- *Corresponding Author:
- H. Haba
Department of Chemistry, Laboratory of Chemistry and Environmental Chemistry, Faculty of Sciences of the Matter, University of Batna 1, Algeria
E-mail: haba.hamada@yahoo.fr
| Date of Received | 18 July 2020 |
| Date of Revision | 23 December 2021 |
| Date of Acceptance | 19 July 2022 |
| Indian J Pharm Sci 2022;84(4):890-901 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study aimed to investigate the phytochemical composition, alpha-amylase and pancreatic lipase inhibitory activities and anti-inflammatory and antioxidant properties of the crude extracts (petroleum ether, ethyl acetate and n-butanol) obtained from the aerial parts of Ephedra altissima. The characterization of the phenolic compounds in crude extracts was performed by high performance liquid chromatographyphotodiode array detection-electrospray ionization-mass spectrometry and the in vitro alpha-amylase and pancreatic lipase inhibitory activities were evaluated using starch and p-nitrophenyl butyrate as substrates, respectively. Furthermore, the anti-inflammatory activity was carried out by bovine serum albumin denaturation method and the antioxidant capacity was assessed using five different assays. Flavonol and flavone glycoside derivatives and phenolic acids, especially isovitexin-2-O-rhamnoside and vicenin II were the major compounds in the n-butanol extract. Otherwise, the ethyl acetate extract presented kaempferol-3-O-rhamnoside, quercetin-3-O-rhamnoside and protocatechuic acid as its main compounds. The n-butanol extract revealed the highest content of total phenolic compounds. The results for antioxidant activity showed that ethyl acetate extract possesses the strongest activity in all the tested methods. Moreover, the ethyl acetate extract displayed the best anti-inflammatory, alpha-amylase and pancreatic lipase inhibitory activities with half-maximal inhibitory concentration values of 126.43, 9.02 and 289.11 μg/ml, respectively. These results give obvious support to the use of Ephedra altissima in traditional medicine for the treatment of diabetic diseases. Also, the moderate alpha-amylase inhibitory activity of Ephedra altissima extracts make advantage of its therapeutic applications compared to the synthetic drugs, since their strong alphaamylase inhibitory effects are associated to many digestive adverse effects including the abnormal bacterial fermentation in the colon.
Keywords
Ephedra altissima, polyphenol, alpha-amylase, pancreatic lipase, anti-inflammatory, antioxidant
Non-Insulin-Dependent Diabetes Mellitus (NIDDM) is a complex metabolic disorder caused by the decrease of insulin secretion or the insulin resistance due to the excessive absorption of glucose[1]. Hyperglycemia is a typical symptom in NIDDM patients, characterized by a rapid increase in postprandial glycaemia due to the excessive hydrolysis of starches[2]. One of the most common therapeutic approaches for the control of postprandial hyperglycemia is the delay of glucose digestion and absorption by the inhibition of carbohydrate hydrolyzing enzymes in the digestive tract including alpha (α)-amylase[3]. Indeed, it has been reported that the inhibition of α-amylase retards the uptake of dietary carbohydrates and suppresses postprandial hyperglycemia resulting in the improvement of type 2 diabetes symptoms. This enzyme slows starch digestion and consequently delays and interrupts the production and the absorption of glucose leading to the decrease of blood glucose level[4,5].
Diabetes complications occur as a result of oxidative stress, the formation of free radicals, glucose oxidation and the subsequent oxidative degradation of glycated proteins[6]. The generation of free radicals due to oxidative stress factors can also be associated with inflammation and other diseases. Furthermore, the supplementation of the body by exogenous antioxidants may partially contribute to inhibiting the progression of complications linked to diabetes and inflammations[7]. Therefore, the research concerning new treatments from natural resources constitutes a promising area to find natural chemical compounds with potential ability for the modulation of digestive enzymes (lipase and α-amylase) and to reduce oxidative stress and its related diseases.
The family Ephedraceae is characterized by a single genus named Ephedra L., including about 68 species[8]. The plants of this genus are generally found in arid and semi-arid climates and distributed mainly in the temperate zones of Europe, Asia, Africa and North America[9]. The important therapeutic applications of plants belonging to the genus Ephedra motivated us to study the species Ephedra altissima Desf. (E. altissima), which is a shrub with climbing stems; the branches are very green and break easily while drying. The cones gather in branched and loose inflorescences, and are red or white at maturity. This endemic species is common to the Sahara (Hoggar and Neighboring massifs, Tefedest)[10]. It is used in folk medicine for the treatment of various diseases such as vascular hypertension and respiratory diseases[11] and also for diabetic diseases[12].
The present work was designed to investigate the phenolic composition and the in vitro evaluation of antioxidant and anti-inflammatory properties and α-amylase and pancreatic lipase inhibitory activities of the extracts (Petroleum Ether (PE), Ethyl Acetate (EtOAc) and n-Butanol (n-BuOH)) obtained from E. altissima aerial parts. To the author’s best knowledge, there are no published reports describing the phenolic constituents, the anti-inflammatory effect and the inhibitory activities of α-amylase and pancreatic lipase of this species.
Materials and Methods
The plant material E. altissima Desf., was collected in November 2016 in Bouhmama Mountain of the vicinity of Khenchela (Aures region), Algeria and was identified by Professor Bachir Oudjehih, Agronomic Institute of the University of Batna-1. A voucher specimen was kept under the number 705/LCCE.
Preparation of crude extracts:
The aerial parts (500 g) of the species of E. altissima Desf., were macerated twice (5 l×2) for 3 d with the solvent mixture of ethanol/water (70/30 v/v) at room temperature. After filtration, the obtained filtrate was evaporated at 40° to give 400 ml of aqueous extract. This latter was subjected to liquid-liquid extraction using solvents with increasing polarity (PE, EtOAc and n-BuOH). Then, the organic fractions were dried with anhydrous sodium sulfate, filtered and evaporated to offer the following extracts: PE (3.24 g), EtOAc (5.7 g) and n-BuOH (10.12 g).
Analysis and quantification of phenolic compounds by High Performance Liquid Chromatography (HPLC)/Mass Spectrometry (MS):
The HPLC Waters Alliance (Manchester, United Kingdom (UK)) system fitted to a mediterraneaTM sea18 reverse-phase analytical column (25 cm length×4.6 mm internal diameter (i.d.), 5 μm particle size; Teknokroma, Barcelona) was used. An elution gradient was used with solvents A (water with 1 % formic acid) and B (acetonitrile with 1 % formic acid). The elution program was as follows. Percentages refer to proportion of eluent B: 5 %-25 % (0-30 min); 25 %-50 % (30-45 min); 50 %-100 % (45-47 min); 100 %-25 % (47-50 min); 25 %-5 % (50-52) and 5 % (52-55 min). The column end was connected directly to a Diode Array Detector (DAD) (Waters 996, Millipore, Manchester, UK) and subsequently, part of the flow (0.4 ml/min) was directed to an online connected quadrupole mass analyzer (ZMD4, Micromass, Waters, Inc., Manchester, UK). Electrospray Ionization (ESI) mass spectra were obtained at the ionization energy of 70 eV, capillary voltage was 3 kV, dissolving temperature 120°, source temperature 80° and extractor voltage 12 V. The flow was maintained at 1 ml/min and the split ratio was 5:1 (Ultraviolet (UV) detector MS) for each analysis.
Stock standard solutions of each compound (gallic acid, p-hydroxybenzaldehyde, p-hydroxybenzoic acid, protocatechuic acid, ferulic acid, coumaric acid, vitexin, isoorientin, vicenin II, quercetin-3-Orhamnoside, kaempferol-3-O-rhamnoside, isovitexin- 2-O-rhamnoside and naringenin) were prepared by dissolving 10 mg of analytical standard in 10 ml of ethanol 80 %, all solutions were stored at -20°. An intermediate solution containing all standard compounds (100 μg/ml) was prepared in 80 % ethanol and dilutions from this solution were made at different levels for calibration curves and validation experiments (matrix effects, precision and accuracy). Triplicate injections were made for each standard and sample. Analytes were identified by comparing Retention time (Rt), UV and mass to charge ratio (m/z) values recorded by MS with those of standards obtained under the same conditions. The calibration curves were used for quantification; peak areas were compared with calibration curves generated by three repeated injections of known standards at seven concentrations (20-100 μg/μl). Linearity ranges for calibration curves were determined.
2,2-Diphenylpicrylhydrazyl (DPPH) scavenging activity:
The scavenging activity of the free radical of DPPH was evaluated spectrophotometrically[13]. 25 μl of sample solutions (extracts and standards) at different concentrations were added to 975 μl of the DPPH solution prepared in methanol. The mixture was kept in the dark at room temperature for 30 min. The blank solution was prepared by adding 25 μl of methanol to 975 μl of DPPH reagent and the absorbance was measured at 517 nm. The percentage of inhibition of each sample was calculated as follows: Activity (%)=[(ABlank- ASample)/ABlank]×100. ABlank is the absorbance of blank solution and ASample is the absorbance of sample. The experiments were performed in triplicate and the results were transmitted as the mean values±Standard Deviation (SD).
Total Antioxidant Capacity (TAC) by Phosphomolybdate (PPM):
The TAC of crude extracts (PE, EtOAc and n-BuOH) was evaluated by the phosphomolybdenum method[13]. 100 μl of different samples (extracts and ascorbic acid) were added to 900 μl of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The tubes containing the reaction mixture were incubated at 95° for 90 min. After incubation, the tubes were left to cool at room temperature and the absorbance was measured at 695 nm. The antioxidant capacity of the extracts was expressed as microgram Equivalents of Ascorbic Acid per mg of dry extract (μg EAA/mg extract).
Ferric Thiocyanate (FTC) assay:
The lipid peroxidation activity of crude extracts and references was measured by the method of FTC[13]. The reaction mixture containing 400 μl of sample (100 μg/ ml), 400 μl of linoleic acid (2.52 % in absolute ethanol) and 800 μl of phosphate buffer (pH 7.4) was incubated at 40° for 1 h. A volume of 100 μl of this solution was added to 5 ml of ethanol (70 %) and 100 μl of ammonium thiocyanate (30 %). After 3 min, 100 μl of Ferrous chloride (FeCl2) prepared in hydrochloric acid (3.5 %) were added to the mixture. A blank solution was created by replacing the samples with distilled water. The absorbance of the resulting solution was read for 7 d at 500 nm using a spectrophotometer. The percentage of inhibition of the lipid peroxidation was calculated.
Hydrogen peroxide (H2O2) scavenging assay:
The ability of E. altissima extracts to scavenge H2O2 was determined spectrophotometrically[13]. A H2O2 solution at a concentration of 40 mM was prepared in phosphate buffer at pH 7.4. A volume of 1 ml of sample solution (100 μg/ml) was added to 0.6 ml of a H2O2 solution. The absorbance of the sample was read at 230 nm after 10 min of incubation against a blank solution containing the phosphate buffer without H2O2. The percentage of inhibition of crude extracts and standard were calculated.
Beta (β)-carotene bleaching activity:
The antioxidant activity of the extracts (PE, EtOAc and n-BuOH) and references (Butylated Hydroxyanisole (BHA) and quercetin) was assessed using β-carotene in a linoleic acid system[13]. The emulsion solution was prepared by dissolving 0.2 mg of β-carotene in 1 ml of chloroform, 200 μl of linoleic acid and 200 μl of Tween 80. After the evaporation of chloroform under vacuum, 50 ml of oxygenated water were added to the reagent mixture and shaken vigorously. 5 ml of aliquots were transferred into tubes containing 200 μl of extracts or standards at the concentration of 1 mg/ml. The absorbance at time zero was measured immediately at 470 nm using a spectrophotometer. The test tubes were then incubated at 50° and the absorbance was read again at 20 min time intervals for 2 h. The rate of β-carotene bleaching (Rt) was calculated according to the following formula: Rt= ln(A0/At)/t. Where ln is natural logarithm, A0 is absorbance at time 0, At is absorbance at time t and t is time at 20, 40, 60, 80, 100 and 120 min. The antioxidant activity of the samples was calculated as percentage of inhibition, using the equation: Activity (%)=[(ABlank-ASample)/ABlank]×100.
Anti-inflammatory activity:
The in vitro evaluation of activity of E. altissima extracts was carried out by the inhibition of protein denaturation method[14] using ibuprofen as standard. 500 μl of sample solutions (extracts or standard) prepared in ethanol by different concentrations were added to 500 μl of Bovine Serum Albumin (BSA) solution (0.2 %, BSA) prepared in tris buffer saline (pH 6.6). A control tube containing a mixture of 500 μl of BSA and 500 μl of ethanol was prepared. The solution tubes were incubated at 37° for 10 min and then heated at 72° for 5 min. After cooling at room temperature for 10 min, the absorbance was read at 660 nm. The percentages of inhibition and the half-maximal Inhibitory Concentration (IC50) value were calculated.
Pancreatic lipase inhibitory activity:
Lipase activity was measured using p-Nitrophenyl Butyrate (p-NPB) as a substrate[15]. Briefly, an enzyme buffer was prepared by the addition of 20 μl of solution of porcine pancreatic lipase (20 mg/ml in Tris buffer, pH 7) to 160 μl of Tris buffer (100 mM Tris-Hydrogen chloride (HCl) and 5 mM Calcium chloride (CaCl2), pH 7.0). Then, increasing concentrations of various extracts (ranging from 0 to 11.25 mg/ml) dissolved in Tris buffer were mixed with 20 μl of the enzyme buffer and incubated for 30 min at 37°. 20 μl of substrate (10 mM p-NPB in dimethylformamide) were then added. Lipase activity was determined by measuring the hydrolysis of p-NPB to p-nitrophenol at 405 nm using an Enzyme-Linked Immunosorbent Assay (ELISA) reader. The inhibition of lipase activity was expressed as the percentage of absorbance decrease when porcine pancreatic lipase was incubated with the tested compounds. Lipase inhibition (%) and the IC50 value were calculated.
α-Amylase inhibitory activity:
Porcine pancreatic α-amylase inhibition was performed using the method of Kwon et al.[16]. A mixture of 200 μl of sample solutions (extracts or reference), 500 μl of a 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M of Sodium chloride (NaCl)) containing α-amylase solution with a concentration of 0.5 mg/ ml was incubated at 25° for 10 min. Then, 500 μl of 1 % starch solution in 0.02 M sodium phosphate buffer were added. The reaction mixture was incubated at 25° for 10 min and the reaction was stopped with the addition of 1 ml of dinitrosalicylic acid. The resulting solution was then incubated in a boiling water bath for 5 min and cooled at room temperature. After cooling, the mixture was then diluted with 10 ml of water and the absorbance was measured at 540 nm. Acarbose was used as positive control; the percentage of inhibition and the IC50 value were calculated.
Statistical analysis:
All the experimental measurements were expressed as the mean±SD for three replicates for each sample. Mean comparisons were determined by one-way Analysis of Variance (ANOVA), followed by the Duncan test. The differences were considered significant at p<0.05. The statistics, 7.0 package was used.
Results and Discussion
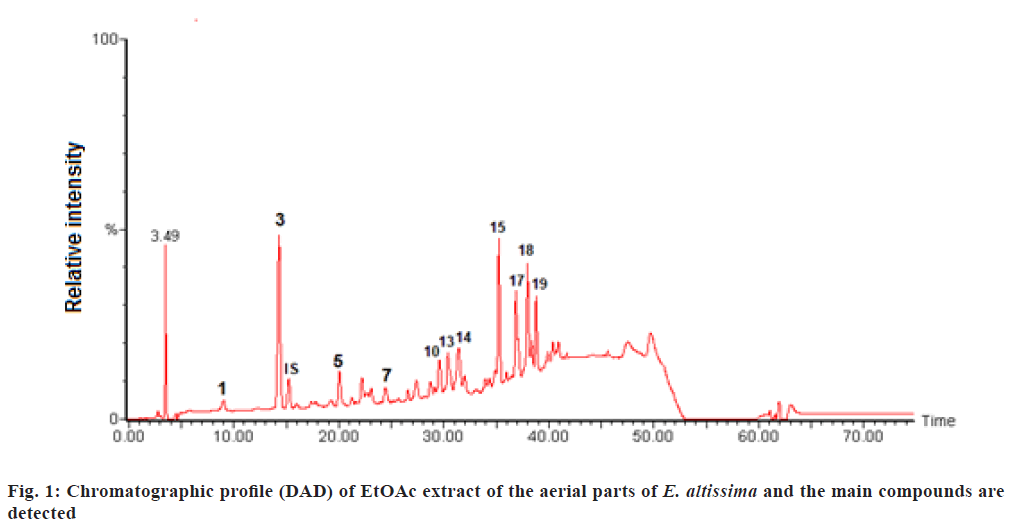
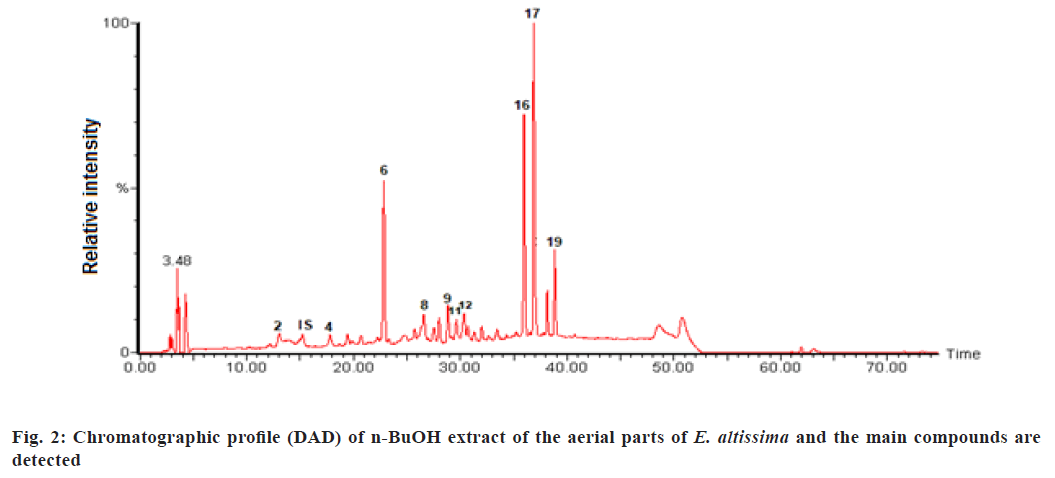
The phenolic compounds of EtOAc and n-BuOH extracts from E. altissima were characterized using the HPLC-DAD-ESI/MS (fig. 1 and fig. 2).
The main compounds detected are shown in fig. 1 which are represented as (1) Gallic acid; (3) Protocatechuic acid; (IS) Internal Standard; (5) p-hydroxybenzoic acid; (7) p-hydroxybenzaldhyde; (10) Coumaric acid; (13) Vitexin; (14) Ferulic acid; (15) Quercetin-3-Orhamnoside; (17) Isovitexin-2-O-rhamnoside; (18) Kaempferol-3-O-rhamnoside and (19) Unknown.
Similarly the main compounds detected are shown in fig. 2 which are represented as (2) Protocatechuic acid glucoside; (IS) Internal Standard; (4) Ferulic acid glucoside; (6) Vicenin II; (8) Isoorientin; (9) Unknown; (11) Unknown; (12) Vitexin-4-O-glucoside; (16) Unknown; (17) Isovitexin-2-O-rhamnoside and (19) Unknown.
Data regarding Rt, wavelength of maximum absorbance (λmax), pseudomolecular ion, main fragment ions in MS, identification and quantification of the individual compounds are summarized in Table 1 and Table 2. The recorded Ultraviolet-Visible (UV-Vis) and mass spectra showed the presence of fifteen different compounds, including eight phenolic acids (compounds 1, 2, 3, 4, 5, 7, 10, 14), and seven flavonoid glycosides (compounds 6, 8, 12, 13, 15, 17, 18). The contents of selected individual and total phenolic compounds in the EtOAc and n-BuOH extracts of E. altissima are summarized in Table 2. The n-BuOH extract displayed the highest phenolic content 123.1 mg/g of extract, while the EtOAc extract contained 68.7 mg/g of extract.
| Peak | Compounds | Rt (min) | [M-H]- | λmax (nm) | [M-H]- fragment m/z |
|---|---|---|---|---|---|
| 1 | Gallic acid | 9.03 | 169 | 271 | 169, 125 |
| 2 | Protocatechuic acid glucoside | 13.13 | 315 | 259, 294 | 315, 153, 109 |
| 3 | Protocatechuic acid | 14.31 | 153 | 259, 294 | 153, 109 |
| 4 | Ferulic acid glucoside | 17.79 | 355 | 223 | 355, 193 |
| 5 | P-Hydroxybenzoic acid | 20.06 | 137 | 254 | 137, 117, 108 |
| 6 | Apigenin-6,8-di-C-glucoside (vicenin II) | 22.81 | 593 | 271, 335 | 593, 575, 503, 473, 383, 353 |
| 7 | P-hydroxybenzaldehyde | 24.44 | 121 | 283 | 121, 117 |
| 8 | Luteolin-6-C-glucoside (isoorientin) | 26.58 | 447 | 269, 347 | 447, 429 |
| 9 | Unknown | 28.83 | 563 | 270, 336 | 563, 503, 473, 353, 443 |
| 10 | Coumaric acid | 29.59 | 163 | 308 | 163, 119 |
| 11 | Unknown | 29.64 | 609 | 264, 340 | 609, 447, 327 |
| 12 | Vitexin-4-O-glucoside | 30.34 | 593 | 267, 336 | 593, 431, 311, 353, 383 |
| 13 | Vitexin | 30.39 | 431 | 370, 336 | 431, 311 |
| 14 | Ferulic acid | 31.41 | 193 | 323 | 193 |
| 15 | Quercetin-3-O-rhamnoside | 35.24 | 447 | 255, 347 | 447, 447, 301 |
| 16 | Unknown | 35.96 | 607 | 271, 334 | 607, 445, 383 |
| 17 | Isovitexin-2-O-rhamnoside | 36.88 | 577 | 273, 331 | 577, 341, 322, 293 |
| 18 | Kaempferol-3-O-rhamnoside | 37.96 | 431 | 264, 346 | 431, 285 |
| 19 | Unknown | 38.84 | 721 | 271, 332 | 445, 283 |
Table 1: Characterization of Phenolic Compounds from EtOAc and n-BuOH Extracts From The Plant E. altissima
| Phenolic compounds | Content mg/g DW | |||
|---|---|---|---|---|
| EtOAc | PE | n-BuOH | Total content | |
| Gallic acid | 3.9±0.1 | ND | ND | 3.9±0.1 |
| 6 % | ||||
| Protocatechuic acid glucoside | ND | ND | 2.8±0.4 | 2.8±0.4 |
| 2 % | ||||
| Protocatechuic acid | 11.8±0.1 | ND | ND | 11.8±0.1 |
| 17 % | ||||
| Ferulic acid glucoside | ND | ND | 1.7±0.1 | 1.7±0.1 |
| 1 % | ||||
| P-hydroxybenzoic acid | 3.5±0.2 | ND | ND | 3.5±0.2 |
| 5 % | ||||
| Vicenin II | ND | Trace | 24.4±0.1 | 24.4±0.1 |
| 20 % | ||||
| P-hydroxybenzaldehyde | 2.1±0.1 | ND | ND | 2.1±0.1 |
| 3 % | ||||
| Luteolin-6-C-glucoside | ND | ND | 4.4±0.2 | 4.4±0.2 |
| (Isoorientin) | 4 % | |||
| Coumaric acid | 2.7±0.2 | ND | ND | 2.7±0.2 |
| 4 % | ||||
| Vitexin-4-O-glucoside | ND | ND | 4.3±0.2 | 4.3±0.2 |
| 3 % | ||||
| Vitexin | 2.5±0.2 | ND | ND | 2.5±0.2 |
| 4 % | ||||
| Ferulic acid | 4.6±0.1 | ND | ND | 4.6±0.1 |
| 7 % | ||||
| Quercetin-3-O-rhamnoside | 6.8±0.4 | ND | ND | 6.8±0.4 |
| 10 % | ||||
| Isovitexin-2-O-rhamnoside | 5.1±0.2 | ND | 41.6±0.2 | 51.9±0.8 |
| 7 % | 34 % | |||
| Kaempferol-3-O-rhamnoside | 14.8±0.4 | ND | ND | 14.8±0.4 |
| 22 % | ||||
| Total phenolic compounds | 68.7±1.6b | - | 123.1±0.9c | 191.8±3.2 |
Note: Data mg/g of EtOAc, PE and n-BuOH extracts are the mean of three replicates. Results correspond to the mean±SD of three replicates; (b,c)Different letters within the same row mean that there are significant differences (p<0.05); ND: Not Detected
Table 2: Content of Selected Individual and Total Phenolic Compounds in Crude Extracts of E. altissima
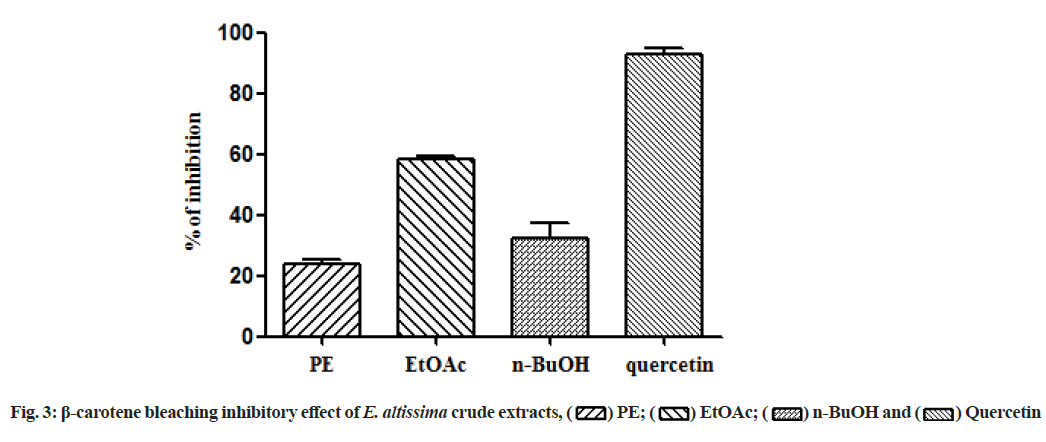
In the present study, all the tested extracts exhibited antioxidant activity in a dose-dependent manner (Table 3). The EtOAc extract showed the highest antioxidant activity in the DPPH radical scavenging and total antioxidant activities with values of 21.0±0.001 μg/ml and 19.2±0.002 μg EAA/mg of extract, respectively. The results of the FTC assay revealed that PE, EtOAc and n-BuOH extracts had an antioxidant potential to inhibit lipid peroxidation with values of 33.9 %, 34.9 % and 31.9 %, respectively. These values are close to BHA (41.9 %) and quercetin (42.1 %) but lower than those of Butylated Hydroxytoluene (BHT) (87.3 %) and ascorbic acid (61.9 %) as standards. In the H2O2 scavenging test, the highest percentage of inhibition was found in the n-BuOH extract (52.2 %) compared to the ascorbic acid as reference (62.4 %) at the concentration of 100 μg/ml (Table 3). According to the results of β-carotene bleaching test illustrated in fig. 3, the EtOAc extract has the greatest antioxidant activity, followed by n-BuOH and PE extracts.
In Table 3, DPPH: DPPH radical scavenging activity; H2O2: Hydrogen peroxide scavenging assay; β-carotene: β-carotene bleaching activity; FTC: Ferric thiocyanate capacity; TAC: Total Antioxidant Capacity; BHA: Butylated Hydroxyanisole; BHT: Butylated Hydroxytoluene; PE: Petroleum Ether; EtOAc: Ethyl Acetate; n-BuOH: n-Butanol; μg EAA/mg of extract: μg equivalents of ascorbic acid per mg of dry extract.
| Antioxidant activities | |||||
|---|---|---|---|---|---|
| Extracts and standards | DPPH assay | β-carotene assay | TAC assay | H2O2 assay | FTC assay |
| IC50 (µg/ml) | EAA % | µg EAA/mg of extract | % inhibition | ||
| PE | 144.0±0.008d | 23.8±1.63a | 8.8±0.03a | 11.17±0.67b | 33.9±3.71a |
| EtOAc | 21.0±0.001b | 58.3±1.10c | 19.2±0.002c | 2.1±0.50a | 34.9±2.31a |
| n-BuOH | 94.0±0.002c | 32.3±5.21b | 12.5±0.05b | 52.2±0.67c | 31.9±1.90a |
| BHA | 3.13±0.12a | 93.3±1.46d | NT | NT | 41.9 ±0.44b |
| BHT | 3.24±0.26a | NT | NT | NT | 87.3±0.27d |
| Ascorbic acid | 3.15±0.05a | NT | NT | 62.4±1.26d | 61.9±1.26c |
| Quercetin | NT | 92.9±1.75d | NT | NT | 42.1±0.25b |
Note: Results correspond to the mean±SD of three replicates. (a, b, c, d)Different letters within the same column mean that there are significant differences (p<0.05). ND: Not Detected and NT: Not Tested
Table 3: Antioxidant Activities of Crude Extracts From E. altissima
In pancreatic lipase inhibitory activity, the results showed that all the tested extracts displayed activity in a dose-dependent manner. Indeed, the EtOAc extract had the best anti-lipase effect with an IC50 value of 289.1±0.53 μg/ml, followed by n-BuOH and PE extracts (Table 4).
The ability of E. altissima extracts to induce the inhibition of α-amylase enzyme in vitro is presented in Table 4. All the tested extracts inhibited α-amylase in a dose-dependent manner. In fact, the EtOAc extract displayed the strongest anti-amylase activity with an IC50 value of 8.07±0.15 μg/ml, followed by n-BuOH extract (IC50 at 14.7±0.003 μg/ml), compared with acarbose (6.4±0.24 μg/ml) as standard. The PE extract exhibited the lowest α-amylase inhibitory activity with a percentage of inhibition at 44.0 % at the concentration of 1000 μg/ml.
| Pancreatic lipase and α-amylase inhibitory activities and anti-inflammatory activity | |||
|---|---|---|---|
| Extracts and standards | Pancreatic lipase inhibitory activity IC50 (µg/ml) | α-amylase inhibitory activity IC50 (µg/ml) | Anti-inflammatory activity IC50 (µg/ml) |
| PE | 525.1±2.05c | ND | ND |
| EtOAc | 289.1±0.53a | 8.07±0.15b | 126.4±2.36b |
| n-BuOH | 327.5±1.18b | 14.7±0.003c | 237.6±4.29c |
| Acarbose | NT | 6.4±0.24a | NT |
| Ibuprofen | NT | NT | 33.6±1.47a |
Note: Results correspond to the mean±SD of three replicates. (a, b, c)Different letters within the same column mean that there are significant differences (p<0.05); ND: Not Detected and NT: Not Tested
Table 4: Anti-Inflammatory Properties, α-Amylase and Pancreatic Lipase Inhibitory Activities of Crude Extracts from E. altissima
All the extracts of E. altissima revealed antiinflammatory activity by the inhibition of BSA denaturation that varied in a dose-dependent manner (fig. 4). The highest anti-inflammatory effect was observed in EtOAc extract with a value of IC50 at 126.4±2.36 μg/ ml, followed by n-BuOH and PE extracts (Table 4). In addition, this activity was lower than that of ibuprofen as a standard drug (IC50: 33.6±1.47).
The identification of the individual phenolic compounds in crude extracts prepared from the species of E. altissima was performed by comparison of the UVVis absorption spectra and mass spectrum with the results of the literature data as well as by comparison with commercial standards. The phenolic acids 1, 3, 5, 7, 10 and 14 were identified respectively as gallic acid, protocatechuic acid, p-hydroxybenzoic acid, p-hydroxybenzaldehyde, coumaric acid and ferulic acid by comparison with commercial standards. Indeed, coumaric acid[17,18], p-hydroxybenzoic and protocatechuic acids[17,19] were previously described in E. alata, E. aphylla and E. equisetina. Peak 2 showed UV maxima absorption at ≈259 nm and 294 nm and a precursor ion at m/z 315 [M-H]− in the negative ionization mode. This precursor ion was fragmented into product ion at m/z 153 (protocatechuic acid), produced by the loss of hexose moiety (162 units), based on comparison of absorption spectra (257, 291 nm) and [M-H]− value (m/z 315) with that reported by Chen et al.[20], peak 2 was then identified as protocatechuic acid glucoside. In addition, peak 4 ([M-H]− at m/z 355, Rt=17.79 min) presented 162 units (glycoside moiety) higher than compound 14, therefore being established as ferulic acid glucoside, which was previously reported in Lingonberry by Ek et al.[21]. Ferulic and protocatechuic acid glucosides were described for the first time in Ephedra plants. The remaining compounds were identified as flavonoids, such as flavonols (quercetin and kaempferol derivatives) and flavones (apigenin and luteolin derivatives). Compounds 6, 12, 13 and 17 were identified as apigenin derivatives. Compound 13 displayed the [M-H]− ion at m/z 431 and the MS spectrum showed typical fragment ions of mono-Cglycosides as they exhibited fragment ions at m/z 311 corresponding to the loss of 120 units. This compound (13) was identified as apigenin-8-C-glucoside (vitexin) by comparison with a commercial standard. Vitexin was also identified in E. campylopoda stem extracts[22].
Comparing with vitexin, compound 12 with a precursor ion at m/z 593 and a daughter ion at m/z 431, was identified as vitexin 4''-O-glucoside, which was previously reported for hawthorn leaves[23]. Compound 17 presented a molecular ion peak at m/z 577 ([MH]− and daughter fragments at m/z 341 and 323 corresponding to the loss of a rhamnose molecule (146), a 90 amu moiety and water (18), which are characteristic of 6-C-glucoside-O-rhamnoside derivatives[24]. It was identified by comparison with a commercial standard as apigenin-6-C-glucoside-2-rhamnoside (isovitexin-2- O-rhamnoside). The MS/MS spectrum of compound 6 in negative ion mode showed a precursor ion at m/z 593 [M-H]− which produced daughter ions at m/z 575 [(MH)- 18]− and 503 [(M-H)-90]− and a base peak at m/z 473 [(M-H)-120]−, exhibiting a fragmentation pattern typical of flavones di-C-glycoside. The ions at m/z 353 [(M-H)-(120+120)]− and 383 [(M-H)-(90+120)]− indicated the presence of apigenin (Molecular Weight (MW): 270) as aglycone and two hexose moieties (glucoses)[24]. It was identified as apigenin-6,8-di-Cglucoside (vicenin II) by comparison with an authentic standard. Compound 8 exhibited [M-H]− ion at m/z 447 with UV maxima at ≈269 and 347 nm. Based on the literature data, it was characterized as luteolin-6-Cglucoside (isoorientin). The presence of a fragment ion at m/z 429 [(M-H)-18]− is characteristic of isoorientin and absent in orientin[24]. Compound 15 was a quercetin derivative (λmax: 255, 347 nm and a MS2 fragment at m/z 301), quercetin-3-O-rhamnoside, while compound 18 was a kaempferol one (λmax: 264, 346 nm and an MS2 fragment at m/z 285), kaempferol-3-O-rhamnoside, both of them identified by comparison with commercial standards.
The total phenolic content of ethanol extract (191.8 mg/g extract) was close to that reported in the plant E. alata (240 mg/g extract)[25]. In fact, variations in the results of total phenolic contents may depend on several factors such as soil characteristics, period and area of harvest, plant storage conditions and quantification methods[26]. To the best of our knowledge, this is the first report on the identification and quantification of different phenolic compounds including phenolic acids, C-flavonoids and O-flavonoids in E. altissima extracts. In the n-BuOH extract, isovitexin-2-O-rhamnoside was the main compound which represents 34 % of the total phenolic compounds followed by vicenin II (20 %). For the other phenolic compounds, the n-BuOH extract contained between 1 % and 8 % of phenols. However, kaempferol-3-O-rhamnoside (22 %) was the predominant compound in the EtOAc extract. In addition, protocatechuic acid (17 %) and quercetin- 3-O-rhamnoside (10 %) were also detected with high contents of phenolic compounds. Moreover, isovitexin- 2-O-rhamnoside (7 %), ferulic acid (7 %), gallic acid (6 %), p-hydroxybenzoic acid (5 %), coumaric acid (4 %), vitexin (4 %) and p-hydroxybenzaldehyde (3 %) were present in the EtOAc extract in small amounts. Furthermore, the presence of apigenin derivatives, such as isovitexin-2-O-rhamnoside and vicenin II, has been reported in the species of E. aphylla[17]. Otherwise, the flavonol glycosides kaempferol-3-O-rhamnoside and quercetin-3-O-rhamnoside were identified previously in E. alata[27].
The antioxidant activities of E. altissima extracts were evaluated using five different methods based on different mechanisms of action including the scavenging of free radicals, reductive capacity and inhibition of lipid peroxidation. The good antioxidant activity of EtOAc extract in most of the tested methods may be associated with the presence of phenolic compounds detected in this plant. Indeed, the number and position of hydroxyl groups in flavonoid structures and the synergistic effects of various bioactive compounds could greatly increase the potency of crude extracts. Studies focused on the antioxidant activity by DPPH of the species from the genus Ephedra such as E. intermedia, E. procera, E. pachyclada and E. sarcocarpa and E. chilensis[27-31] showed differences in their potential. Also, the results of the antioxidant capacity from the plant E. altissima reported previously by Rached et al.[32] indicated a higher activity (IC50 at 12.0±0.23 μg/ml) compared to the results of our study. The observed variations in the results of the antioxidant activity of extracts from the same species are probably due to the chemical composition, the amount of the secondary metabolites and the used solvents in the extraction procedures.
The observed anti-lipase activity of E. altissima could be attributed to the presence of chemical compounds such as flavonoids and polyphenols known for their ability to inhibit the enzymatic activity of pancreatic lipase. In fact, several phenolic acids (p-coumaric and protocatechuic) and flavonoids (rutin and quercetin) previously isolated from the Ephedra taxa were considered as great inhibitors of pancreatic lipase[17,18]. Several studies reported on species of the genus Ephedra exhibited a potential capacity to promote weight loss by efficiently decreasing body weight, fasting glucose and insulin levels in healthy overweight and obese populations[33]. The administration of E. sinica reduced body weight gain, total visceral weight and food intake[34]. E. herba significantly reduced fat percentage, total cholesterol and triglyceride levels[35]. The inhibitory effect of E. altissima extracts on lipase may be due to the combined actions of the various phenolic acids which cause synergistic effects. Studies by Moreno et al.[36] have shown that there are synergistic effects when two or more polyphenols are combined. To prove such synergistic effects Cai et al.[37] tested the lipase inhibition interactions of each binary combination of ferulic, p-coumaric and caffeic acids. Synergy was observed, especially at high concentrations. However, the interactions of binary combinations of these phenolic acids were additive rather than synergistic when they were tested at low concentrations. Previous research conducted on species of the genus Ephedra indicated the effectiveness of these species in the treatment of obesity-related type 2 diabetes. E. alata and E. foliata possessed antioxidant and anti-diabetic activities[38], and E. distachya showed a remarkable anti-diabetic effect[39].
The observed α-amylase inhibitory activity could be due to the antioxidant potential of the extracts. Indeed, several studies showed that antioxidant activities correlated reasonably with the antidiabetic effects and that the effectiveness of anti-diabetic drugs increased when they were associated with antioxidants[6]. E. altissima extracts exhibited a mild inhibitory activity against α-amylase in agreement with an earlier study showing that plant phytochemical compounds are mild inhibitors of α-amylase and strong inhibitors of α-glucosidase activity, a property that confers a benefit over synthetic drugs that strongly inhibit α-amylase[16]. It has been suggested that side effects such as abdominal distention, flatulence, meteorism and possibly diarrhea could be caused by the excessive inhibition of pancreatic α-amylase resulting in the abnormal bacterial fermentation of undigested carbohydrates in the colon[16]. Food-grade phenolic α-amylase inhibitors derived from dietary plant extracts are potentially safer and can be a preferred alternative to modulate the digestion of carbohydrates and to control the glycemic index of food products.
Protein denaturation is a pathological process that causes inflammation. Most proteins lose their biological functions by the alteration of their tertiary and secondary structures. This usually occurs when proteins are exposed to external stress such as heat and a strong acid or a base[40]. In vivo protein denaturation occurs in the case of an autoimmune inflammatory process by the production of auto-antigens inducing a multitude of diseases including rheumatoid arthritis, diabetes and obesity[41]. Indeed, it is proven that anti-inflammatory drugs inhibit not only the synthesis of pro-inflammatory mediators but also the denaturation of proteins. However, these drugs usually have many adverse side effects[42]. Therefore, it is necessary to search for new alternative substances in natural sources that prevent the denaturation of proteins and constitute effective anti-inflammatory drugs with few side effects[43]. In the present study, the crude extracts prepared from the species, E. altissima moderately prevent the BSA denaturation compared to the reference drug. The observed anti-inflammatory activity of E. altissima extracts could be due to its chemical composition, mainly phenolic compounds. These metabolites are recognized for their anti-inflammatory properties by reducing the arthritis index and decreasing Interleukin 1 beta (IL-1β) and Tumor Necrosis Factor alpha (TNF-α) level in inflamed arthritic rat tissues[44]. However, the antioxidant power of the different extracts from this plant can increase the importance of the obtained results. Indeed, several studies have identified oxidative stress as the leading cause of chronic inflammation and autoimmune diseases[45]. Thus, the fight against oxidative stress can prevent the onset of this disease and reduce the severity of the observed symptoms. It can be concluded that the species E. altissima, is a rich source of polyphenols that has both α-amylase and pancreatic lipase inhibitory activities and therefore the use of its extracts may be a good strategy for treating obesity-related type 2 diabetes.
Acknowledgements:
The authors wish gratefully to express thanks to the Directorate General for Scientific Research and Technology Development (DGRSDT) of the Algerian Ministry of Higher Education and Scientific Research for PRFU project (B00L01UN050120180001), and the Ministry of Science and Innovation of Spain (AGL2017-82428-R).
Conflict of interests:
The authors declared no conflict of interest.
References
- Hameed I, Masoodi SR, Mir SA, Nabi M, Ghazanfar K, Ganai BA. Type 2 diabetes mellitus: From a metabolic disorder to an inflammatory condition. World J Diabetes 2015;6(4):598-612.
[Crossref] [Google scholar] [PubMed]
- Deshpande MC, Venkateswarlu V, Babu RK, Trivedi RK. Design and evaluation of oral bioadhesive controlled release formulations of miglitol, intended for prolonged inhibition of intestinal α-glucosidases and enhancement of plasma glucagon like peptide-1 levels. Int J Pharm 2009;380(1):16-24.
[Crossref] [Google scholar] [PubMed]
- Tucci SA, Boyland EJ, Halford JC. The role of lipid and carbohydrate digestive enzyme inhibitors in the management of obesity: A review of current and emerging therapeutic agents. Diabetes Metab Syndr Obes 2010;3:125-43.
[Crossref] [Google scholar] [PubMed]
- Agarwal P, Gupta R. Alpha-amylase inhibition can treat diabetes mellitus. Res Rev J Med Health Sci 2016;5(4):1-8.
- Bashary R, Vyas M, Nayak SK, Suttee A, Verma S, Narang R, et al. An insight of alpha-amylase inhibitors as a valuable tool in the management of type 2 diabetes mellitus. Curr Diabetes Rev 2020;16(2):117-36.
[Crossref] [Google scholar] [PubMed]
- Mehta JL, Rasouli N, Sinha AK, Molavi B. Oxidative stress in diabetes: A mechanistic overview of its effects on atherogenesis and myocardial dysfunction. Int J Biochem Cell Biol 2006;38(5-6):794-803.
[Crossref] [Google scholar] [PubMed]
- Arulselvan P, Fard MT, Tan WS, Gothai S, Fakurazi S, Norhaizan ME, et al. Role of antioxidants and natural products in inflammation. Oxid Med Cell Longev 2016;2016:1-15.
[Crossref] [Google scholar] [PubMed]
- Christenhusz MJ, Byng JW. The number of known plants species in the world and its annual increase. Phytotaxa 2016;261(3):201-17.
- Pirbalouti AG, Amirmohammadi M, Azizi S, Craker L. Healing effect of hydro-alcoholic extract of Ephedra pachyclada Boiss. in experimental gastric ulcer in rat. Acta Pol Pharm 2013;70(6):1003-9.
[Google scholar] [PubMed]
- Quezel P, Santa S. New flora of Algeria and southern desert regions. Paris: National Center for Scientific Research; 1963.
- Hammiche V, Maiza K. Traditional medicine in Central Sahara: Pharmacopoeia of Tassili N’ajjer. J Ethnopharmacol 2006;105(3):358-67.
[Crossref] [Google scholar] [PubMed]
- Idm’hand E, Msanda F, Cherifi K. Ethnopharmacological review of medicinal plants used to manage diabetes in Morocco. Clin Phytoscience 2020;6(1):1-32.
- Alam MN, Bristi NJ, Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J 2013;21(2):143-52.
[Crossref] [Google scholar] [PubMed]
- Karthik K, Kumar BRP, Priya VR, Kumar SK, Rathore RSB. Evaluation of anti-inflammatory activity of Canthium parviflorum by in vitro method. Indian J Res Pharm Biotechnol 2013;1(5):729-31.
- Kim J, Jang DS, Kim H, Kim JS. Anti-lipase and lipolytic activities of ursolic acid isolated from the roots of Actinidia arguta. Arch Pharm Res 2009;32(7):983-7.
[Crossref] [Google scholar] [PubMed]
- Kwon YI, Apostolidis E, Kim YC, Shetty K. Health benefits of traditional corn, beans, and pumpkin: In vitro studies for hyperglycemia and hypertension management. J Med Food 2007;10(2):266-75.
[Crossref] [Google scholar] [PubMed]
- Hussein SA, Barakat HH, Nawar MA, Willuhn G. Flavonoids from Ephedra aphylla. Phytochemistry 1997;45(7):1529-32.
- Nawwar MA, Barakat HH, Buddrust J, Linscheidt M. Alkaloidal, lignan and phenolic constituents of Ephedra alata. Phytochemistry 1985;24(4):878-9.
- Chumbalov TK, Chekmeneva LN, Polyakov VV. Phenolic acids of Ephedra equisetina. Chem Nat Compd 1977;13(2):238-9.
- Chen HJ, Inbaraj BS, Chen BH. Determination of phenolic acids and flavonoids in Taraxacum formosanum Kitam by liquid chromatography-tandem mass spectrometry coupled with a post-column derivatization technique. Int J Mol Sci 2011;13(1):260-85.
[Crossref] [Google scholar] [PubMed]
- Ek S, Kartimo H, Mattila S, Tolonen A. Characterization of phenolic compounds from lingonberry (Vaccinium vitis-idaea). J Agric Food Chem 2006;54(26):9834-42.
[Crossref] [Google scholar] [PubMed]
- Kallassy H, Fayyad-Kazan M, Makki R, Yolla EM, Hamade E, Rammal H, et al. Chemical composition, antioxidant, anti-inflammatory, and antiproliferative activities of the plant Lebanese Crataegus azarolus L. Med Sci Monit Basic Res 2017;23:270-84.
- Zhang W, Xu M, Yu C, Zhang G, Tang X. Simultaneous determination of vitexin-4″-O-glucoside, vitexin-2″-O-rhamnoside, rutin and vitexin from hawthorn leaves flavonoids in rat plasma by UPLC–ESI-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 2010;878(21):1837-44.
[Crossref] [Google scholar] [PubMed]
- Hassan WH, Abdelaziz S, Al Yousef HM. Chemical composition and biological activities of the aqueous fraction of Parkinsonea aculeata L. growing in Saudi Arabia. Arab J Chem 2019;12(3):377-87.
- Ziani BE, Heleno SA, Bachari K, Dias MI, Alves MJ, Barros L, et al. Phenolic compounds characterization by LC-DAD-ESI/MSn and bioactive properties of Thymusalgeriensis Boiss. and Reut. and Ephedra alata Decne. Food Res Int 2019;116:312-9.
[Crossref] [Google scholar] [PubMed]
- Ibragic S, Sofić E. Chemical composition of various Ephedra species. Bosn J Basic Med Sci 2015;15(3):21-7.
[Crossref] [Google scholar] [PubMed]
- Nawwar MA, El-Sissi HI, Barakat HH. Flavonoid constituents of Ephedra alata. Phytochemistry 1984;23(12):2937-9.
- Grippo AA, Capps K, Rougeau B, Gurley BJ. Analysis of flavonoid phytoestrogens in botanical and ephedra-containing dietary supplements. Ann Pharmacother 2007;41(9):1375-82.
[Crossref] [Google scholar] [PubMed]
- Parsaeimehr A, Sargsyan E, Javidnia K. A comparative study of the antibacterial, antifungal and antioxidant activity and total content of phenolic compounds of cell cultures and wild plants of three endemic species of Ephedra. Molecules 2010;15(3):1668-78.
[Crossref] [Google scholar] [PubMed]
- Gul R, Jan SU, Faridullah S, Sherani S, Jahan N. Preliminary phytochemical screening, quantitative analysis of alkaloids and antioxidant activity of crude plant extracts from Ephedra intermedia indigenous to Balochistan. Sci World J 2017;2017:1-7.
- Mellado M, Soto M, Madrid A, Montenegro I, Jara-Gutiérrez C, Villena J, et al. In vitro antioxidant and antiproliferative effect of the extracts of Ephedra chilensis K Presl aerial parts. BMC Complement Altern Med 2019;19(1):1-10.
[Crossref] [Google scholar] [PubMed]
- Rached W, Benamar H, Bennaceur M, Marouf A. Screening of the antioxidant potential of some Algerian indigenous plants. J Biol Sci 2010;10(4):316-24.
- Hackman RM, Havel PJ, Schwartz HJ, Rutledge JC, Watnik MR, Noceti EM, et al. Multinutrient supplement containing ephedra and caffeine causes weight loss and improves metabolic risk factors in obese women: A randomized controlled trial. Int J Obes 2006;30(10):1545-56.
[Crossref] [Google scholar] [PubMed]
- Kim BS, Song MY, Kim H. The anti-obesity effect of Ephedra sinica through modulation of gut microbiota in obese Korean women. J Ethnopharmacol 2014;152(3):532-9.
[Crossref] [Google scholar] [PubMed]
- Lee SE, Lim C, Lim S, Lee B, Cho S. Effect of Ephedrae herba methanol extract on high-fat diet-induced hyperlipidaemic mice. Pharm Biol 2019;57(1):676-83.
[Crossref] [Google scholar] [PubMed]
- Moreno DA, Ilic N, Poulev A, Brasaemle DL, Fried SK, Raskin I. Inhibitory effects of grape seed extract on lipases. Nutrition 2003;19(10):876-9.
[Crossref] [Google scholar] [PubMed]
- Cai S, Wang O, Wang M, He J, Wang Y, Zhang D, et al. In vitro inhibitory effect on pancreatic lipase activity of subfractions from ethanol extracts of fermented oats (Avenasativa L.) and synergistic effect of three phenolic acids. J Agric Food Chem 2012;60(29):7245-51.
[Crossref] [Google scholar] [PubMed]
- Al-Snafi AE. Therapeutic importance of Ephedra alata and Ephedra foliata-A review. Indo Am J Pharm Sci 2017;4(2):399-406.
- Chauhan A, Sharma PK, Srivastava P, Kumar N, Dudhe R. Plants having potential antidiabetic activity: A review. Der Pharm Lett 2010;2(3):369-87.
- Ingle PV, Patel DM. C-reactive protein in various disease condition-an overview. Asian J Pharm Clin Res 2011;4(1):9-13.
- Sangeetha G, Vidhya R. In vitro anti-inflammatory activity of different parts of Pedaliummurex (L.). Int J Herb Med 2016;4(3):31-6.
- Chatterjee P, Chandra S, Dey P, Bhattacharya S. Evaluation of anti-inflammatory effects of green tea and black tea: A comparative in vitro study. J Adv Pharm Technol Res 2012;3(2):136-8.
- Ghasemian M, Owlia S, Owlia MB. Review of anti-inflammatory herbal medicines. Adv Pharmacol Sci 2016;2016:1-11.
[Crossref] [Google scholar] [PubMed]
- Rahman H, Eswaraiah MC, Dutta AM. In vitro anti-inflammatory and anti-arthritic activity of Oryza sativa Var. joha rice (an aromatic indigenous rice of Assam). Am Eurasian J Agric Environ Sci 2015;15(1):115-21.
- Del Buono M, Abbate A, Toldo S. Interplay of inflammation, oxidative stress and cardiovascular disease in rheumatoid arthritis. Heart 2018;104(24):1991-2.
[Crossref] [Google scholar] [PubMed]
 )PE; (
)PE; ( ) EtOAc; (
) EtOAc; ( )n-BuOH and (
)n-BuOH and ( ) Quercetin
) Quercetin 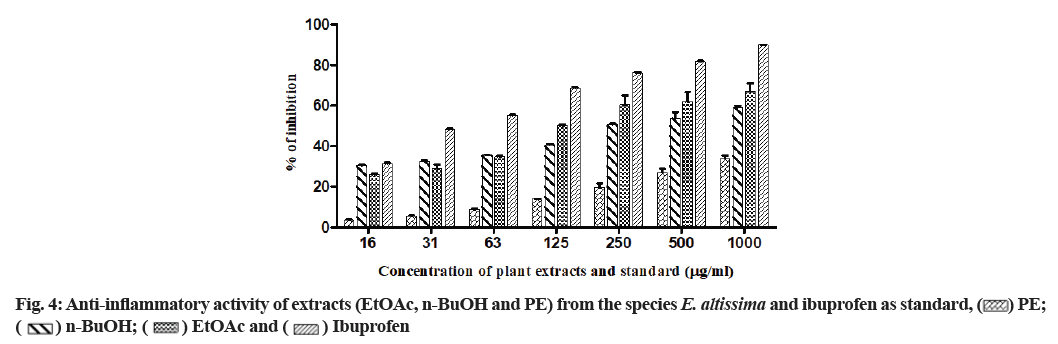
 ) PE;
(
) PE;
( ) n-BuOH; (
) n-BuOH; ( ) EtOAc and (
) EtOAc and ( ) Ibuporfen
) Ibuporfen