- *Corresponding Author:
- C. Ozay
Department of Biology, Faculty of Science and Literature, Turkey
E-mail: cennetozay@hotmail.com
| Date of Submission | 03 October 2016 |
| Date of Revision | 23 January 2017 |
| Date of Acceptance | 24 May 2017 |
| Indian J Pharm Sci 2017;79(4):585-590 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Plants of the Prospero genus have been used for promoting blood circulation, treating nervous conditions, infertility in women and rheumatic fever in the traditional folk medicine. The aim of present research was to investigate the phenolic composition, antioxidant and cytotoxic activities of the aerial and underground parts of Prospero autumnale. While β-carotene/linoleic acid, metal chelating and phosphomolybdenum assays were used for the determining antioxidant activity, Folin-Ciocalteu assay, aluminium colorimetric and vanillin-sulfuric acid method were used to detect total phenolic, flavonoid and saponin contents in the extracts, respectively. The phenolic compounds of P. autumnale were estimated by high performance liquid chromatography analysis. Nine phenolics were identified in the extract. Quercetin was the major constituent in the extract and is mainly responsible for biological activities observed. Aerial parts of the plant showed higher antioxidant activity than the underground parts and this activity may be related to a good total phenolic content. In vitro cytotoxic activity was determined by a luminometric method against non-small cell lung carcinoma cells. Underground parts of the plant exhibited more than fifty percent mortality on H1299 cells at a concentration of 500 µg/ml. This cytotoxicity could be due to the saponins, which have been suggested as possible anticarcinogens in plants. Therefore, further in depth studies are needed elucidate the mechanism involved in cytotoxic activity of P. autumnale extract along with isolation and identification of active principles.
Keywords
Antioxidant activity, Prospero autumnale, cytotoxicity, HPLC
Nature has been a source of traditional or modern medicinal products for thousands of years. Since ancient times, plants have been used by humans for treatment of various diseases and a great number of active ingredients of modern drugs have been isolated from natural sources. Especially, the plant-based, traditional medicine systems have become popular again because of the adverse effects of synthetic drugs [1].
Geophyte plants are those, which develop underground parts such as tubers, bulbs and rhizomes specifically to store nutrients. Eight hundred and sixteen geophyte species belonging to 73 genera have been registered in Flora of Turkey [2]. Scilla autumnalis L. is a synonym of Prospero autumnale (L.) Speta, an autumnal flowering plant of the family Asparagaceae, is found in the Mediterranean region from Portugal to Turkey and the Caucasus [3].
There has been no report on the possible medical uses of P. autumnale (as S. autumnalis) in the literature but it is known that Scilla species are widely used in folk medicine to treat different illness related to inflammation and pain. Phytochemical studies have demonstrated the presence of triterpene and triterpenoid saponins derived from alkaloids, eucosterol, stilbenoids and lignan in the plants of this genus. In relation to these substances, several bioactivities such as antioxidant, antitumor, antiinflammatory, cardioprotective and glycosidase inhibitory activities, have been reported [4-6]. S. scilloides has been used for a long time by traditional Chinese healers to treat abscesses and to promote circulation. The bulb extract has been evaluated for its potential as an antimicrobial agent, as an antiinflammatory and as an antioxidant [7]. Some genera of the Asparagaceae have been investigated, with the bulbs receiving most attention. Investigations into the chemistry of the bulbs of Scilla has yielded a large number of nortriterpenoids, cardiac glycosides and homoisoflavanones, many of which have been evaluated for biological activity [8-10].
While most of the studies represent the biological activities of underground parts (bulbs) of the P. autumnale, there are few studies about the aerial parts of this plant. In the light of all information mentioned above, the present study aimed to investigate the potential antioxidant and cytotoxic activities of aerial and underground parts of the ethanolic extract of P. autumnale. The phenolic compounds of aerial parts of the plant were also detected by reversed-phase high performance liquid chromatography (RP-HPLC) in this study.
Materials and Methods
P. autumnale was collected in October 2015 from Denizli, Turkey and authenticated in the Department of Biology, Pamukkale University, Denizli, Turkey. A voucher specimen (RM1002) has been deposited in the Herbarium of the Department of Biology. Aerial and underground parts of the plant were air-dried in shade at 25°, powdered to a fine grain and then extracted with 100 ml ethanol at 50° for 6 h in temperature controlled shaker [11]. The ethanol extract was filtered with Whatman filter paper (No. 1) and evaporated to remove the ethanol or dryness under vacuum at temperature below 50° using the rotary evaporator (IKA RV10D, Staufen, Germany). A minimum volume of distilled water was added to the dry ethanol extract and the resulting material was freeze-dried (Labconco FreeZone, Kansas City, MO). Obtained extracts were stored at –20° until use.
β-carotene/linoleic acid assay
β-carotene/linoleic acid assay was carried out by the method reported by Sokmen et al. [12]. A stock solution of β-carotene-linoleic acid mixture was prepared by dissolving 0.5 mg β-carotene in 1 ml chloroform. About 25 μl linoleic acid and 200 mg Tween 40 were added. Chloroform was completely evaporated using a vacuum evaporator. Then 100 ml of distilled water was added with vigorous shaking. Also, 2.5 ml of this reaction mixture was dispensed into test tubes and 350 μl portion (1 mg/ml) of the extract was added and the emulsion system was incubated for up 2 h at 50°. The same process was done again with synthetic antioxidant, butylated hydroxytoluene (BHT), as positive control, and a blank. The absorbance of the mixtures was measured at 490 nm after the incubation period, and inhibition ratio was calculated.
Total antioxidant capacity
The total antioxidant capacity of extract was assessed by phosphomolybdenum method according to Prieto et al. [13]. About 0.3 ml of extract solution (1 mg/ml) was mixed with 3 ml of reagent solution (6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The reaction mixture was incubated for 90 min at 95°. Then, the absorbance of the solution was measured at 695 nm against blank. The antioxidant capacity of extract was expressed as equivalents of ascorbic acid (mg AEs/g).
Metal chelating activity on ferrous ions
The metal chelating activity on ferrous (Fe+2) ions of the extract was estimated by the method previously reported by Aktumsek et al. [14]. Shortly, sample solution (2 ml) was added to FeCl2 solution (0.05 ml, 2 mM). The reaction was started immediately by adding 5 mM of ferrozine (0.2 ml). In the same way, a blank was prepared by adding sample solution (2 ml) to FeCl2 solution (0.05 ml, 2 mM) and water (0.2 ml) without ferrozine. Then, the sample and blank were left at room temperature for 10 min and the absorbance’s were measured at 562 nm. The metal chelating activity was expressed as equivalents of EDTA (mg EDTAs/g).
Total phenolic content
Total phenolic content of the extract was determined by Folin-Ciocalteu method [15] with a slight modification. About 1 ml of extract solution (1 mg) was added to 46 ml of distilled water and 1 ml of Folin-Ciocalteu reagent and was mixed properly. After 3 min, the mixture was added to 3 ml of sodium carbonate (2%) and shaken intermittently for 2 h. The absorbance was measured at 760 nm. Gallic acid was used as a standard for calibration curve. The total phenolic content was expressed as gallic acid equivalents (mg GAEs/g).
Total flavonoids content
Total flavonoids content of the extract was determined by aluminium colorimetric method according to Arvouet-Grand, et al. [16]. Briefly, 2000 μg (1 ml) extract was mixed with 1 ml of 2% aluminium trichloride (AlCl3) in methanol. Similarly, a blank was prepared by adding extract solution (1 ml) to methanol (1 ml) without AlCl3. After 10 min incubation at room temperature, the blank and extract absorbance’s were measured at 415 nm. The total flavonoids content was expressed as quercetin equivalents (mg QEs/g).
Total saponin content
Total saponin content was determined by the vanillinsulphuric acid method. The extracts were mixed with the same amount of vanillin (8%, w/v) and twice the amount of sulphuric acid (72%, w/v). The mixture was incubated at 60° for 10 min followed by cooling in an ice water bath for 15 min. Absorbance was measured at 535 nm. The total saponin content was expressed as equivalents of Quillaja (mg QAEs/g) [17].
Quantification of phenolic components by RPHPLC
Phenolic components were evaluated by RP-HPLC. Detection and quantification were carried out with a LC-20AT pump, a Diode Array Detector (SPD-M20A), a CTO-10ASVp column heater, SCL-10Avp system controller, DGU-14A degasser and SIL-10ACHT auto sampler. Separations were conducted at 30° on C-18 reversed-phase column (250×4.6 mm length, 5 μm particle size). The eluates were identified at 278 nm. The mobile phases were 3.0% formic acid in distilled water and methanol. Ethanol was used to dissolve samples, and then 20 μl of this solution was injected into the column. Phenolic composition of the extract was determined according to the method of Caponio et al. [18] with a slight modification. Kaempferol, quercetin, (-)- epicatechin, myricetin, quercetin-3-Oglucoside, caffeic acid, rutin, (+)- catechin, sinapic acid, ferulic acid, chlorogenic acid, gallic acid and vanillic acid (Sigma Chemical Co.) were used as standard. The differentiation and quantitative analysis were made by comparing the standards. The quantity of each phenolic compound was expressed as mg/g of the extract.
Cytotoxicity assay
H1299 cells were cultured in RPMI 1640 medium (Sigma Aldrich, St. Louis, MO, USA) at 37° in a CO2 incubator. When the cells were grown to about 90% confluence, the medium was aspirated. Cells were washed, trypsinized, counted with a hemocytometer, and seeded into 96-well plates (2×103 cells/well). After 24 h incubation, the medium was removed from the well leaving the adherent cells and cells were treated with plant extract in different concentrations (1000, 500, 250, 100, 50, 10 and 1 μg/ml) for 72 h. For the untreated control group, cells were not treated with any extracts. At the end of incubation time, medium was removed, and cytotoxicity of plant extract-treated and untreated control groups was determined by the luminometric method using a CytotoxGlo kit [22].
Results and Discussion
Antioxidant activities of the aerial and underground parts of P. autumnale were analysed by using β-carotene/ linoleic acid, metal chelating and phosphomolybdenum assays. β-carotene/linoleic acid bleaching test analysed by the power to neutralize the free radicals formed in the system, which attack the highly unsaturated β-carotene models [19] and these results are shown in Table 1. Aerial parts of the plant demonstrated higher antioxidant activity than underground parts with the inhibition value of 70.34% and 65.71%, respectively, which indicated the role of structural features of polyphenolic compounds with respect to their antioxidant potential. Inhibition rate of oxidation of linoleic acid of S. maritima bulbs was reported as 19.77%, which was lower than P. autumnale [20]. A previous study reported that ethanol extract of the bulbs of S. scilloides exhibited significant antioxidant activity. Antioxidant activity was assessed by monitoring the oxidation of linoleic acid and good activity was found (antioxidative index of 33.2 at a concentration of 1%) [7]. Data obtained from the phosphomolybdenum assay was found in correlation with those obtained from total phenolic assay. In phosphomolybdenum assay, aerial parts of the plant (55.22 mg AEs/g), showed higher antioxidant activity than the underground parts (47.15 mg AEs/g) (Table 2).
| Sample | ß-carotene/linoleic acid assay (%) |
Metal chelating activity (mg EDTAEs/g)a |
|---|---|---|
| Aerial part | 70.34±0.04b | 7.14±0.11 |
| Underground part | 65.71±0.03 | 5.03±0.07 |
| BHA | 92.04±0.10 | nt |
| BHT | 93.02±0.21 | nt |
aEDTAEs: EDTA equivalents, nt: no tested. bValues expressed are means ±SD
Table 1: Antioxidant activities of P. autumnale
| Material | TPCa | TFCb | TACc |
|---|---|---|---|
| Aerial part | 16.03±0.05d | 25.01±0.08 | 55.22±0.09 |
| Underground part | 10.01±0.04 | 15.34±0.02 | 47.15±0.08 |
aTotal phenolic content (TPC) expressed as gallic acid equivalents (mg GAEs/g; btotal flavonoid content (TFC) expressed as quercetin equivalents (mg QEs/g); ctotal antioxidant capacity (TAC) expressed as ascorbic acid equivalents (mg AEs/g); dvalues expressed are means±SD
Table 2: Total antioxidant capacity, total phenolic and flavonoid contents of P. autumnale
While natural bioactive compounds such as plant phenolics could reverse the pathological conditions, reactive oxygen species cause harmful effects leading to several disorders [21]. Total phenolics and flavonoids content were found to be maximum (16.03 mg GAEs/g, 25.01 mg QEs/g) in aerial part of the plant in relation to its antioxidant activity (Table 2). In a previous study, Scilla hanburyi was evaluated for the total phenolic constituents. Total phenolic contents of the aqueous and methanolic extracts of S. hanburyi were detected as 17.3 and 6.9 mg GAEs/g, respectively. The results of total phenolic contents demonstrated a uniform tendency to those of the antioxidant capacities. For this reason, the high content of total phenolics in the extract might be state the strong antioxidant capacity of P. autumnale. These results are in good agreement with previously published reports in the literature, which exhibited strong connection between antioxidant activities and total phenolic contents [22,23].
Plant extracts were also assessed for their metal chelating activities, which are linked to antioxidant capabilities. Metal chelating assay was based on the measurement of iron-ferrozine absorbance at 562 nm. The chelating activities of the extracts were established using EDTA as a standard (mg EDTAEs/g extract). In good agreement with results of other antioxidant assays, potent chelation capacities were again detected in the aerial parts of the plant with 7.14 mg EDTAEs/g (Table 1). Tripathi et al. [24] examined the antioxidant capacity of the bulb of S. indica in the way of its effect on ferrous sulphate-induced lipid peroxidation and concentration of reduced glutathione in mice liver homogenate and on hydroxyl radicals, superoxide radical scavenging and iron chelation in a chemical model. The results exhibited that the ethanolic bulb extract of S. indica inhibited lipid peroxidation and chelated transition metals in the body.
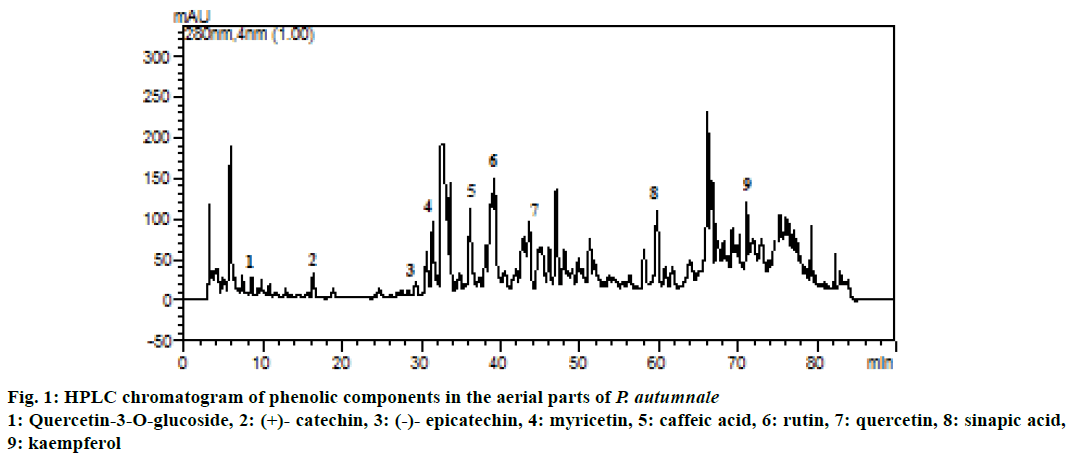
Phenolic components are a diverse group of phytochemicals widely distributed in the plant kingdom [25], therefore the phenolic components contained in the aerial parts of the P. autumnale ethanolic extract were characterized using HPLC. Out of the 13 standard phenolics analysed, 9 phenolic components were identified in the extract and listed in Table 3. Also, HPLC chromatograms of phenolic components in the P. autumnale were shown in Figure 1. Major phenolic components in the extract were determined as quercetin (2.33 mg/g extract) and caffeic acid (2.15 mg/g extract). These data indicated that the antioxidant activities of P. autumnale could be ascribed to their polyphenol components. Quercetin is considered to be a strong antioxidant due to its ability to scavenge free radicals and bind transition metal ions [26]. Therefore, high concentration of quercetin was thought to be responsible for the antioxidant activities performed. As a result, quercetin may aid in the prevention of certain diseases, such as cancer, atherosclerosis, and chronic inflammation [27]. A previous study reported that eighteen polyphenols were identified from extracts of the aerial and underground parts of S. bifolia (caftaric acid, isoquercitin, routine, myricetol, fistein, quercetol, patuletin, gentisic acid, caffeic acid, chlorogenic acid, p-coumaric acid, ferulic acid, hyperoside, quercitrin, luteolin, kaempferol, apigenin and sinapic acid) [28].
| Phenolic components | mg/g extract |
|---|---|
| Kaempferol | 1.02±0.10 |
| Quercetin | 2.33±0.19 |
| (-)- Epicatechin | 0.09±0.06 |
| Myricetin | 0.08±0.02 |
| Quercetin-3-O-glucoside | 0.02±0.02 |
| Caffeic acid | 2.15±0.08 |
| Rutin | 0.03±0.02 |
| (+)-Catechin | 0.07±0.05 |
| Sinapic acid | 0.44±0.07 |
| Ferulic acid | nda |
| Chlorogenic acid | nd |
| Gallic acid | nd |
| Vanillic acid | nd |
aValues not determined
Table 3: Phenolic components in the aerial parts of P. autumnale (mean±sd)
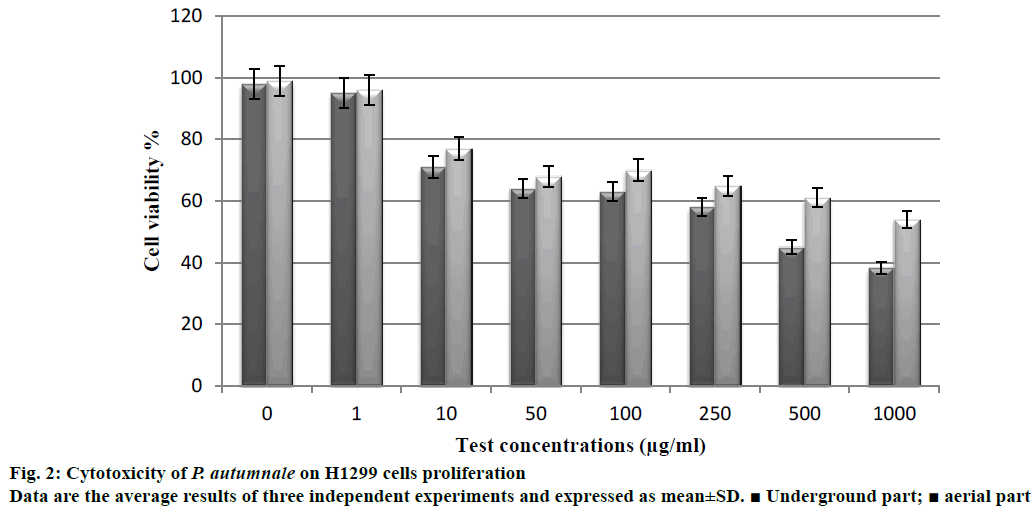
The extracts were evaluated for cytotoxicity against H1299 human non-small cell lung carcinoma cell line using the luminometric test. The underground parts of P. autumnale were found to be more cytotoxic than the aerial parts. Cytotoxicity of the underground parts on the proliferation of H1299 cell line was determined as 56.7% (Figure 2). The cell viability of the extract decreased with increasing the concentration. Saponins are natural glycosides, which possess a wide range of pharmacological properties including cytotoxic activity [29]. The saponin contents of the underground parts of P. autumnale extract were found as 148 mg QAEs/g, while the aerial parts of extract were 51 mg QAEs/g. In a previous study, the toxic effects of the aqueous extract obtained from the underground parts of S. nervosa in HepG2 liver cells, were investigated [30]. Results indicated that extracts of underground parts had cytotoxic activity on liver cells. An ethanolic extract of S. indica, has reportedly shown good inhibition of the Semliki Forest Virus and the active principal found to be a diosgenin saponin [31]. Sparg et al. [32] investigated anticancer activity of aqueous, ethanolic, dichloromethane and n-hexane extracts of Scilla natalensis. The phytochemical screening of underground parts of S. natalensis revealed the presence of saponins and bufadienolides within its underground parts [33]. It is tempting to speculate that the observed cytotoxic activity of the underground parts of P. autumnale is contributed to the presence of saponins.
Biological activity and chemical composition of P. autumnale have been performed for the first time in this study. In terms of the biological activity assays, antioxidant and cytotoxic activities of the extracts were exhibited. Aerial and underground parts of P. autumnale extracts possess remarkable biological properties. Traditional uses of some bulbous plant species, mostly belonging to the Asparagaceae, Amaryllidaceae and Hyacinthaceae could provide beneficial leads in novel pharmaceutical progressions. By reason of the pharmacological action and biochemical content of several of this species still remains poorly understood, future investigations should be a valuable aid in this respect.
Acknowledgements
The authors thank Professor Dr. R. Mammadov, Department of Biology, Pamukkale University for his support in authenticating the plant.
Conflicts of interest
There are no conflicts of interest
Financial support and sponsorship
Nil.
References
- Owolabi OJ, Omogbai EKI, Obasuyi O. Antifungal and antibacterial activities of the ethanolic and aqueous extract of Kigella africana (Bignoniaceae) stem bark. Afr J Biotechnol 2007;1677-80.
- Sargin SA, Selvi S, Akçiçek E. Investigations of ethnobotanical aspect of some geophytes growing in Alaşehir (Manisa) and surrounding area. Erciyes Univ J Ins Sci Technol 2013;29:170-77.
- Parker J, Lozano S, Taylor R, Rejón S, Ruiz M. Chromosomal structure of populations of Scilla autumnalis in the Iberian Peninsula. Heredity 1991;67:287-97.
- Lee SM, Chun HK, Lee CH, Min BS, Lee ES, Kho YH. Eucosterol oligoglycosides isolated from Scilla scilloides and their antitumor activity.Chem Pharm Bull 2002;50:1245-9.
- Asano N, Ikeda K, Kasahara M, Arai Y, Kizu H. Glycosidase-inhibiting pyrrolidines and pyrrolizidines with a long side chain in Scilla peruvian. J Nat Prod 2004;67:846-50.
- Senthilkumar B. Evaluation of cardioprotective and antioxidant activity phytoconstituents from Scilla hyacinthinaagainst doxorubicin-induced myocardial necrosis in albinorats. J Bioequiv Availab 2013;5:150.
- Yeo EJ, Kim KT, Han YS, Nah SY, Paik HD. Antimicrobial, antiinflammatory, and antioxidative activities of Scilla scilloides (Lindl.) Druce root extract. Food Sci Biotechnol 2006;15:639-42.
- Bezabih M, Famuyiwa SO, Abegaz BM. HPLC analysis and NMR identification of homoisoflavonoids and stilbenoids from the inter-bulb surfaces of Scilla nervosa. Nat Prod Commun 2009;4:1367-70.
- Mulholland DA, Schwikkard SL, Crouch NR. The chemistry and biological activity of the Hyacinthaceae. Nat Prod Rep 2013;30:1165-210.
- Fan MY, Wang YM, Wang ZM, Gao HM. Advances on chemical constituents and pharmacological activity of genus Scilla. China J Chinese Materia Medica 2014;39:162-70.
- Ozay C, Mammadov R, Tasdelen G, Karagur ER, Akca H. Potential antioxidant, antiproliferative and hepatoprotective effects of Crataegus meyeri. J Food Biochem2015;39:548-53.
- Sokmen A, Gulluce M, Akpulat HA, Daferera D, Tepe B, Polissiou M, et al. The in vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of endemic Thymus spathulifolius. Food Control 2004;15:627-34.
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphormolybdenum complex: specific application to the determination of vitamin E. Anal Biochem1999;269:337-41.
- Aktumsek A, Zengin, G, Guler GO, Cakma YS, Duran A. Antioxidant potentials and anticholinesterase activities of methanolic and aqueous extracts of three endemic Centaurea L. species. Food Chem Toxicol 2013;55:290-6.
- Slinkard K, Singleton VL. Total phenol analyses: automation and comparison with manual methods. Am J Enol Vitic1977; 28:49-55.
- Arvouet-Grand A, Vennat B, Pourrat A, Legret P. Standardization of propolis extract and identification of principal constituents. J Pharm Belg 1994;49:462-8.
- Hiai S, Oura H, Nakajima T. Color reaction of some sapogenins and saponins with vanillin and sulfuric acid. Planta Med 1976;29:116-22.
- Caponio F, Alloggio V, Gomes T. Phenolic compounds of virgin olive oil: influence of paste preperation techniques. Food Chem 1999;64:203-9.
- Barros L, Heleno SA, Carvalho AM, Ferreira ICFR. Systematic evaluation of the antioxidant potential of different parts of Foeniculum vulgare Mill. from Portugal. Food Chem Toxicol 2009;47:2458-64.
- Amessis-Ouchemoukh N, Madani K, Falé PLV, Serralheiro ML, Araújo MEM. Antioxidant capacity and phenolic contents of some Mediterranean medicinal plants and their potential role in the inhibition of cyclooxygenase-1 and acetylcholinesterase activities. Ind Crop Prod 2014;53:6-15.
- Kostyuk V, Potapovich A, De Luca C. The promise of plant polyphenols as the golden standard skin antiinflammatory agent. Curr Drug Metab 2010;11:414-24.
- Ozay C, Mammadov R. Assessment of some biological activities of Alyssum L. known as madwort. Acta Pol Pharm 2016;73:1213-20.
- Amudha M, Rani S. Evaluation of in vitro antioxidant potential of Cordia retusa. Indian J Pharm Sci 2016;78:80-6.
- Tripathi YB, Singh AV, Dubey GP. Antioxidant property of the bulb of Scilla indica. Curr Sci 2001;80:1267-9.
- Cartea ME, Francisco M, Soengas P, Velasco P. Phenolic compounds in brassica, vegetables. Molecules 2011;16:251-80.
- Salem Alrawaiq N, Abdullah A. A review of flavonoid quercetin: metabolism, bioactivity and antioxidant properties. Int J Pharm Tech Res 2014;6:933-41.
- Hollman PCH, Katan MB. Absorption, metabolism and health effects of dietary flavonoids in man. Biomed Pharmacother 1997;51:305-10.
- Baskaran P, Ncube B, Van Staden J. In vitro propagation and secondary product production by Merwilla plumbea (Lindl.) Speta. Plant Growth Regul 2012;67:235-45.
- Podolak I, Galanty A, Sobolewska D. Saponins as cytotoxic agents: a review. Phytochem Rev 2010;9:425-74.
- Pillay P, Phulukdaree A, Chuturgoon AA, Du Toit K, Bodenstein J. The cytotoxic effects of Scilla nervosa (Burch.) Jessop (Hyacinthaceae) aqueous extract on cultured HepG2 cells. J Ethnopharmacol 2013;145:200-4.
- Abid Ali Khan MM, Zaidi SNH, Musanna SA, Singh N. Identification of a new Semliki Forest Virus inhibitory diosgenin saponin from Scilla indica Baker plant,Asian J Exp Biol Sci 2012;3:476-80.
- Sparg SG, Van Staden J, Jager AK. Pharmacological and phytochemical screening of two Hyacinthaceae species: Scilla natalensis and Ledebouria ovatifolia. J Ethnopharmacol 2002;80:95-101.
- Waller GR, Yamasaki K. Saponins used in traditional and modern medicine series: Advances in experimental medicine and biology. New York: Springer US;1996. p. 606.