- Corresponding Author:
- Veena Sharma
Department of Bioscience and Biotechnology, Banasthali University, Jaipur-304 022, India
E-mail: veenasharma61@gmail.com
| Date of Submission | 14 September 2014 |
| Date of Revision | 03 February 2015 |
| Date of Acceptance | 22 November 2015 |
| Indian J Pharm Sci 2015;77(6):729-734 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Free radicals or reactive oxygen indices give rise to oxidative injury, which is a fundamental mechanism underlying a number of disease like diabetes, cancer, neurodegenerative disorders. Deleterious effects of reactive oxygen species can be nullified by using different natural antioxidants derived from plants Indigofera tinctoria is such plant. This study was planned in order to trace and determine the antioxidant capability of Indigofera tinctoria. All the reagents and chemicals used in this study were obtained from reliable firms. The plant extracts were subjected to phytochemical screening, quantitative assays and antioxidant profiling. The results suggested that plant extracts contained all pharmacologically important phytoconstituents in appreciable amounts and having excellent antioxidant capabilities.
Keywords
Antioxidants, Indigofera tinctoria, pharmacology, reactive oxygen species.
Medicinal plants are highly conventional and one of the foremost sources of novel medicinal therapeutics due to the presence of various bioactive components or phytochemicals[1,2]. Most of these components are antioxidant in nature. The antioxidants from plants usually are secondary metabolites that have the ability to cope up oxidative stress resulted by the reactive oxygen species (ROS). These antioxidants produced in the plant as a result for their own defense mechanisms. From the past two or three decades, a number of medicinally important plants have been studied for their ability of neutralizing specific ROS, as the hydroxyl radical, the superoxide radicals, nitric oxide radicals[3].
India is a hub of such pharmacologically important known medicinal plants. Indigofera tinctoria belongs to the family Fabaceae and is an annual herb with a height of 4-6 feet. It is cultivated in India, China and other countries as a source of dyeing agent i.e. indigo. The herb is extensively used in the Indian system of medicine for neurological disorders, epilepsy, bronchitis and hepatic ailments[4].
The roots, stems and leaves of Indigofera tinctoria are bitter, thermogenic, laxative, trichogenous expectorant, hepatoprotective, anticancer, antihelminthic and used in treating gastropathy, splenomegaly, cephalalgia, cardiopathy, epilepsy, neuropathy, chronic bronchitis, asthma, ulcers, skin diseases, diuretic and are useful for promoting the growth of hair[5].
Due to the less available data and reports to trace out the rich antioxidant abilities of Indigofera tinctoria, this study was designed in order to experimentally find out the content of various phytochemicals and antioxidant profiling of methanol and hydrometholic extracts of Indigofera tinctoria.
Materials and Methods
Phenol reagent, dragendorff’s reagent, methanol, gallic acid, aluminium chloride, HCl, sodium nitroprusside, hydrogen peroxide were purchased from Merck, USA. Reduced NADH, phenazine methosulpahte, nitroblue tetrazolium, L-ascorbic Acid were obtained from HiMedia, Mumbai. All other chemicals and reagents were high purity grade analytical reagents.
Collection and processing of plant material
Botanically identified, experimental plant was procured from SM Heena Industries, Rajasthan. The plant was shade dried, milled to coarse powder. 40 g of plant material was placed in soxhlet thimble and was refluxed with solvents for 48-72 at 40-60º to obtain sequential methanol and nonsequential hydromethanol extracts. Extracts were collected and cooled at room temperature and poured in china dishes and then evaporated at 40º using hot air oven. Dried extracts were kept in desiccators for two days and stored at 5º in air tight containers. For the various assays, plant extracts were prepared with a concentration of 1 mg/ml.
Preliminary phytochemical screening
The methanol and hydromethanol extracts were subject to screen the various phytoconstituents as flavonoids, glycosides, saponins, alkaloids, amino acids, proteins, carbohydrates, steroids, tannins and phenols present in them using standard protocols (Table 1)[6-8].
Total phenolic content
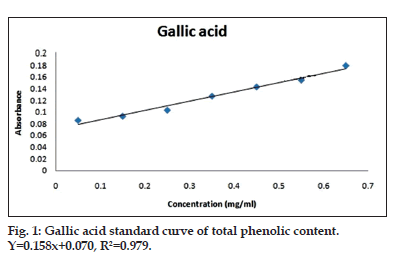
Freshly prepared plant extract and gallic acid standard were treated with 1 ml of folin ciocalteu’s reagent and mixed well. After 5 min, 4 ml of 7% Na2CO3 solution and 4 ml distilled water was added to it. This reaction mixture was incubated for 90 min in dark and then centrifuged at 10 000 rpm for 5 min. The supernatant was taken and its absorbance was recorded at 750 nm and the total phenolic content was expressed as mg gallic acid equivalent (GAE) per g of plant sample (fig. 1 and Table 2)[9].
| Phytochemicals | Methanol extract | Hydromethanol extract |
|---|---|---|
| Alkaloids | + | - |
| Amino acids | - | + |
| Carbohydrates | + | + |
| Flavonoids | + | + |
| Glycosides | + | + |
| Proteins | + | + |
| Saponins | + | + |
| Steroids | + | + |
| Tannins and phenols | + | + |
Table 1: Phytochemical Screeing Of Indigoferatincoria.
Total saponin content
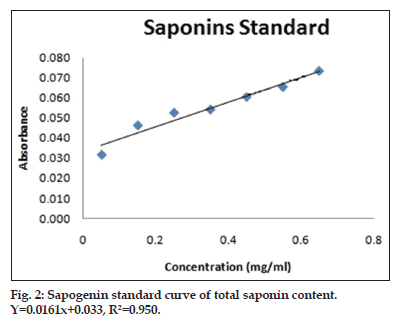
Sample and standard (saponin) were treated with 400 μl vanillin-acetic acid reagent and 1.6 ml of perchloric acid. This reaction mixture was kept on water bath at 70-75° for several min. It was than cooled on ice bath for 2 min and 2.5 ml of glacial acetic acid was poured into it. Absorbance was taken at 550 nm after mixing it well. The total saponin content was expressed as mg sapogenin equivalent (SE) per g of plant sample (fig. 2 and Table 2)[10].
Total flavonoid content
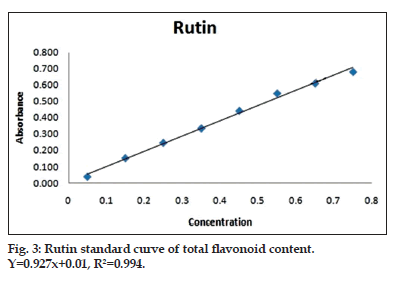
To 0.5 ml sample and standard (rutin), 2 ml distilled water and 0.15 ml 5% NaNO2 solution were added. After 6 min, 0.15 ml 10% AlCl3 solution was added and kept for another 6 min. To this reaction mixture, 2 ml 4% NaOH solution and 0.2 ml water was added to make up the final volume 5 ml. The reaction mixture was mixed well and allowed to stand for 15 min after which absorbance was recorded at 510 nm. Total flavonoid content was expressed as mg rutin equivalent (RE)/g plant sample (fig. 3 and Table 2)[11].
Total tannin content
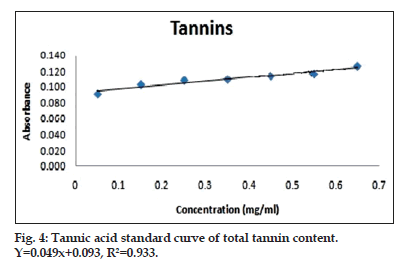
Sample and standard (tannic acid) were diluted with 8 ml distilled water and added 6.5 ml Folin–Ciocalteu reagent, 1.5 ml 20% Na2CO3 solution was to them. Absorbance was recorded at 775 nm and its content was expressed as mg tannic acid equivalents (TAE) per g of plant sample (fig. 4 and Table 2)[12].
Total proanthocynidin content
Extract and standard (rutin) were treated by 1 ml ethanol (70%, v/v), 1.5 ml 25% HCl and 1 ml H2O. This reaction mixture was heated at 85-90° for 80 min and cooled to add 1.5 ml n-butanol. The absorbance was than recorded at 545 nm. Total proanthocynidin content was expressed as mg rutin equivalent (RE) per g plant sample sample (fig. 5 and Table 2)[13].
| Parameter analyzed | Met | HM |
|---|---|---|
| Total phenolic content (mg GAE/g) | 207.67±0.002 210.62±0.005 | |
| Total saponin content (mg SE/g) | 89.9±0.001 | 87.5±0.001 |
| Total flavonoid content (mg RE/g) | 96.4±0.003 | 104.16±0.008 |
| Total tannin content (mg TAE/g) | 229±0.001 | 230±0.001 |
| Total proanthocynidin content (mg RE/g) | 6.107±0.002 | .17±0.001 |
Table 2: physico chemical quantitative assays of methanol and hydromethanol extracts of Indigofera tincoria.
DPPH scavenging assay
Extract and standards 250 μl were treated with 2.5 ml of 0.004% DPPH solution and incubated at room temperature for 30 min in dark. The absorbance was recorded at 517 nm[14].
FRAP (feric reducing antioxidant power) assay
The stock solutions included 300 mM acetate buffer (pH=3.6), 10 mM TPTZ (2,4,6-tripyridyl-s-triazine) solution in 40 mM HCl and 20 mM FeCl3.6H2O solutions were prepared. The fresh working solution was prepared by mixing 25 ml acetate buffer, 2.5 ml TPTZ and 2.5 ml FeCl3.6H2O. The temperature of the solution was raised to 37° before using. Plant extract (150 μl) and standard (FeSO4) were allowed to react with 2850 μl working solution for 30 min in dark. The absorbance of the colored product was recorded at 593 nm[15].
Total antioxidant capacity
Extracts and standard were treated with reagent mixture (0.6 M H2SO4, 28 mM NaPO4, 4 mM ammonium molybdate; 1:1:1). The reaction mixture was incubated at 95° for 90 min and absorbance was taken at 695 nm[16].
Hydroxyl radical scavenging capacity
Reaction mixture consists of 200 μl 10 mM FeSO4. 7H2O, 200 μl 10 mM EDTA and 200 μl 10 mM deoxyribose was mixed with 0.5 ml sample. The volume was made up to 1.8 ml by phosphate buffer. To it, 200 μl 10 mM H2O2 was also added and incubation was provided at 37° for 1 h. This reaction mixture was treated with 1 ml 0.5% TBA and 1 ml of ice cold TCA (2.8% in 25 mM NaOH) and incubated at 80° for 30 min. The absorbance was than recorded at 532 nm[17].
Superoxide radical Scavenging activity
Samples were treated with 1 ml NBT, 1 ml NADH and 100 μl PMS solution and incubated at 25° for 3 min. The absorbance was recorded at 560 nm[18].
Metal chelating capacity
Samples and EDTA standard (0.5 ml) were allowed to react with 1 mM FeSO4, 0.5 ml Tris-HCl buffer (0.2 M; pH=7.4), 0.5 ml 0.1% bipyridyl solution, 0.4 ml 10% hydroxylamine HCl and 2 ml ethanol. The reaction mixture kept for 2 min at 25° and absorbance was recorded at 522 nm[19].
Reducing power assay
Plant extracts and rutin standards were treated with 2.5 ml 0.2 M phosphate buffer of pH 6.6 and 2.5 ml 1% potassium ferricyanide. Incubation was given at 50° for 20 min. To it, 2.5 ml 10% TCA solution was added. 2.5 ml of the upper layer was taken of the above mixture, mixed with distilled water and 0.5 ml 0.1% FeCl3. Absorbance was taken at 700 nm[20].
Nitric oxide radical scavenging activity
Samples (0.5 ml) were mixed with 0.5 ml 0.1 M phosphate buffer of pH 7.4 and 2 ml 10 mM sodium nitroprusside solution. Incubation was provided at 25° for 2.5 h. Taken 0.5 ml of above reaction mixture and 1 ml 0.33% sulphanilic acid in 20% glacial acetic acid was added to it. Kept it at room temperature for 5 min, added 1 ml 5% N-NED-HCl, mixed and incubated at room temperature for 5 to 30 min. Absorbance was recorded at 564 nm of pink color[21].
Results and Discussion
Phytochemical investigation of the methanol and hydromethanol extract of Indigofera tinctoria revealed the occurrence of pharmacologically important phytoconstituents as flavonoids, saponins, glycosides, steroids, tannins and phenols. Alkaloids were found to be absent in hydromethanol extract while present in low amount in methanol extract. Both extracts were also found to possess amino acids, proteins and carbohydrates. Flavonoids produced in plants due to microbial response. They are most potent and excellent antioxidants possess the capability of acting as anticancer agents[22]. Saponins, which have hemolytic and antiinflammatory ability, were also present in both extracts[23]. Plant extracts also possess glycosides, which are known to lower the blood pressure[24]. Steroids have been reported to have antibacterial activity[25] and antidiarrhoeal activity as it increase intestinal absorption sodium ions and water[26].
The total phenolic content in methanol content was 207.69 mg/g of dry weight of extract and 210.62 mg/g of dry weight for hydromethanol extract. Such amount of phenolic components is able to scavenge free radicals by donating active hydrogen and thus help reducing the detrimental effects of oxidative stress. This property of plant phenolic makes them a key group of compounds that perform as free radical scavengers or primary antioxidants. Total saponin content was 89.9 mg/g for methanol extract and 87.5 mg/g for the hydromethanol extract.
Flavonoid content was found to be 96.4 mg/g of dry weight for methanol extract and 104.16 mg/g of dry weight for hydro methanol extract. Flavonoids are chemically characterized by two benzene rings joined by a linear carbon chain. Various reports indicate that regular flavonoids may trim down the risk of several chronic diseases including neurodegenerative diseases, atherosclerosis, and cancer[27]. The flavonoids also reported to have antiviral, antiallergic, antiplatelet and antiinflammatory properties.
Tannins are phenolic compounds having antioxidant and antibacterial properties and also have ability to form complexes with macromolecules and metal ion[28]. The amount of tannins present in methanol and hydromethanol extract was 229 and 230 mg/g of dry weight of respective extracts.
Acid butanol assay is used to determine the total proanthocynidin content and it was observed this is an easy, simple and rapid test. Total proanthocynidin content was 6.107 mg/g of dry weight of extract in methanol extract and 7.17 mg/g of dry weight of extract in hydro methanol extract. Literature have indicated that proanthocynidins exhibit antimicrobial, anticarcinogenic and DPPH test present information on the reactivity of the test sample with a stable free radical entity. DPPH gives a strong absorption band in visible spectrum at 517 nm wavelength. When an odd electron becomes paired off in the presence of a free radical scavenging agent, the DPPH solution is decolorized and its color changes from deep violet to light yellow as the absorption reduces. The degree of reduction in absorbance measurement is pinpointing of the antioxidant ability of the sample extract. To scavenge DPPH radicals, inhibition concentration of methanol extract was 0.709 and 0.881 mg/ml of hydromethanol extract, which was found to be much higher when compared to the IC50 of tocopherol standard (0.94 mg/ml) (Table 3).
FRAP is an excellent method in order to determine the free radical scavenging activity. Low pH causes reduction of ferric-TPTZ to ferrous-TPTZ, which gives an intense blue color, measured at 593 nm using spectrophometer. The FRAP value for methanol extract and hydromethanol extract were 10.807 and 18.256 mM Fe (II) ions/mg of plant extracts (Table 3).
Total antioxidant capacity or TAC is based on phosphomolybdenum model in which formation of a green phosphate-Mo (V) complex occurs after the reduction of Mo (VI) to Mo (V) at low pH. Total antioxidant capacity for methanol extract of Indigofera tinctoria was 622.74 mg/g of plant extract and 722.72 mg/g of hydromethanol extract (Table 3).
| Parameters analyzed | Met | HM |
|---|---|---|
| DPPH scavenging activity (IC50-mg/ml) | 0.709±0.018 | 0.881±0.027 |
| FRAP activity (mM Fe (II) ions/mg | 10.807±0.016 | 18.256±0.036 |
| dried plant extract) | ||
| Total antioxidant capacity (mg GAE/g) 622.74±0.002 | 722.72±0.007 | |
| Hydroxyl radical scavenging activity | 0.020±0.003 | 0.028±0.002 |
| (IC50-mg/ml) | ||
| Superoxide radical scavenging activity | 0.394±0.004 | 0.457±0.004 |
| (IC50-mg/ml) | ||
| Metal chelating capacity (IC50-mg/ml) | 0.758±0.017 | 0.391±0.021 |
| Reducing power assay (mg RE/g) | 2803±0.015 | 2494.74±0.028 |
| Nitricoxide radical scavenging activity | 0.709±0.002 | 0.709±0.004 |
| (IC50-mg/ml) | ||
Table 3: Antioxidant Assays Of Methanol And Hydromethanol Extracts Of Indigoferatincoria.
The ability to scavenge hydroxyl radicals is measured by the ability of extracts to prevent degradation of deoxyribose by the hydroxyl radicals generated in the reaction mixture. Highly reactive hydroxyl radicals can be generated in biological systems by fenton reaction. The IC50 to scavenge the hydroxyl radicals for methanol and hydromethanol extract were 0.020 and 0.028 mg/ml, respectively (Table 3).
Superoxide radical scavenging activity was determined by PMS-NADH system. Superoxide radicals play an important role in living cells as they on decomposition, produced more harmful free radical entities as hydroxyl radicals and singlet oxygen. IC50 value to scavenge superoxide radicals was almost similar for hydromethanol extract of Indigofera tinctoria (0.457 mg/ml) and ascorbic acid standard (0.462 mg/ml). For methanol extract it was found to be 0.394 mg/ml (Table 3).
Metal chelating capacity is important as it reduce the concentration of transition metals as iron, which accelerate rate of lipid peroxidation by fenton reaction. According to the results, the plant extracts showed good metal chelating activity. IC50 values of 0.75 and 0.39 mg/ml were observed for metal chelating capacity, respectively in methanol and hydromethanol extract. The standard EDTA IC50 value was 0.29 mg/ml (Table 3).
Reducing power for methanol extract was found to be 2494.74 mg/g of plant extract and 2803 mg/g plant extract for hydromethanol extract of Indigofera tinctoria. Like the antioxidant activity, the reducing power increased with increasing amount of the extract. For the measurement of the reductive ability, the Fe3+-Fe2+ transformation was investigated in presence of the extract. Presence of various antioxidants results in the reduction of the ferricyanide complex to the ferrous form (Table 3).
In biological systems, nitric oxide (NO) plays a potent role of physiological process such as smooth muscle relaxation, neuronal signalling, inhibition of platelet aggregation and regulation of cell mediated toxicity. It is a diffusible free radical, which plays many roles as an effector molecule in diverse biological systems including neuronal messenger, vasodilation and antimicrobial and antitumor activities. IC50 values of 0.035 mg/ml for nitric oxide radical scavenging activity was observed for both methanol and hydromethanol extract (Table 3).
This study put forwarded that methanol and hydromethanol extracts of Indigofera tinctoria possess antioxidant properties when compared with the ability of well exemplified and widely used antioxidant standards. The high potential of being good antioxidant and free radical scavengers of these two extracts of Indigofera tinctoria is due to the presence of phytoconstituents, which possess a number of therapeutic and pharmacological properties and are promising source of herbal medicinal cure of various ailments including cancers and neurogenerative disorders.
The studies are further warranted for the isolation and structural characterization of these phytoconstituents present in the plant extract. Furthermore, in vivo studies are also needed to be performed in order to under the mechanism of action of these antioxidant components and before the access to preceding clinical trials.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Uddin G, Rehman TU, Arfan M, Liaqatwaliullah W, Rauf A, Khan I, et al. Antimicrobial, insecticidal and phytotoxic activities of Indigoferaheterantha roots. J Med Plants Res 2011;5Suppl 24:5835-9.
- Sharam V, Agarwal A, Chaudhary U, Singh M. Phytochemical investigation of various extracts of leaves and stems of Achyranthesaspera Linn. Int J Pharm PharmSci 2013;5Suppl 1:317-20.
- Pietta P, Simonetti P, Mauri P. Antioxidant activity of selected medicinal plants. J Agric Food Chem 1998;46:4487-90.
- Magesh V, Yuvaraj G, Deecaraman M, Kalaichelvan PT. Methanol extract of Indigoferatinctoria induces apoptosis in HCT116 cells. J ApplBiosci 2009;14:768-74.
- Asuntha G, Prasannaraju Y, Prasad KV. Effect of ethanol extract of Indigoferatinctoria Linn (Fabaceae) on lithium/pilocarpine induced status epilepticus and oxidative stress in wistar rats. Trop J Pharm Res 2010;9Suppl 2:149-56.
- Kokate CK. Practical Pharmacognosy. 11th ed. Pune: NiraliPrakashan; 2008.
- Siddiqui AA, Ali M. Identification tests of organic drugs. In: Practical Pharmaceutical Chemistry. 1st ed. New Delhi: CBS Publishers and Distributers; 2008. p. 127-31.
- Khandelwal KR. Preliminary phytochemical screening. In: Practical Pharmacognosy. 12th ed. Pune: NiraliPrakashan; 2004. p. 149-55.
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J EnolVitic 1965;16:144-58.
- Madland E. Extraction, Isolation and Structure Elucidation of Saponins from Herniarisincana. Trondheim, Norwegian University of Science and Technology; 2013. Available from: http://www.diva-portal. org/smash/get/diva2:610888/FULLTEXT01.pdf. [Last accessed on 2015 Feb 03].
- Zhinsen J, Mengcheng T, Jianming W. The determination of flavonoidal contents in mulberry and their scavenging effects on superoxide radical. Food Chem 1999;64:555-9.
- Tamilselvi N, Krishnamoorthy P, Dhamotharan R, Arumugam P, Sagadevan E. Analysis of plant phenols, total tannins and screening of phytocomponents in Indigoferaaspalathoides. J Chem Pharm Res 2011;4Suppl 6:3259-62.
- Hiermann A, Kartnig T, Azzam S. A contribution to the quantification of the proanthocyanidins in Crataegus. Sci Pharm 1986;54:331-7.
- Braca A, De Tommasi N, Di Bari L, Pizza C, Politi M, Morelli I. Antioxidant principles from Bauhinia tarapotensis. J Nat Prod 2001;64:892-5.
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem 1996;239:70-6.
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of Vitamin E. Anal Biochem 1999;269:337-41.
- Halliwell B, Gutteridge JM, Aruoma OI. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 1987;165:215-9.
- Robak J, Gryglewski RJ. Flavonoids are scavengers of superoxide anions. BiochemPharmacol 1988;37:837-41.
- Yamaguchi K, Mori H, Nishimura M. A novel isoenzyme of ascorbate peroxidase localized on glyoxysomal and leaf peroxisomal membranes in pumpkin. Plant Cell Physiol 1995;36:1157-62.
- Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jap J Nutr 1986;44:307-15.
- Sangameswaran B, Balakrishnan BR, Deshraj C, Jayakar B. In vitro antioxidant activity of roots of Thespesia lampas Dalz and Gibs. Pak J Pharm Sci 2009;22:368-72.
- Yadav RN, Agarwala M. Phytochemical analysis of some medicinal plants. J Phytol 2011;3:10-4.
- Just MJ, Recio MC, Giner RM, Cuéllar MJ, Máñez S, Bilia AR, et al. Anti-inflammatory activity of unusual lupanesaponins from Bupleurumfruticescens. Planta Med 1998;64:404-7.
- Nyarko AA, Addy ME. Effects of aqueous extract of Adeniacissampeloides on blood pressure and serum analyte of hypertensive patients. Phytother Res 1990;4:25-8.
- Epand RF, Savage PB, Epand RM. Bacterial lipid composition and the antimicrobial efficacy of cationic steroid compounds (Ceragenins). BiochimBiophysActa 2007;1768:2500-9.
- Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: A review. Int Pharm Sci 2011;1:98-106.
- Mahesh AR, Ranganath MK, Kumar H. Enrichment of flavonoids from the methanol extract of Boerhaaviadiffusa roots by partitioning technique. Res J ChemSci 2013;3:43-7.
- Nitao JK, Birr BA, Nair MG, Herms DA, Mattson WJ. Rapid quantification of proanthocyanidins (Condensed tannins) with a continuous flow analyzer. J Agric Food Chem 2001;49:2207-14.
- Chung KT, Stevens SE, Lin WF. Growth inhibition of selected food-borne bacteria by tannic acid, propyl gallate and related compounds. LettApplMicrobiol 1993;17:29-35.
- Katiyar SK, Mukhtar H. Tea antioxidants in cancer chemoprevention. J Cell BiochemSuppl 1997;27:59-67.
- Amarowicz R, Pegg RB, Bautista DA. Antibacterial activity of green tea polyphenols against Escherichia coli K 12. Nahrung 2000;44:60-2.