supervision of animal experiments and the national institutional animal care.
Experimental design:
The rats used in the experiment were divided into three groups of ten and randomly placed in cages.
Negative control group (G1): For 4 w, rats were given 1 ml/kg Body Weight (BW) of normal saline solution (0.9 % Sodium chloride (NaCl)) intraperitoneal (i.p.) three times a week at 24 h intervals, in addition to 3 ml/kg BW of normal saline solution orally once a day.
Positive control group (G2): Rats were injected i.p. with TAA at the dose of (200 mg/kg BW) 3 times weekly at 24 h intervals for 4 w to induce testicular toxicity[25].
TAA+SBN group (G3): Rats received silibinin (100 mg/kg BW, orally by gavage, daily) for 4 w[26] concurrently with i.p. injection of TAA in the same dose as G2.
Testicular weight measurements:
After 4 w of experimentation, the animals final body weights were recorded and they fasted for 12 h before being sacrificed. At the sampling time, the right and left testes were dissected from the epididymis and the relative testis weight was estimated as (testis weight/body weight)×100 weighed[27].
Sample collection:
Phenobarbital at 60 mg/kg doses is used to anesthetize rats by injection through the IP route[28]. Blood was collected from the retro-orbital sinus in which 3 cm of blood into a plain tube was taken and centrifuged at 3000 rpm at 4° for 5 min to get the serum for biochemical analysis of sexual hormones. The abdomen was opened and the testis and epididymis were carefully dissected. The right testes were prepared for histological and immunohistochemical analysis. The left testes were prepared for biochemical analysis of oxidative stress markers.
Biochemical analysis:
Assay of LH, FSH and testosterone concentrations: The separated sera were used for the estimation of serum levels of TH, LH and FSH.Rat ELISA kits identified serum levels according to the instructions of the manufacturer.
Determination of the testicular oxidative stress status and antioxidant enzymes: The left testis was homogenized from each rat in ice-cold (10 % w/v) Phosphate-Buffered Saline (PBS) (50 mM Dipotassium phosphate (K2HPO4), pH 7.4). The supernatant was separated by centrifugation at 1000 g for 20 min at 4°. Tissue MDA, GSH, SOD and CAT levels were determined by spectrophotometer using ELISA assay kits according to the manufacturer’s instructions[29-32].
Histological and immunohistochemical study: Right testes were processed for light microscopic studies. After 24 h at room temperature in Bouin's solution, the right testes were fixed. After that, ascending grades of alcohol (70 %, 95 % and 100 %) were used to dehydrate the specimens, cleared in xylene then embedded into paraffin wax. Paraffin blocks were cut at 5 µm thickness using a Leica rotator microtome (Germany). For evaluation of testicular changes with exposure to the following stains; Hematoxylin and Eosin (H & E) stain[33], Masson trichrome stain demonstrates collagen fibers[34]. Immunohistochemical staining using the avidin-biotin-peroxidase complex technique[35] for the detection of Sertoli cells with an anti-Vimentin antibody and Leydig cells with p53, a marker for apoptosis.
Deparaffinized sections (6 µm) were mounted on positively charged slides for immunohistochemical staining to detect Vimentin and p53 reaction in testicular tissue using anti-Vimentin and anti-p53 antibodies. They were soaked in PBS and pre-treated with 3 % hydrogen peroxide in distilled water to inactivate endogenous peroxidase activity. Primary antibodies were applied and the samples were incubated in a humidified environment for 60 min. Then the slides were stained with 3,3-diaminobenzidine kit (purchased from Boster Bio-Technology Co., Ltd) as the chromogenic and counterstained with Mayer’s haematoxylin slide dehydrated in 95 % ethanol, cleared in xylene. Coverslips were mounted using two drops of dibutylphthalate polystyrene xylene mounting medium. The positively stained nuclei were brown. On the other hand, one of the testicular sections was used as a negative control bypassing the step of applying the primary antibody.
Johnsen’s mean testicular biopsy score count H and E 40x: Johnsen's mean testicular biopsy score (Table 1) was used to evaluate testicular histological damage and spermatogenesis with light microscopy[36,37]. Each testis's 30 tubules were given a grade. Histological evaluation of testicular seminiferous tubules was performed by assigning a score between 1 and 10 to each tubule based on the presence or absence of several germ cell types such as spermatozoa, spermatids, spermatocytes, spermatogonia, germ cells and Sertoli cells. The Johnsen test reflects the health of spermatogenesis on a scale from 0 to 100, with higher scores indicating better health and lower scores indicating a more severe malfunction. With a score of 1, inactive tubules are not even evaluated for epithelial development. Alternatively, a score of 10 indicates that the tubules are functioning at their peak activity level and that the epithelium has fully matured.
| Score | Description |
|---|---|
| 10 | Full maturation of the sperm cells results in many spermatozoa. The thickness of the germinal epithelium is uniformly structured, creating a patent lumen |
| 9 | The germinal epithelium is disordered and there is significant sloughing or obliteration of the lumen despite numerous spermatozoa |
| 8 | There were few spermatozoa in the section |
| 7 | There are no spermatozoa but plenty of spermatids |
| 6 | Neither spermatozoa nor even many spermatids may be found |
| 5 | No spermatozoa or spermatids are present; however, there are many spermatocytes |
| 4 | The sperm count is low, with few spermatocytes but no spermatids or spermatozoa |
| 3 | The only type of germ cell found is spermatogenic |
| 2 | Sertoli cells are present, but no germ cells can be seen |
| 1 | There are no cells in the tubule |
Table 1: Cross-Sectional Histological Analysis of the Seminiferous Tubules utilizing the Johnsen Scoring System.
Morphometric analysis:
Image analysis was performed with the image analyzer Olympus Image J, NIH, 1.41b, America Inc, Melville, New York, USA, on tissue samples from all rats in each group (n=10). Ten non-overlapping randomly chosen fields per section were quantified for the following morphometric measurements were determined; the height of seminiferous epithelium extending from the basement membrane of the seminiferous tubule towards the lumen. The mean area percentage (%) collagen fibers content in Masson trichrome stained sections (20x). The mean number of Vimentin-immunopositive Sertoli cells (20x). The mean number of p53-immunopositive Leydig cells (20x).
Statistical analysis:
A one-way Analysis of Variance (ANOVA) was used to compare the means and Standard Deviations (SDs) of the quantitative data. The post hoc Tukey test was employed to determine which pairwise comparisons between groups explained any significant ANOVA results. Probability value p<0.05 indicated significant differences. Using Pearson's correlation coefficient, researchers analyzed the relationship between the average number of Leydig cells and blood testosterone level and the number of Sertoli cells. Calculations were made on Statistical Package of Social Science (SPSS) software version 26 (IBM Corp., Armonk, New York, USA). Data was represented graphically using GraphPad® Prism 9 (2022), a statistical package.
Results and Discussion
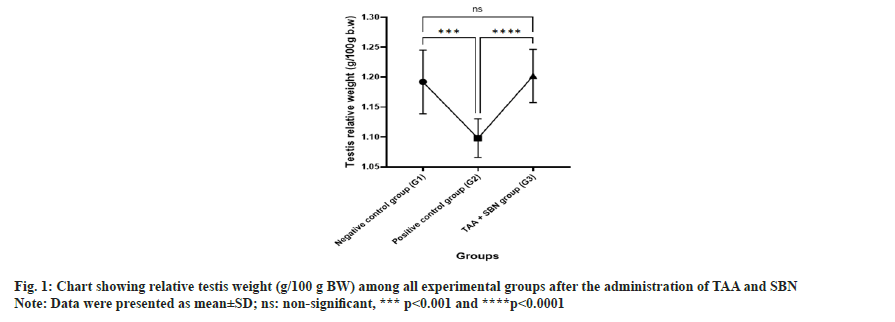
Table 2 and fig. 1 show the effect of TAA and SBN on relative testis weight in negative control and experimental rats (positive control group and SBN treated group). A significant (p<0.0001) decrease in relative testis weights was noted in TAA-treated rats compared to the negative control. Significant (p<0.0001) changes were observed in relative testis weights between negative control and SBN-treated rats. However, all these changes induced by TAA intoxication were significantly (p<0.0001) improved on oral treatment with SBN.
| Groups | Negative control group (G1) | Positive control group (G2) | TAA+SBN group (G3) | p value |
|---|---|---|---|---|
| n | 10 | 10 | 10 | |
| Testis relative weight (g/100 g BW) | 1.19±0.05 | 1.098±0.03a*** | 1.20±0.04ansb**** | 0.0002, 0.9434<0.0001 |
Note: Data were presented as mean±SD, (n): Number of rats/group, (a): Compared to negative control group (G1); (b): Compared to positive control group (G2) and (ns): non-significant, ***p<0.001 and ****p<0.0001.
Table 2: Relative testis weight of all experimental groups.
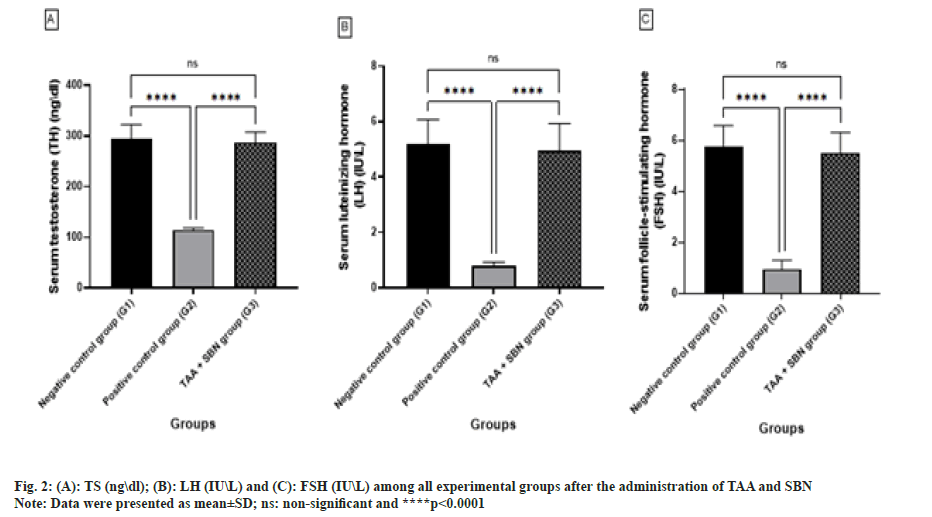
After 4 w of the experiment, there was a highly significant decrease (p<0.0001, p<0.0001 and p<0.0001; respectively) in TAA (G2) compared to the negative control group (G1) regarding serum TH, LH and FSH levels(Table 3 and fig. 2). However, all these changes induced by TAA intoxication were significantly (p<0.0001, p<0.0001 and p<0.0001, respectively) increased after oral treatment with SBN. G3 (TAA+SBN) treated rats showed a non-significant difference compared to the negative control group.
| Groups | Negative control group (G1) | Positive control group (G2) | TAA+SBN group (G3) | p value |
|---|---|---|---|---|
| n | 10 | 10 | 10 | |
| TH (ng\dl) | 294.4±28.10 | 114.9±3.957a**** | 287.6±20.29ansb**** | <0.0001, 0.83<0.0001 |
| LH (IU\L) | 5.18±0.88 | 0.78±0.15a**** | 4.960±0.96ansb**** | <0.0001, 0.89<0.0001 |
| FSH (IU\L) | 5.76±0.82 | 0.95±0.34a**** | 5.52±0.79ansb**** | <0.0001, 0.82<0.0001 |
Note: Data were presented as mean±SD, (n): Number of rats/group, (a): Compared to negative control group (G1); (b): Compared to positive control group (G2) and (ns): non-significant, ****p<0.0001.
Table 3: Comparison between all experimental groups according to the levels of TH (ng\dl), LH (IU/L) and FSH (IU/L).
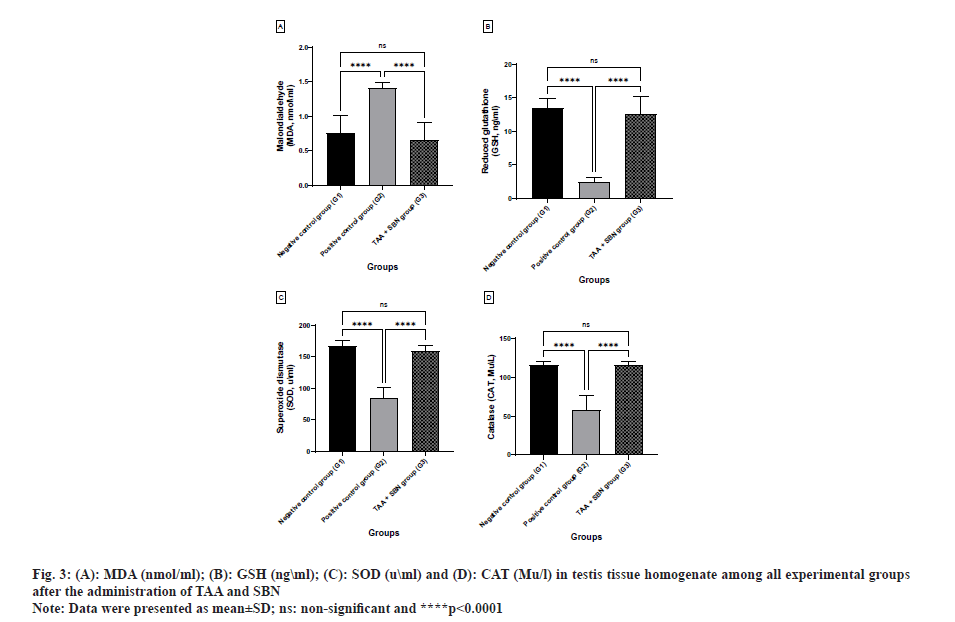
After 4 w of the experiment, there was a highly significant difference (p<0.0001, p<0.0001 and p<0.0001; respectively) in TAA (G2) compared to the negative control group (G1) regarding testicular MDA, GSH, SOD and CAT levels. However, all these changes induced by TAA intoxication were significantly (p<0.0001, p<0.0001 and p<0.0001, respectively) improved after oral treatment with SBN. Otherwise, G3 (TAA+SBN) treated rats showed a non-significant difference compared to the negative control group (Table 4 and fig. 3).
| Groups | Negative control group (G1) | Positive control group (G2) | TAA+SBN group (G3) | p value |
|---|---|---|---|---|
| n | 10 | 10 | 10 | |
| MDA (nmol\ml) | 0.75±0.25 | 1.41±0.08a**** | 0.66±0.24ansb**** | <0.0001, 0.70<0.0001 |
| GSH (ng\ml) | 13.52±1.34 | 2.48±0.69a**** | 12.60±2.55ansb**** | <0.0001, 0.5631 <0.0001 |
| SOD (u\ml) | 167.40±8.30 | 84.50±16.97a**** | 159.0±9.47ansb**** | <0.0001, 0.99 <0.0001 |
| CAT (Mu\l) | 115.70±4.523 | 58.70±18.07a**** | 116.20±3.67ansb**** | <0.0001, 0.99 <0.0001 |
Note: Data were presented as mean±SD, (n): Number of rats/group, (a): Compared to negative control group (G1); (b): Compared to positive control group (G2) and (ns): non-significant, ***p<0.001 and ****p<0.0001.
Table 4: Comparison between all experimental groups according to the levels of oxidative stress enzyme MDA (nmol\ml) and the antioxidant enzymes such as reduced GSH (ng\ml), SOD (u\ml) and CAT (Mu\l) in testis tissue homogenate.
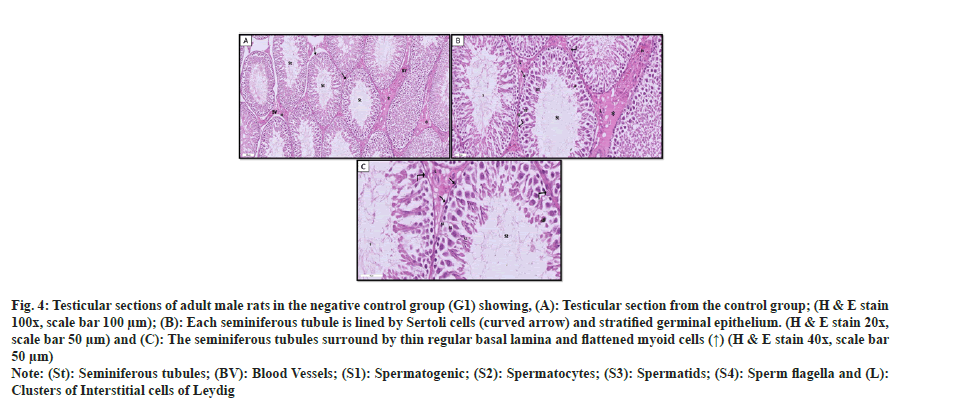
Seminiferous tubules bordered by spermatogenic cells and Sertoli cells were seen near one another in the testicles of the G1 negative control group. Spermatogenic cells included spermatogonia, primary spermatocytes, spermatids and spermatozoa. Spermatogonia appeared as small rounded cells with rounded nuclei resting on the regular basement membrane. Primary spermatocytes were the largest spherical cells that had large rounded nuclei with partially condensed chromosomes. Spermatids appeared as small round cells with pale rounded nuclei and prominent nucleoli. Spermatozoa with deeply stained heads were also observed near the lumen of the tubules. Flattened, smooth muscle-like myoid cells were found external to the basal lamina of the seminiferous tubules. Sertoli cells appeared as tall cells lodged between spermatogenic cells with cytoplasmic extension. The interstitial tissue between the tubules showed Leydig's blood vessels and interstitial cells that appeared as large polygonal cells with vesicular nuclei and acidophilic cytoplasm containing small lipid droplets as shown in fig. 4.
Fig. 4: Testicular sections of adult male rats in the negative control group (G1) showing, (A): Testicular section from the control group; (H & E stain 100x, scale bar 100 µm); (B): Each seminiferous tubule is lined by Sertoli cells (curved arrow) and stratified germinal epithelium. (H & E stain 20x, scale bar 50 µm) and (C): The seminiferous tubules surround by thin regular basal lamina and flattened myoid cells (↑) (H & E stain 40x, scale bar 50 µm).
Note: (St): Seminiferous tubules; (BV): Blood Vessels; (S1): Spermatogenic; (S2): Spermatocytes; (S3): Spermatids; (S4): Sperm flagella and (L): Clusters of Interstitial cells of Leydig.
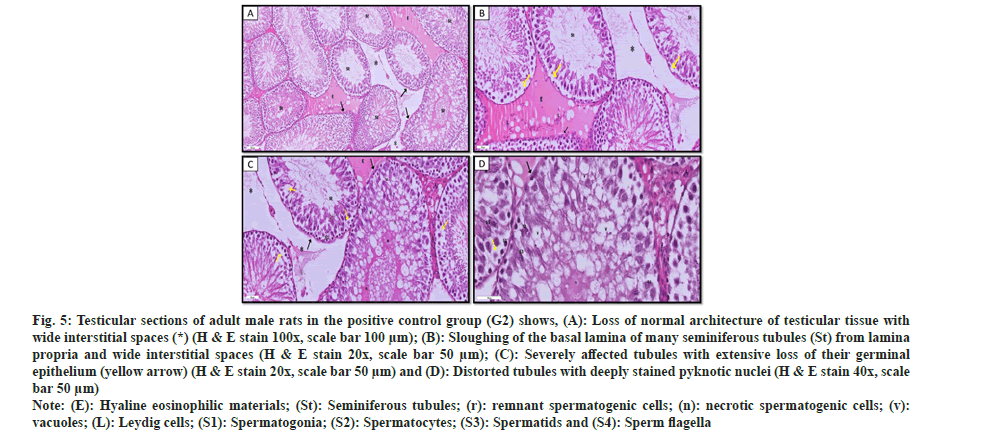
In the positive control group (G2), examination of testicular sections demonstrated distorted histological architecture with widening interstitial spaces. Some of the seminiferous tubules had uneven basement membranes and the spermatogenic cells had been detached, leaving empty areas. Most spermatogenic cells showed apoptotic alterations, including pyknotic or irregular darkly pigmented nuclei and highly acidophilic cytoplasm. Other spermatogenic cells showed cytoplasmic vacuolation. In addition, some seminiferous tubules showed sloughed homogenous acidophilic necrotic material inside the lumen. Interstitial tissue contained most Leydig cells that appeared with darkly stained nuclei as shown in fig. 5.
Fig. 5: Testicular sections of adult male rats in the positive control group (G2) shows, (A): Loss of normal architecture of testicular tissue with wide interstitial spaces (*) (H & E stain 100x, scale bar 100 µm); (B): Sloughing of the basal lamina of many seminiferous tubules (St) from lamina propria and wide interstitial spaces (H & E stain 20x, scale bar 50 µm); (C): Severely affected tubules with extensive loss of their germinal epithelium (yellow arrow) (H & E stain 20x, scale bar 50 µm) and (D): Distorted tubules with deeply stained pyknotic nuclei (H & E stain 40x, scale bar 50 µm).
Note: (E): Hyaline eosinophilic materials; (St): Seminiferous tubules; (r): remnant spermatogenic cells; (n): necrotic spermatogenic cells; (v): vacuoles; (L): Leydig cells; (S1): Spermatogonia; (S2): Spermatocytes; (S3): Spermatids and (S4): Sperm flagella.
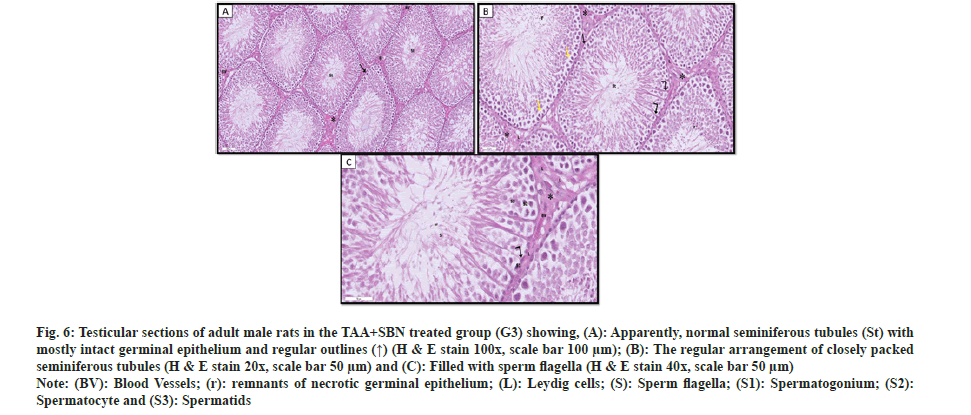
Regarding TAA+SBN treated group (G3), testicular sections showed apparently normal-packed seminiferous tubules lined by several layers of spermatogenic cells and Sertoli cells resting on regular basement membranes surrounded by myoid cells with flattened nuclei. The spermatogenic epithelium still had a few gaps here and there. The interstitial gaps contained blood veins and polygonal Leydig cells with pale nuclei and acidophilic vacuolated cytoplasm. Yet, only a small percentage of cells displayed heavily stained nucleias shown in fig. 6.
Fig. 6: Testicular sections of adult male rats in the TAA+SBN treated group (G3) showing, (A): Apparently, normal seminiferous tubules (St) with mostly intact germinal epithelium and regular outlines (↑) (H & E stain 100x, scale bar 100 µm); (B): The regular arrangement of closely packed seminiferous tubules (H & E stain 20x, scale bar 50 µm) and (C): Filled with sperm flagella (H & E stain 40x, scale bar 50 µm).
Note: (BV): Blood Vessels; (r): remnants of necrotic germinal epithelium; (L): Leydig cells; (S): Sperm flagella; (S1): Spermatogonium; (S2): Spermatocyte and (S3): Spermatids.
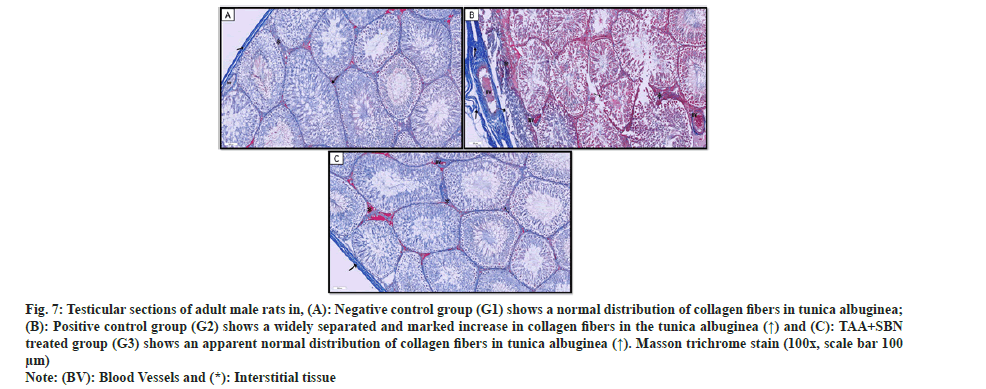
Sections from the negative control group (G1) revealed the normal distribution of bluish-stained collagen fibers in the testicular capsules (tunica albuginea), basal laminae of the seminiferous tubules and interstitial tissue in-between (fig. 7a). However, TAA-treated rats (G2) showed widely separated collagen fibers (by edema) in the tunica albuginea and disrupted collagen fibers in the basal laminae of seminiferous tubules and interstitial tissue. Moreover, there was a marked increase in the collagen fibers in the testicular capsules, basal laminae of seminiferous tubules and interstitium (fig. 7b). TAA+SBN-treated rats showed an apparent normal distribution of collagen fibers compared to the control group as shown in fig. 7c.
Fig. 7: Testicular sections of adult male rats in, (A): Negative control group (G1) shows a normal distribution of collagen fibers in tunica albuginea; (B): Positive control group (G2) shows a widely separated and marked increase in collagen fibers in the tunica albuginea (↑) and (C): TAA+SBN treated group (G3) shows an apparent normal distribution of collagen fibers in tunica albuginea (↑). Masson trichrome stain (100x, scale bar 100 µm).
Note: (BV): Blood Vessels and (*): Interstitial tissue.
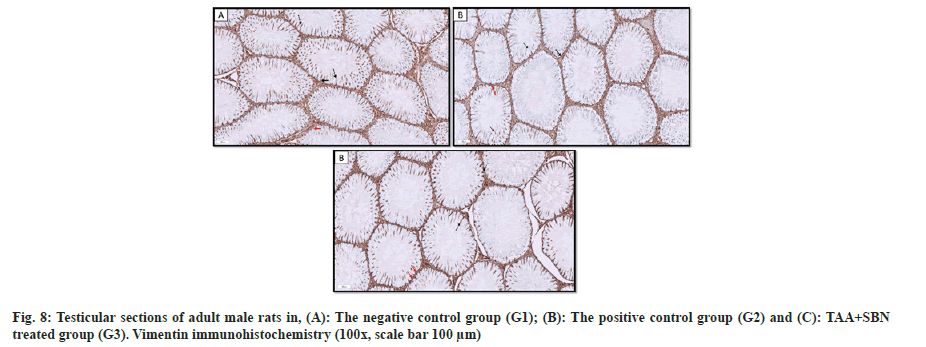
In the negative control group, Vimentin filaments positive reaction within Sertoli cells was concentrated around the nucleus (perinuclear) in the basal part of the cell, then radiating apically toward the apical part of the cell (fig. 8a). In the positive control group (TAA), the number of Sertoli cells presenting positive Vimentin reaction was reduced with fragmented cytoplasmic extensions and marked irregularity of the basement membrane of the seminiferous tubule (fig. 8b and fig. 8c). These findings were improved in SBN-treated rats (G3), as many Sertoli cells showed normally distributed Vimentin filaments perinuclear and apical with cytoplasmic extensions. In addition, a regular basement membrane of the seminiferous tubule was detected (fig. 8d).
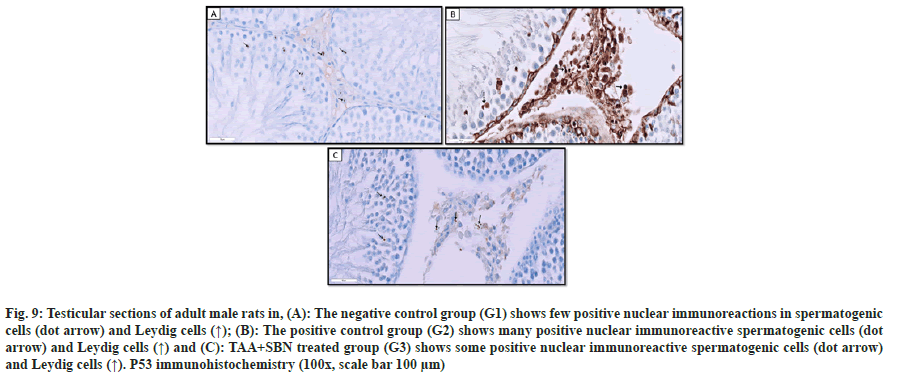
Sections of the negative control group (G1) and TAA+SBN treated rats (G3) stained with anti-p53 showed positive brown nuclear immunoreaction in a few spermatogenic cells and Leydig cells. However, those of TAA-treated rats (G2) showed many immunoreactive spermatogenic and Leydig cells compared to G1. Regarding TAA+SBN (G3) treated rats, some positive immunoreactive spermatogenic and Leydig cells were observed as shown in fig. 9.
Fig. 9: Testicular sections of adult male rats in, (A): The negative control group (G1) shows few positive nuclear immunoreactions in spermatogenic cells (dot arrow) and Leydig cells (↑); (B): The positive control group (G2) shows many positive nuclear immunoreactive spermatogenic cells (dot arrow) and Leydig cells (↑) and (C): TAA+SBN treated group (G3) shows some positive nuclear immunoreactive spermatogenic cells (dot arrow) and Leydig cells (↑). P53 immunohistochemistry (100x, scale bar 100 µm).
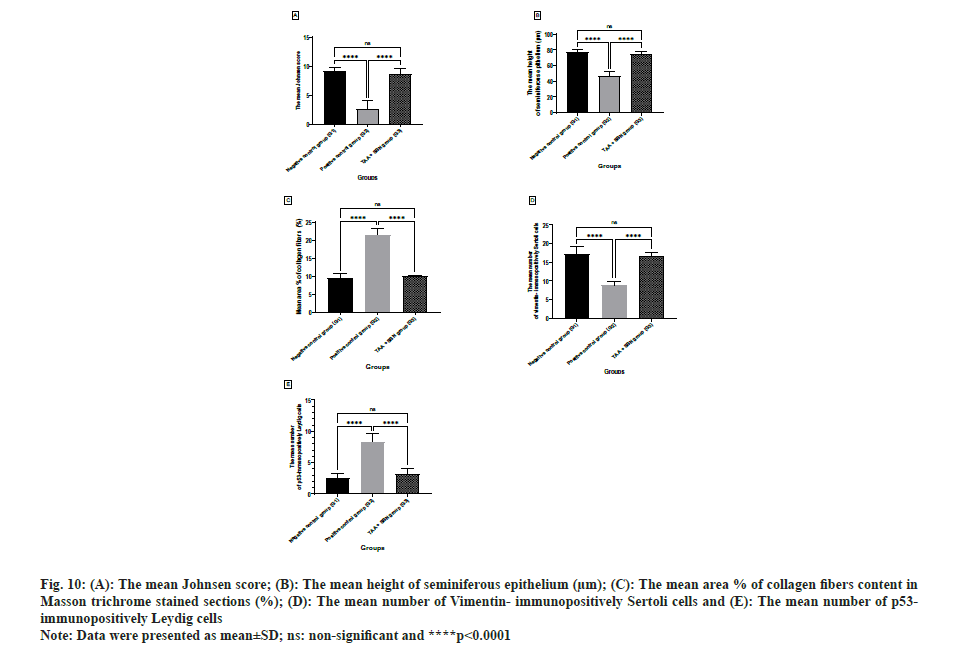
The mean Johnsen score, the mean height of seminiferous epithelium, the mean number of Vimentin-immunopositive Sertoli cells and the mean number of p53-immunopositive Leydig cells values in the positive control group (TAA, G2) showed a statistically significant (p<0.0001, p<0.0001, p<0.0001 and p<0.0001; respectively) decrease as compared with the negative control group (G1) and SBN-treated group (G3). While the mean area percent (%) of collagen fibers content showed a statistically significant (p<0.0001) increase as compared with the negative control group (G1) and SBN-treated group (G3). The non-significant difference in all morphometric parameters was recorded in SBN-treated rats vs. the negative control group as shown in Table 5 and fig. 10.
| Groups | Negative control group (G1) | Positive control group (G2) | TAA+LUT group (G3) | p value |
|---|---|---|---|---|
| n | 10 | 10 | 10 | |
| The mean Johnsen score | 9.200±0.6325 | 2.600±1.430a**** | 8.600±1.075ansb**** | <0.0001, 0.5457 <0.0001 |
| The mean height of seminiferous epithelium (µM) | 77.95±2.306 | 47.08±4.929a**** | 74.59±3.620ansb**** | <0.0001, 0.1595 <0.0001 |
| The mean area % of collagen fibers content in Masson trichrome stained sections (%) | 9.526 ±1.208 | 21.46±1.768a**** | 10.00±0.3034ansb**** | <0.0001, 0.7867 <0.0001 |
| The mean number of Vimentin-immunopositively Sertoli cells | 17.10±2.132 | 8.900±0.9944a**** | 16.66±0.9915ansb**** | <0.0001,0.8810 <0.0001 |
| The mean number of p53-immunopositively Leydig cells | 2.600±0.6992 | 8.300±1.337a**** | 3.100±0.9944ansb**** | <0.0001, 0.6473 <0.0001 |
Note: Data were presented as mean±SD, (n): Number of rats/group, (a): Compared to negative control group (G1); (b): Compared to positive control group (G2) and (ns): non-significant, ***p<0.001 and ****p<0.0001.
Table 5: Morphometric Analysis in all experimental groups according to the mean diameter of seminiferous tubules (MM), mean thickness of germinal epithelium (µM), mean area % of collagen fibers content in masson trichrome stained sections (%), and mean area % of vimentin-positive cells (%).
Fig. 10: (A): The mean Johnsen score; (B): The mean height of seminiferous epithelium (µm); (C): The mean area % of collagen fibers content in Masson trichrome stained sections (%); (D): The mean number of Vimentin-immunopositively Sertoli cells and (E): The mean number of p53-immunopositively Leydig cells.
Note: Data were presented as mean±SD; ns: non-significant and ****p<0.0001.
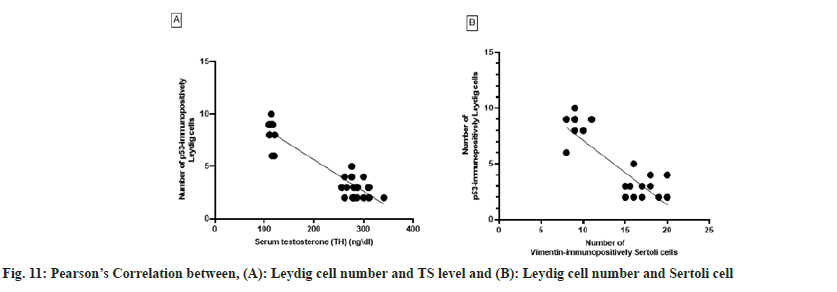
Leydig cell number was negatively correlated with Sertoli cell number (r=-0.8415; p<0.0001) when data from rats in the negative control, positive control and SBN-treated groups were pooled. Therefore, the number of Sertoli cells generated in the experiment might predict the number of Leydig cells in the rat testis. When the number of Leydig cells was artificially decreased in experimental rats, a significant negative association was found between the serum TH level and the number of Leydig cells (r=-0.9283, p<0.0001) as shown in fig. 11.
It is widely believed that oxidative stress contributes significantly to sperm failure. Due to a high concentration of Polyunsaturated Fatty Acids (PUFA) in their membranes, spermatocytes are particularly susceptible to the damaging effects of ROS[38]. Researchers from all over the world have concluded that an excessive amount of ROS in men's sperm is directly responsible for their inability to conceive. Because of a discrepancy between its synthesis and breakdown, ROS levels have risen in spermatozoa and seminal plasma. Diverse antioxidant scavengers in the cell are responsible for breaking ROS[39]. Healing ailments using plants has a long history, dating back to prehistoric times. The antioxidant and anti-inflammatory properties of SBN have made it a popular choice for treating hepatic diseases, with promising outcomes[24].
The present study investigated the biochemical, histological, immunohistochemical and morphometric changes that may develop in the rat testis exposed to oxidative stress and the possible protective role of SBN.
Oxidative stress is one of the most important causes of induction of different organ toxicity. In the present study, testicular homogenate content of GSH, SOD and CAT was significantly decreased in the positive control group (TAA-treated rats). At the same time, MDA was significantly increased than other groups, indicating oxidative stress’s role on testicular toxicity, therefore expecting these changes to be alleviated by the antioxidant treatment as in the present work. That supports a recent study by Celik et al.[11]. They reported that TAA exposure induced lipid peroxidation and decreased the efficacy of the antioxidant defense system via a reduction of CAT, SOD, GPx activity and GSH levels. Some studies have suggested that CAT and GSH were decreased with TAA[40]. The MDA is one of the most popular and reliable markers determining Overall Survival (OS) in clinical situations. It is produced by the peroxidation of PUFA. So, an increased MDA level is considered an important indicator of lipid peroxidation and subsequently, loss of membrane structures and functions. Testes, rich in PUFA and having high metabolism rates and cell replication and poor antioxidant defenses, are much more vulnerable to peroxidation injury than other tissues[41]. Increased MDA levels in rat testes following TAA exposure were similarly reported in previous studies[42]. Our results show that SBN protects rat testicular homogenate from the oxidative stress caused by TAA by boosting the animal’s antioxidant defenses. SBN’s cytoprotective impact may arise from several mechanisms, each of which acts on a different cellular level. Since SBN is a powerful antioxidant, ROS-based cell regulation mechanisms may be involved in some of its radical-scavenging activities[22]. Further, decreased height of lining spermatogenic epithelium and level of TH in the positive control group (TAA-treated rats) against the negative control may account for the loss of histological architecture of seminiferous tubules. The same characteristics have been noted before[25]. This explanation was enforced by the ability of MDA to react with multiple biomolecules, such as proteins or Deoxyribonucleic acid (DNA), leading to DNA damage, cell cycle arrest and apoptosis of germ cells. The high susceptibility of spermatozoa to DNA damage was attributed to the loss of their cytoplasm, which contains antioxidant enzymes and consequently, the loss of their capacity for DNA repair after spermiation[42].
Both endocrine and paracrine variables can affect testicular function. When it comes to the male reproductive system, the hypothalamic-pituitary-testicular axis is a major player. The hypothalamus regulates pituitary gonadotropin production by secreting gonadotropin-releasing hormone, which stimulates the production of the glycoprotein gonadotropins LH and FSH. These hormones then enter the bloodstream and travel to the testes, where they act directly on the somatic cells of the testis[43-45]. Leydig cells in the testicular interstitial tissue express LH receptor, whereas Sertoli cells bind FSH. Cholesterol in the Leydig cells is where LH triggers the creation of TH[46,47]. Androgens function in the testis to stimulate the development of both Leydig and Sertoli cells. Androgen has opposing effects on adult Leydig cells and adult Sertoli cells, partially suppressing steroid genesis in the former but boosting the latter’s actions that are essential for spermatogenesis[48,49]. Serum TH reduction in the present study might be due to a disturbed oxidant antioxidant state[25]. Previously, it was suggested that necrosis of Leydig cells or diminished stimulation of the cells by interstitial cells stimulating hormone is the reason for testosterone reduction[50]. In the present study, treatment with 100 mg/kg SBN significantly restored sperm serum TH and the levels of gonadotrophins to near normal, which denotes the stimulation of spermatogenesis by SBN[51]. After 5 d of treatment with (50, 100, or 150) mg/kg/day SBN, it was observed that the mice's TH levels had increased in a dose-dependent manner relative to the control group[21]. They reported that SBN has an aromatase inhibitor property[21] which could explain the increase in the TH level.
Previous research has demonstrated the importance of Sertoli cells in the testis ability to differentiate, mature and function normally in adulthood. They regulate the maturation of Leydig cells, the activity of peritubular myoid cells and the survival and development of germ cells[52,53]. According to Vimentin immunoreactivity, in the negative control group, there was positive staining of Vimentin in Sertoli cells either located around the nucleus or extended to the apical region of Sertoli cells as its extension, which extended between spermatogonia and primary spermatocytes. In their study, ElGhamrawy et al. observed that Vimentin immunostaining was present in the middle and tips of Sertoli cell walls and neighboring germ cells and spermatozoa[54]. Rats administered TAA showed a decrease in immunoreactivity staining, but rats given SBN, in addition to TAA, showed an improvement. This decrease in vimentin expression in injured testicular tissue occurred because Vimentin filaments disintegrated away from the Sertoli cell membrane. Detached spermatogenic cells may then incur apoptosis due to insufficient nourishment and support from nearby Sertoli cells[55]. This was represented in the present study by histopathological results, which showed close Sertoli cytoplasmic extensions in TAA and SBN groups but, conversely, loss of these relations in TAA-treated rats. Vimentin filaments in Sertoli cells, as determined by the research of ElGhamrawy et al., were crucial in the upkeep of spermatogenesis, with their disruption or restoration linked to the repair and restoration of spermatogenesis[56].
Reduced serum TH levels in TAA-treated rats in this study could be attributed to the impairment of Leydig cells. This assumption was supported in the present work by the apoptotic appearance and positive expression of p53 of many Leydig cells in G2 (TAA-treated rats). That was furtherly established by a significant increase in the number of p53-positive Leydig cells decrease and serum TH. The current study also found that the number of Sertoli and Leydig cells was significantly lower in the TAA group. The research also uncovered an essential connection between Leydig cell count, Sertoli cell count and TH levels. Rebourcet et al. found that the size of the Sertoli cell population is predictive of the final testicular cell composition across all ages, which may explain this phenomenon. A decrease in Sertoli cell quantity or proliferation at any age will result in a corresponding fall in germ cell and Leydig cell populations, potentially negatively impacting fertility and health[49]. Results from this study corroborate those of Oufi et al.[22]. They found a positive association between TH level and the mean number of Leydig cells after administering SBN at a dose of 100 mg/kg intraperitoneally to rats[22].
Our study detected that TAA caused a marked decline in the testicular, body and testis relative weights compared to the negative control group. These results agree with prior studies that documented the same finding confirming the gonadotoxicity of TAA and the testicular atrophy results from the suppression of spermatogenesis[40]. However, TAA+SBN treatment raised testicular, body and testis relative weights compared to TAA-treated rats. Similar findings were reported by Muthumani et al., who reported that all changes induced by arsenic intoxication significantly improved the oral administration of SBN (75 mg/kg)[39]. Our histological studies also coincided with the biochemical and morphometric findings. TAA-induced injurious testicular changes. Similar findings were reported by Karabulut et al.[27]. They described a washed-out appearance of the seminiferous tubules, reflecting the degeneration of germinal epithelium and extensive cell loss after TAA administration.
Apoptosis, caused by oxidative stress, which permeabilizes the mitochondrial outer membrane and releases apoptotic proteins for diffusion into the cytosol, may explain the presence of small dark nuclei in the spermatogonia and the spermatids, which had shrunken nuclei and abnormally distributed mitochondria[57]. Rats given TAA exhibited severely damaged spermatogenic germ epithelium, as measured by the Johnsen score. The germinal epithelium lining the seminiferous tubules was patchy and atrophy was present in some of the tubules in this sample of testis tissue. Vascular congestion, edema and vacuolization have been observed in seminiferous tubules near the capsule[25]. We observed an improvement in testicular histology in the groups treated with TAA+SBN. This improvement was more statistically significant in TAA+SBN than in TAA treated group. This result was clarified by other authors who stated that the antioxidant properties of SBN afforded significant protection and restored the normal architecture of the testis[22].
Examination of Masson’s trichrome-stained sections of the TAA+SBN treated rats showed that SBN administration improved the previous effect in the TAA-treated group as decreased collagen fibers distribution was much less in the capsule and around the blood vessels. Ezhilarasan et al., who looked into the preventive role of SBN against liver fibrosis, came to similar conclusions. At a dose of 100 mg/kg/day, SBN was found to block the messenger RNA expression of collagen types 1 and 3, thereby lowering the enhanced collagen fibers deposition seen in N-nitrosodimethylamine-induced liver necrosis, inflammatory alterations and hepatic fibrosis[17].
In conclusion,this study showed that Silibinin might increase testosterone and gonadotropin levels and enhance several testicular indices (LH and FSH). Furthermore, the protective effects of SBN on reproductive toxicity and oxidative stress were evidenced by the antioxidant activities and improvement of the histological structures of both Leydig and Sertoli cells. As a result, it can be used as a supplement to improve male reproductive function, particularly spermatogenesis.
Conflict of interests:
The authors declared no conflict of interests.
References
- Aebi H. Catalase in vitro. Methods Enzymol 1984;105:121-6.
[Crossref] [Google Scholar] [PubMed]
- Agarwal A, Virk G, Ong C, Du Plessis SS. Effect of oxidative stress on male reproduction. The World J Men Health 2014;32(1):1-7.
[Crossref] [Google Scholar] [PubMed]
- Alam MS, Ohsako S, Tay TW, Tsunekawa N, Kanai Y, Kurohmaru M. Di (n-butyl) phthalate induces vimentin filaments disruption in rat sertoli cells: A possible relation with spermatogenic cell apoptosis. Anat Histol Embryol 2010;39(3):186-93.
[Crossref] [Google Scholar] [PubMed]
- Almeer RS, Abdel Moneim AE. Evaluation of the protective effect of olive leaf extract on cisplatin-induced testicular damage in rats. Oxid Med Cell Longev 2018;2018:8487248.
[Crossref] [Google Scholar] [PubMed]
- Asadi N, Bahmani M, Kheradmand A, Rafieian-Kopaei M. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: A review. J Clin Diagnostic Res 2017;11(5):IE01.
[Crossref] [Google Scholar] [PubMed]
- Atta MS, Almadaly EA, El-Far AH, Saleh RM, Assar DH, Al Jaouni SK, et al. Thymoquinone defeats diabetes-induced testicular damage in rats targeting antioxidant, inflammatory and aromatase expression. Int J Mol Sci 2017;18(5):919.
[Crossref] [Google Scholar] [PubMed]
- Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. 7th ed. Elsevier health sciences; 2008.
- Bello TH, Idris OA. The effect of antioxidant (gallic acid) on the testes of lead acetate induced Wistar rat. Toxicol Environ Health Sci 2018;10(5):261-7.
- Beutler E. Improved method for the determination of blood glutathione. J Lab Clin Med 1963;61:882-8.
[Google Scholar] [PubMed]
- Bijak M. Silybin, a major bioactive component of milk thistle (Silybum marianum L. Gaernt.)-chemistry, bioavailability and metabolism. Molecules 2017;22(11):1942.
[Crossref] [Google Scholar] [PubMed]
- Celik H, Camtosun A, Ciftci O, Cetin A, Aydin M, Gürbüz S. Beneficial effects of nerolidol on thioacetamide-induced damage of the reproductive system in male rats. Biomed Res 2016;27(3):725-30.
- Cho CL, Esteves SC, Agarwal A. Novel insights into the pathophysiology of varicocele and its association with reactive oxygen species and sperm DNA fragmentation. Asian J Androl 2016;18(2):186.
[Crossref] [Google Scholar] [PubMed]
- Corradi PF, Corradi RB, Greene LW. Physiology of the hypothalamic pituitary gonadal axis in the male. Urol Clin 2016;43(2):151-62.
[Crossref] [Google Scholar] [PubMed]
- Dang-Cong T, Nguyen-Thanh T. Testicular histopathology and spermatogenesis in mice with scrotal heat stress. Male Reproduct Anatomy 2021.
- Dupuis ML, Conti F, Maselli A, Pagano MT, Ruggieri A, Anticoli S, et al. The natural agonist of estrogen receptor β silibinin plays an immunosuppressive role representing a potential therapeutic tool in rheumatoid arthritis. Front Immunol 2018;9:1903.
[Crossref] [Google Scholar] [PubMed]
- ElGhamrawy TA, Helmy D, Elall HF. Cadherin and vimentin immunoexpression in the testis of normal and induced infertility models of albino rats. Folia Morphol 2014;73(3):339-46.
[Crossref] [Google Scholar] [PubMed]
- Ezhilarasan D, Karthikeyan S, Vivekanandan P. Ameliorative effect of silibinin against N-nitrosodimethylamine-induced hepatic fibrosis in rats. Environ Toxicol Pharmacol 2012;34(3):1004-13.
[Crossref] [Google Scholar] [PubMed]
- Ezhilarasan D, Karthikeyan S. Silibinin alleviates N-nitrosodimethylamine-induced glutathione dysregulation and hepatotoxicity in rats. Chin J Nat Med 2016;14(1):40-7.
[Google Scholar] [PubMed]
- Farkhondeh T, Samarghandian S. The hepatoprotective effects of curcumin against drugs and toxic agents: An updated review. Toxin Rev 2016;35(3-4):133-40.
- Gulcin İ. Antioxidants and antioxidant methods: An updated overview. Arch Toxicol 2020;94(3):651-715.
[Crossref] [Google Scholar] [PubMed]
- Gurung P, Yetiskul E, Jialal I. Physiology, male reproductive system. StatPearls Publishing; 2021.
- Hauptman D, Perić MH, Marić T, Bojanac AK, Sinčić N, Zimak Z, et al. Leydig cells in patients with non-obstructive azoospermia: Do they really proliferate? Life 2021;11(11):1266.
[Crossref] [Google Scholar] [PubMed]
- Heinrich A, DeFalco T. Essential roles of interstitial cells in testicular development and function. Andrology 2020;8(4):903-14.
[Crossref] [Google Scholar] [PubMed]
- Johnsen SG. Testicular biopsy score count–a method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Hormone Res Paediatr 1970;1(1):2-5.
[Crossref] [Google Scholar] [PubMed]
- Kang JS, Morimura K, Toda C, Wanibuchi H, Wei M, Kojima N, et al. Testicular toxicity of DEHP, but not DEHA, is elevated under conditions of thioacetamide-induced liver damage. Reprod Toxicol 2006;21(3):253-9.
[Crossref] [Google Scholar] [PubMed]
- Kaprara A, Huhtaniemi IT. The hypothalamus-pituitary-gonad axis: Tales of mice and men. Metabolism 2018;86:3-17.
[Crossref] [Google Scholar] [PubMed]
- Karabulut D, Akın AT, Sayan M, Kaymak E, Ozturk E, Yakan B. Effects of melatonin against thioacetamide-induced testicular toxicity in rats. Int J Morphol 2020;38(5):1455-62.
- Kaur C, Kapoor HC. Antioxidants in fruits and vegetables–The millennium’s health. Int J Food Sci Technol 2001;36(7):703-25.
- Kavitha CV, Deep G, Gangar SC, Jain AK, Agarwal C, Agarwal R. Silibinin inhibits prostate cancer cells and RANKL-induced osteoclastogenesis by targeting NFATc1, NF-κB and AP-1 activation in RAW264. 7 cells. Mol Carcinog 2014;53(3):169-80.
[Crossref] [Google Scholar] [PubMed]
- Khan I, Ahmad S. The impact of natural antioxidants on human health. Funct Food Prod Sustainable Health 2020;11-24.
- Kiernan JA. Histological and histochemical methods: Theory and practice. Shock 1999;12(6):111-62.
- Kim TH, Woo JS, Kim YK, Kim KH. Silibinin induces cell death through reactive oxygen species–dependent down regulation of notch-1/ERK/Akt signaling in human breast cancer cells. J Pharmacol Exp Ther 2014;349(2):268-78.
[Crossref] [Google Scholar] [PubMed]
- López-Alarcón C, Denicola A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal Chim Acta 2013;763:1-10.
[Crossref] [Google Scholar] [PubMed]
- Marouf BH, Zalzala MH, Al-Khalifa II, Aziz TA, Hussain SA. Free radical scavenging activity of silibinin in nitrite-induced hemoglobin oxidation and membrane fragility models. Saudi Pharm J 2011;19(3):177-83.
- McCord JM, Fridovich I. Superoxide dismutase: An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 1969;244(22):6049-55.
[Crossref] [Google Scholar] [PubMed]
- Mohammadi M, Ariafar S, Talebi-Ghane E, Afzali S. Comparative efficacy of silibinin and nano-silibinin on lead poisoning in Male Wistar rats. Toxicology 2022;475:153242.
[Crossref] [Google Scholar] [PubMed]
- Mohasseb M, Ebied S, Yehia MA, Hussein N. Testicular oxidative damage and role of combined antioxidant supplementation in experimental diabetic rats. J Physiol Biochem 2011;67(2):185-94.
[Crossref] [Google Scholar] [PubMed]
- Muthumani M, Miltonprabhu S. Silibinin ameliorates oxidative stress-mediated testicular damage by arsenic in rats. Asian Pacific J Tropic Biomed 2012;1:1-7.
- Muthumani M, Prabu SM. Silibinin potentially protects arsenic-induced oxidative hepatic dysfunction in rats. Toxicol Mech Methods 2012;22(4):277-88.
[Crossref] [Google Scholar] [PubMed]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95(2):351-8.
[Crossref] [Google Scholar] [PubMed]
- Oufi HG, Al-Shawi NN, Hussain SA. What are the effects of silibinin on testicular tissue of mice? J Appl Pharm Sci 2012;2(11):009-13.
- Oufi HG, Al-Shawi NN. The effects of different doses of silibinin in combination with methotrexate on testicular tissue of mice. Eur J Pharmacol 2014;730:36-40.
[Crossref] [Google Scholar] [PubMed]
- Pourghasem M, Nasiri E, Shafi H. Early renal histological changes in alloxan-induced diabetic rats. Int J Mol Cell Med 2014;3(1):11-5.
[Google Scholar] [PubMed]
- Rebourcet D, Darbey A, Monteiro A, Soffientini U, Tsai YT, Handel I, et al. Sertoli cell number defines and predicts germ and Leydig cell population sizes in the adult mouse testis. Endocrinology 2017;158(9):2955-69.
[Crossref] [Google Scholar] [PubMed]
- Rebourcet D, O’Shaughnessy PJ, Monteiro A, Milne L, Cruickshanks L, Jeffrey N, et al. Sertoli cells maintain Leydig cell number and peritubular myoid cell activity in the adult mouse testis. PloS One 2014;9(8):e105687.
[Crossref] [Google Scholar] [PubMed]
- Roque M, Esteves SC. Effect of varicocele repair on sperm DNA fragmentation: A review. Int Urol Nephrol 2018;50(4):583-603.
[Crossref] [Google Scholar] [PubMed]
- Rugamba A, Kang DY, Sp N, Jo ES, Lee JM, Bae SW, et a. Silibinin regulates tumor progression and tumorsphere formation by suppressing PD-L1 expression in non-small cell lung cancer (NSCLC) cells. Cells 2021;10(7):1632.
[Crossref] [Google Scholar] [PubMed]
- Saadh MJ. Hypoglycemic and hypolipidemic activity of combined milk thistle and fenugreek seeds in alloxan-induced diabetic albino rats. Vet World 2020;13(8):1732-6.
[Crossref] [Google Scholar] [PubMed]
- Sahoo DK, Roy A, Chainy GB. Protective effects of vitamin E and curcumin on L-thyroxine-induced rat testicular oxidative stress. Chem Biol Interact 2008;176(2-3):121-8.
[Crossref] [Google Scholar] [PubMed]
- Shi JF, Li YK, Ren K, Xie YJ, Yin WD, Mo ZC. Characterization of cholesterol metabolism in Sertoli cells and spermatogenesis. Mol Med Rep 2018;17(1):705-13.
[Crossref] [Google Scholar] [PubMed]
- Shiraishi K, Matsuyama H. Gonadotoropin actions on spermatogenesis and hormonal therapies for spermatogenic disorders. Endocr J 2017;64(2):123-31.
[Crossref] [Google Scholar] [PubMed]
- Srinivasan R, Ramprasath C. Beneficial role of silibinin in monitoring the cadmium induced hepatotoxicity in Albino Wistar rats. Recent Res Sci Technol 2012;4(1):46-52.
- Suvarna KS, Layton C, Bancroft JD, editors. Bancroft's theory and practice of histological techniques E-Book. Elsevier health sciences; 2018.
- Bisht S, Faiq M, Tolahunase M, Dada R. Oxidative stress and male infertility. Nat Rev Urol 2017;14(8):470-85.
[Crossref] [Google Scholar] [PubMed]
- Wu PY, Scarlata E, O’Flaherty C. Long-term adverse effects of oxidative stress on rat epididymis and spermatozoa. Antioxidants 2020;9(2):170.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Wang H, Yu L, Chen J. The Puerarin improves renal function in STZ-induced diabetic rats by attenuating eNOS expression. Ren Fail 2015;37(4):699-703.
[Crossref] [Google Scholar] [PubMed]
- Zhou R, Wu J, Liu B, Jiang Y, Chen W, Li J, et al. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell Mol Life Sci 2019;76(14):2681-95.
[Crossref] [Google Scholar] [PubMed]