- *Corresponding Author:
- O. Goshain
Department of Pharmaceutical Chemistry, College of Pharmacy, Teerthanker Mahaveer University, Moradabad, Uttar Pradesh 244001
E-mail: omprakashgoshain@gmail.com
| Date of Received | 28 May 2021 |
| Date of Revision | 03 August 2021 |
| Date of Acceptance | 03 June 2022 |
| Indian J Pharm Sci 2022;84(3):703-711 |
Abstract
A series of hydrazones were synthesized by condensing substituted hydrazides with appropriate carbonyl compounds. The chemical structures of the synthesized compounds were confirmed by infrared, proton nuclear magnetic resonance, carbon-13 nuclear magnetic resonance, mass ((4-chloro-phenylamino)-acetic acid [1-(4-chlorophenyl)-phenyl-methylene]-hydrazide and (2,4-dichloro-phenylamino)-acetic acid (3-oxo- 1,3-dihydro-indole-2-ylidene)-hydrazide only) spectral data and carbon hydrogen nitrogen analysis. The title compounds were screened for their anticonvulsant activity against mice at doses of 30 mg/kg, 100 mg/kg and 300 mg/kg. The preliminary anticonvulsant screening data of this series resulted in the identification of two lead compounds (2,4-dichloro-phenylamino)-acetic acid (3-oxo-1,3-dihydro-indole- 2-ylidene)-hydrazide and (2,4-dichloro-phenylamino)-acetic acid [1-(4-chlorophenyl)-phenyl-methylene]- hydrazide with excellent preliminary anticonvulsant profile with no neurotoxicity in maximal electroshock seizure and subcutaneous metrazol mice model.
Keywords
Hydrazides, semicarbazones, hydrazones, anticonvulsant, molecular modelling
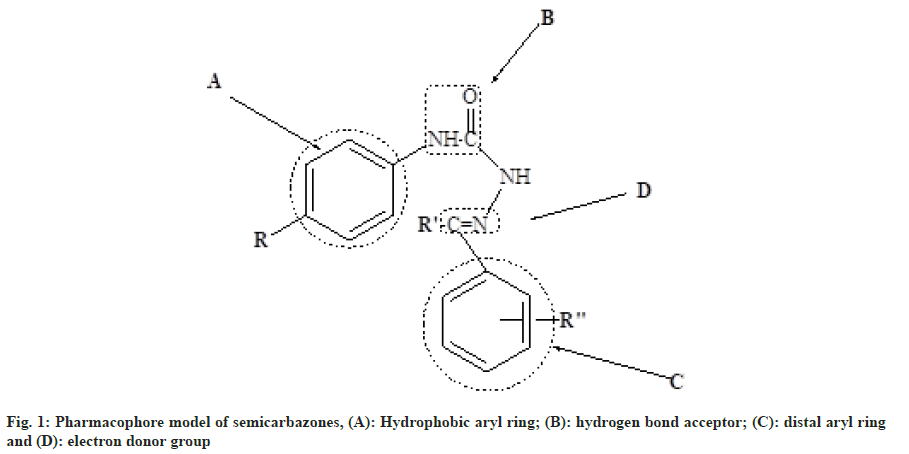
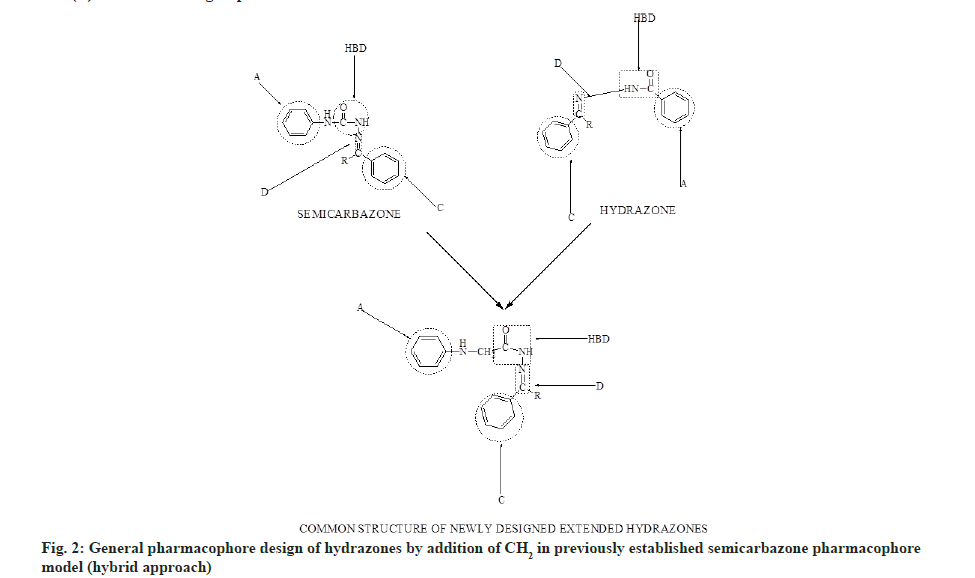
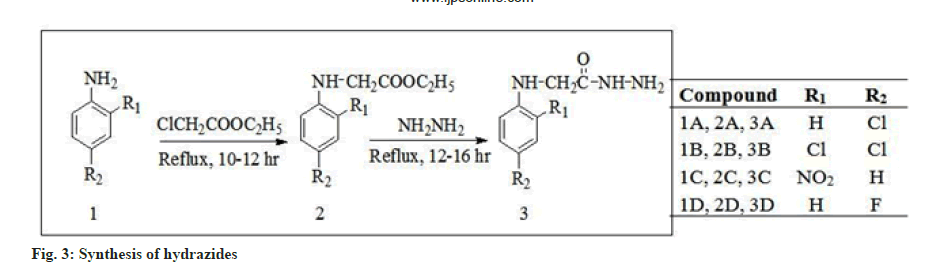
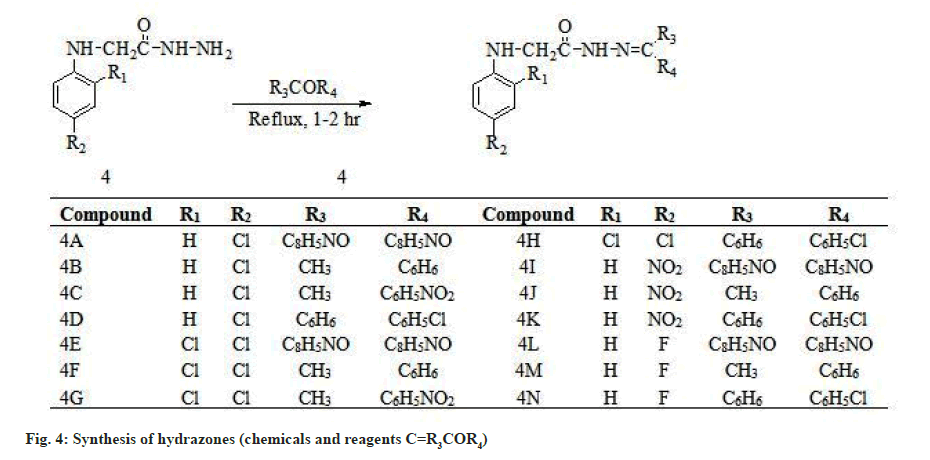
Hydrazone derivatives have been reported to possess a wide range of biological properties including antimicrobial[1-3], antitubercular[4-6], antifungal[3-7], anticancer[8,9], anticonvulsant[10] and anti-inflammatory[11] activities. The hydrazones were synthesized from aryl amines (para (p)-chloroaniline, 2,4-dichloroaniline, p-nitroaniline and p-fluoroaniline) hydrazides. Both semicarbazones and hydrazones were well documented as novel templates for development of newer anticonvulsants and they almost share the common structural features (common binding sites) which are present in the currently available drugs such as phenytoin, zonisamide and rufinamide[12,13]. In order to identify and optimize the pharmacophore model of semicarbazone[14] as shown in fig. 1, several structural modifications were attempted by incorporating the appropriate fragments to fit to the model. In the present work, we attempted to design newer region–specific structural analogs based on the semicarbazone pharmacophore by doing the following modifications: Introduction of -CH2 between B and NH in the pharmacophore model of semicarbazone as shown in fig. 2; incorporation of chloro, nitro and fluoro substituted phenyl ring at position A of semicarbazone pharmacophore (fig. 3); inclusion of p-substituted or unsubstituted aryl ring at site (C) and R’ (fig. 4). These modifications resulted in the identification of two lead compounds 4E and 4H. The title compounds were investigated for their anticonvulsant activity against mice. In general most of the compounds have shown mild to good activity by protecting 25 % to 100 % tested animals. Few compounds like 4A, 4C and 4K were found to be inactive in all tests at all tested doses.
Materials and Methods
The melting points were determined in one side sealed melting point capillary tubes using Thomas Hoover melting point apparatus and are uncorrected. The reactions were monitored by Thin Layer Chromatography (TLC) using iodine vapours as visualizing agents. Infrared (IR) spectra were determined on JASCO FT/IR-5300 IR spectrophotometer by Potassium bromide (KBr) disc method. Proton Nuclear Magnetic Resonance (1H NMR) (300 MHz) and Carbon-13 Nuclear Magnetic Resonance (13C-NMR) (75.46 MHz) spectral studies were done on JEOL Fourier Transform Nuclear Magnetic Resonance (FTNMR) spectrophotometer using chloroform as solvent. The Mass Spectrometry (MS) data for compounds 4D and 4E were recorded on a Quattro micro™ API Waters’ mass spectrometer method-Electrospray Ionization Mass Spectrometry (ESI-MS). Elemental analyses were carried out on a Perkin Elmer 2400 Carbon Hydrogen Nitrogen (CHN) elemental analyzer.
Chemistry
Synthesis of hydrazides:
A mixture of arylamine (0.017 mol), ethylchloroacetate (0.017 mol) and Potassium carbonate (K2CO3) (0.017 mol) was dissolved in acetone and placed in a dried and cleaned round bottom flask and was refluxed for 10-12 h on water bath. After refluxing, benzene was added to the mixture and it was washed with water. Then organic layer was separated with the help of separating funnel and it was dried by using anhydrous sodium sulphate. After this, the solvent was evaporated and the resultant crude product was recrystallized from ethanol. Ester (0.017 mol) was dissolved in ethyl alcohol and placed in a dried round bottomed flask. Hydrazine hydrate[15] (0.017 mol) was added to the solution and then the reaction mixture was refluxed for 12-16 h. After cooling, the mixture was transferred in a dry petri dish and left it for simple evaporation. The residue obtained after evaporation was collected and recrystallized from ethanol.
Synthesis of hydrazones:
Hydrazide (0.017 mol) and carbonyl compound (0.034 mol) was taken in 1:2 molar ratios in a dried round bottom flask. Propanol was added to the flask as a solvent. Sodium acetate or glacial acetic acid was added to the reaction mixture as catalyst. Now reaction mixture was refluxed for 1-2 h. After cooling the mixture was transferred to a dry petri dish and left it for evaporation. The solid product obtained after evaporation was recrystallized from ethanol.
T(4-chloro-phenylamino)-acetic acid hydrazide (3A): Molecular formula: C8H10ClN3O; molecular weight: 199.64; yield: 74.20 %; melting point (m.p): 58°; IR (KBr, ν, cm-1): 3471 (-NH2), 3381 (NH-), 2924 (CH2), 1672 (-C=O), 823 (aryl, C-H) 638 (-Cl); 1H NMR (CDCl3-d1, Deuterium oxide (D2O) exchange, 300 MHz): δ 7.94 ( m, 1H, CONH), δ 6.37-7.05 (m, 4H, Ar-CH), δ 4.03 (m, 1H, Ar-NH), δ 3.95 (m, 2H, methylene-H); 13C NMR (CDCl3-d1): δ 170.3 (CONH), δ 145.7 (Ar-CCl), δ 114.9 –129.7 (Ar- CH), δ 122.7 (Ar-CNH), δ 57.3 (CH2); anal: (calculated: C: 48.13, H: 05.05, N: 21.05; found: C: 48.24, H: 04.91, N: 21.32) %.
(2,4-dichloro-phenylamino)-acetic acid hydrazide (3B): Molecular formula: C8H9Cl2N3O; molecular weight: 234.08; yield: 73.24 %; m.p: 51°; IR (KBr, ν, cm-1): 3419 (NH-), 3319 (-NH2) 2934 (CH2), 1728 (-C=O), 812 (Aryl-CH) 640 (-Cl); 1H NMR (CDCl3-d1, D2O exchange, 300 MHz): δ 6.37-7.56 (m, 8H, Ar-CH), δ 7.26 (s, 1H, CONH), δ 4.92 (s, 1H, Ar-NH). δ 3.91(m, 2H, methylene-H), δ 2.11 (m, 2H, amine-NH); 13C NMR (CDCl3-d1): δ 170.3 (CONH), δ 142.0 (Ar-CNH), δ 116.3–131.2 (Ar-CH), δ 123.8-124.1 (Ar-CCl) δ 56.8 (CH2); anal: (calculated: C: 41.05, H: 03.88, N: 17.95; found: C: 41.07, H: 04.17, N: 17.63) %.
(4-nitro-phenylamino)-acetic acid hydrazide (3C): Molecular formula: C8H10N4O3; molecular weight: 210.19; yield: 73.20 %; m.p: 110°; IR (KBr, ν, cm-1): 3419 (NH-), 3319 (-NH2) 2934 (CH2), 1633 (-C=O), 1535 (-NO2), 858 (aryl-CH); 1H NMR (CDCl3-d1, D2O exchange, 300 MHz): δ 6.37-7.56 (m, 8H, Ar-CH), δ 7.26 (s, 1H, CONH), δ 4.92 (s, 1H, Ar-NH), δ 3.91 (m, 2H, methylene-H); 13C NMR (CDCl3-d1): δ 170.3 (CONH), δ 153.7 (Ar-CNH), δ 114.4–121.9 (Ar-CH), δ 136.8 (Ar-CNO2), δ 57.3 (CH2); anal: (calculated: C: 45.71, H: 04.80, N: 22.84; found: C: 45.43, H: 04.39, N: 23.17) %.
(4-fluoro-phenylamino)-acetic acid hydrazide (3D): Molecular formula: C8H10FN3O; molecular weight: 183.18; yield: 78.36 %; m.p: 80°; IR (KBr, ν, cm-1): 3471 (-NH2), 3381 (NH-), 2924 (CH2), 1672 (-C=O), 823 (Aryl, C-H), 638 (-F); 1H NMR(CDCl3-d1, D2O exchange, 300 MHz): δ 6.37-7.56 (m, 8H, Ar-CH), δ 7.26 (s, 1H, CONH), δ 4.92 (s, 1H, Ar-NH), δ 3.91 (m, 2H, methylene-H); 13C NMR (CDCl3-d1): δ 170.3 (CONH), δ 151.3 (Ar-CF), δ 143.2 (Ar-CNH), 115.1– 116.3 (Ar-CH), δ 57.3 (CH2); anal: (calculated: C: 52.45, H: 05.50, N: 22.94; found: C: 52.15, H: 05.57, N: 21.83) %.
(4-chloro-phenylamino)-acetic acid (3-oxo-1,3-dihydro-indole-2-ylidene)-hydrazide (4A): Molecular formula: C16H13ClN4O2; molecular weight: 328.75; yield: 60.83 %; m.p: charred; IR (KBr, ν, cm-1): 3259 (NH), 2928 (-CH2-), 1726 (-C=O), 1570 (-C=N), 833 (aryl C-H), 644 (Cl); 1H NMR (CDCl3-d1, D2O exchange, 300 MHz): δ 6.37-7.56 (m, 8H, Ar-CH), δ 7.26 (s, 1H, CONH), δ 4.92 (s, 1H, Ar-NH) δ 3.91 (m, 2H, methylene-H); 13C NMR (CDCl3-d1): δ 190.0 (CO), δ 173.0 (CONH), δ 156. 0 (CNH), δ 154.0 (CN), δ 145.7(Ar-CNH), δ 114.9–135.4 (Ar-CH), δ 122.7 (Ar-CCl), δ 112.5 (indole-C), δ 57.6 (CH2); anal: (calculated: C: 58.45, H: 03.99, N: 17.04; found: C: 57.94, H: 03.33, N: 18.34) %.
(4-chloro-phenylamino)-acetic acid (1-phenylethylidene)-hydrazide (4B): Molecular formula: C16H16ClN3O; molecular weight: 301.77; yield: 79.73 %; m.p: 96°; IR (KBr, ν, cm-1): 3377 (NH), 3001 (-CH2-), 1722 (-C=O), 1570 (-C=N), 838 (aryl, C-H), 646 (Cl); 1H NMR (CDCl3-d1, D2O exchange, 300 MHz): δ 6.37- 7.60 (m, 9H, Ar-CH), δ 7.26 (s, 1H, CONH), δ 4.12 (s, 1H, Ar-NH), δ 3.91 (m, 2H, methylene-H), δ 0.93(s, 3H, methyl-H); 13C NMR (CDCl3-d1): δ 173.0 (CONH), δ 168.7 (CN), δ 145.7 (Ar-CNH), δ 131.1 (Ar-C), δ 114.9–131.1 (Ar-CH), δ 122.7 (Ar-CCl), δ 57.6 (CH2), δ 13.5 (CH3); anal: (calculated: C: 63.68, H: 05.34, N: 13.92; found: C: 63.41, H: 05.34, N: 13.69) %.
(4-chloro-phenylamino)-acetic acid [1-(4-nitrophenyl)-ethylidene)]-hydrazide (4C): Molecular formula: C16H15ClN4O3; molecular weight: 346.77; yield: 90.90 %; m.p: 56°; IR (KBr, ν, cm-1): 3362 (NH), 3001 (-CH2-), 1693 (-C=O), 1608 (-C=N), 1525 (NO2), 856 (aryl, C-H), 692 (Cl); 1H NMR (CDCl3-d1, D2O exchange, 300 MHz): δ 6.37–8.12 (m, 8H, Ar- CH), δ 7.26 (s, 1H, CONH), δ 4.32 (m, 1H, Ar-NH), δ 3.91 (m, 2H, methylene-H) δ 0.93 (s, 3H, methyl-H); 13C NMR (CDCl3-d1): δ 173.0 (CONH), δ 168.7 (CN), δ 150.7 (Ar-CNO2), 145.7 (Ar-CNH), δ 122.7 (Ar- CCl), δ 57.6 (CH2), δ 13.5 (CH3); anal: (calculated: C: 55.42, H: 04.36, N: 16.16; found: C: 55.01, H: 04.73, N: 15.89) %.
(4-chloro-phenylamino)-acetic acid [1-(4-chlorophenyl)-phenyl-methylene]-hydrazide (4D): Molecular formula: C21H17Cl2N3O; molecular weight: 398.29; yield: 83.69 %; m.p: 54°; IR (KBr, ν, cm-1): 3417 (NH), 2924 (-CH2-), 1651 (-C=O), 1585 (-C=N), 844 (aryl-CH), 696 (Cl); 1H NMR (CDCl3-d1, D2O exchange, 300 MHz): δ 6.37–7.84 (m, 13H, Ar- CH), δ 7.13 (s, 1H, CONH), δ 4.17 (m, 1H, Ar-NH). δ 3.91 (m, 2H, methylene-H); 13C NMR (CDCl3-d1): δ 170.3 (CONH), δ 145.7 (Ar-CCl), δ 114.9–129.7 (Ar- CH), δ 122.7 (Ar-CNH), δ 57.3 (CH2); ESI-MS m/z 399.29 [M+1]+; anal: (calculated: C: 63.33, H: 04.30, N: 10.55; found: C: 63.31, H: 03.98, N: 10.11) %.
(2,4-dichloro-phenylamino)-acetic acid (3-oxo-1,3-dihydro-indole-2-ylidene)-hydrazide (4E): Molecular formula: C16H12Cl2N4O2; molecular weight: 363.20; yield: 98.79 %; m.p: 111°; IR (KBr, ν, cm-1): 3348 (-NH), 2927 (-CH2-), 1745 (-C=O), 1618 (-C=N),813 (aryl, C-H), 661 (Cl); 1H NMR (CDCl3-d1, D2O exchange, 300 MHz): δ 6.31–7.56 (m, 7H, Ar-CH), δ 7.13 ( s, 1H, CONH), δ 4.11 (m, 1H, Ar-NH), δ 3.91 (m, 2H, methylene-H); 13C NMR (CDCl3-d1): δ 190.0 (CO), δ 173.0 (CONH), δ 156. 0 (CNH), δ 154.0 (CN), δ 142.0 (Ar-CNH), δ 116.3–135.4 (Ar-CH), δ 124.1 (ArpCCl), δ 123.8 (Ar-oCCl), δ 112.5 (indole-C), δ 57.1 (CH2); ESI-MS m/z 382.21 [M+1]+ anal: (calculated: C: 52.91, H: 03.33, N: 15.43; found: C: 53.21, H: 02.95, N: 15.71) %.
(2,4-dichloro-phenylamino)-acetic acid (1-phenylethylidene)-hydrazide (4F): Molecular formula: C16H15Cl2N3O; molecular weight: 336.22; yield: 29.32 %; m.p: 56°; IR (KBr, ν, cm-1): 3319 (NH), 2951(-CH2-), 1728 (-C=O), 1624 (-C=N), 862 (aryl, C-H), 646 (Cl); 1H NMR (CDCl3-d1, D2O exchange, 300 MHz): δ 6.31– 7.60 (m, 8H, Ar-CH), δ 7.13 (s, 1H, CONH), δ 4.01 (m, 1H, Ar-NH), δ 3.91 (m, 2H, methylene-H); 13C NMR (CDCl3-d1): δ 173.0 (CONH), δ 168.7 (CN), δ 142.0 (Ar-CNH), δ 134.0 (Ar-C), δ 116.3–131.2 (Ar-CH), δ 124.1 (Ar-pCCl), δ 123.8 (Ar-oCCl), δ 57.1 (CH2), δ 13.5 (CH3); anal: (calculated: C: 57.16, H: 04.50, N: 12.50; found: C: 57.16, H: 04.10, N: 12.22) %.
(2,4-dichloro-phenylamino)-acetic acid [1-(4-nitrophenyl)-ethylidene)]-hydrazide (4G): Molecular formula: C16H14Cl2N4O3; molecular weight: 381.21; yield: 75.78 %; m.p: 56°; IR (KBr, ν, cm-1): 3362 (NH), 2922 (-CH2-), 1693 (-C=O), 1608 (-C=N), 1527 (NO2), 856 (aryl-CH), 692 (Cl); 1H NMR (CDCl3-d1, D2O exchange, 300 MHz): δ 6.34-8.10 (m, 7H, Ar-CH), δ 7.16 (s, 1H, CONH), δ 4.01 (m, 1H, Ar-NH), δ 3.87 (m, 2H, methylene-H) δ 0.91 (s, 3H, methyl-H); 13C NMR (CDCl3-d1): δ 173.0 (CONH), δ 168.7 (CN), δ 150.7 (Ar-CNO2), δ 142.0 (Ar-CNH), δ 116.3-131.2 (Ar-CH), δ 124.1 (Ar-pCCl), δ 123.8 (AroCCl), δ 57.1 (CH2), δ 13.5 (CH3); anal: (calculated: C: 50.41, H: 03.70, N: 14.70; found: C: 50.76, H: 04.02, N: 14.39) %.
(2,4-dichloro-phenylamino)-acetic acid [1-(4-chlorophenyl)–phenyl-methylene]-hydrazide (4H): Molecular formula: C21H16Cl3N3O; molecular weight: 432.13; yield: 92.56 %; m.p: 56°; IR (KBr, ν, cm-1): 3419 (NH), 2924 (-CH2-), 1651 (-C=O), 1597 (-C=N), 844 (Aryl, C-H), 696 (Cl); 1H NMR (CDCl3-d1, D2O exchange, 300 MHz): δ 6.31-7.84 (m, 12H, Ar- CH), δ 7.13 (s, 1H, CONH), δ 4.15 (m, 1H, Ar-NH), δ 3.95 (m, 2H, methylene-H); 13C NMR (CDCl3-d1): δ 173.0 (CONH), δ 142.0 (Ar-CNH), δ 116.3-131.1 (Ar- CH), δ 136.6 (R4-pCCl), δ 124.1 (Ar-pCCl), δ 123.8 (Ar-oCCl), δ 57.1 (CH2); anal: (calculated: C: 58.29, H: 03.73, N: 09.71; found: C: 57.84, H: 04.09, N: 10.03) %.
(4-nitro-phenylamino)-acetic acid (3-oxo-1,3-dihydro-indole-2-ylidene)-hydrazide (4I): Molecular formula: C16H13N5O4; molecular weight: 339.31; yield: 98.81 %; m.p: 122°; IR (KBr, ν, cm-1): 3363 (NH), 2920 (-CH2-), 1693 (-C=O), 1600 (-C=N), 1525 (NO2), 856 (aryl, C-H); 1H NMR (CDCl3-d1, D2O exchange, 300 MHz): δ 6.63–7.98 (m, 7H, Ar-CH), δ 7.13 (s, 1H, CONH), δ 4.05 (m, 1H, Ar-NH), δ 3.95 (m, 2H, methylene-H); 13C NMR (CDCl3-d1): δ 190.0 (CO), δ 173.0 (CONH), δ 156.0 (CNH), δ 154.0 (CN), δ 153.7 (Ar-CNH), δ 136.8 (Ar-CNO2), δ 114.4–135.4 (Ar- CH) δ 57.6 (CH2); anal: (calculated: C: 51.42, H: 03.24, N: 18.74; found: C: 51.00, H: 02.77, N: 18.74) %.
(4-nitro-phenylamino)-acetic acid (1-phenylethylidene)-hydrazide (4J): Molecular formula: C16H16N4O3; molecular weight: 312.32; yield: 96.05 %; m.p: 99°; IR (KBr, ν, cm-1): 3363 (NH), 2924 (-CH2-), 1634 (-C=O), 1600 (-C=N), 1535(NO2), 840 (aryl, C-H); 1H NMR(CDCl3-d1, D2O exchange, 300 MHz): δ 6.61–7.18 (m, 8H, Ar-CH), δ 7.03 (s, 1H, CONH), δ 4.07 (m, 1H, Ar-NH), δ 3.95 (m, 2H, methylene-H); 13C NMR (CDCl3-d1): δ 173.0 (CONH), δ 168.7 (CN), δ 153.7 (Ar-CNH), δ 136.8 (Ar-CNO2), δ 114.4–131.1 (Ar-CH), δ 134.0 (Ar-C), δ 57.6 (CH2), δ 13.5 (CH3); anal: (calculated: C: 55.42, H: 04.36, N: 16.16; found: C: 55.10, H: 03.99, N: 16.16) %.
(4-nitro-phenylamino)-acetic acid [1-(4-chlorophenyl)–phenyl-methylene]-hydrazide (4K): Molecular formula: C21H17ClN4O3; molecular weight: 408.84; yield: 73.38 %; m.p: 50°; IR (KBr, ν, cm-1): 3363 (NH), 2920 (-CH2-), 1651 (-C=O), 1585 (-C=N), 1535 (NO2), 844 (aryl, C-H), 696 (Cl); 1H NMR (CDCl3-d1, D2O exchange, 300 MHz): δ 6.69– 7.84 (m, 13H, Ar-CH), δ 7.01 (s, 1H, CONH), δ 4.00 (m, 1H, Ar-NH), δ 3.95 (m, 2H, methylene-H); 13C NMR (CDCl3-d1): δ 173 (CONH), δ 153.7 (Ar-CNH), δ 136.8 (Ar-CNO2), δ 136.6 (Ar-CCl) δ, δ 114.4–131.1 (Ar-CH), 57.6 (CH2); anal: (calculated: C: 61.69, H: 04.19, N: 13.70; found: C: 61.67, H: 04.19, N: 13.78) %.
(4-fluoro-phenylamino)-acetic acid (3-oxo-1,3-dihydro-indole-2-ylidene)-hydrazide (4L): Molecular formula: C16H13FN4O2; molecular weight: 312.31; yield: 38.42 %; m.p: 99°; IR (KBr, ν, cm-1): 3408 (NH), 2926 (-CH2-), 1697 (-C=O), 1585 (-C=N), 1521 (NO2), 856 (aryl-CH), ,756 (F); 1H NMR (CDCl3-d1, D2O exchange, 300 MHz): δ 6.41–7.56 (m, 8H, Ar-CH), δ 7.04 (s, 1H, CONH), δ 4.00 (m, 1H, Ar-NH). δ 3.95 (m, 2H, methylene-H); 13C NMR (CDCl3-d1): δ 190.0 (CO), δ 173.0 (CONH), δ 156.0 (CNH), 154.0 (CN) δ, δ 151.3 (Ar-CF), δ 143.2 (Ar-CNH), δ 115.1-135.4 (Ar-CH), δ 112.5 (indole-C), δ 57.6 (CH2); anal: (calculated: C: 61.53, H: 04.20, N: 17.94; found: C: 61.49, H: 03.89, N: 18.11) %.
(4-fluoro-phenylamino)-acetic acid (1-phenylethylidene)-hydrazide (4M): Molecular formula: C16H16FN3O; molecular weight: 285.32; yield: 46.26 %; m.p: 124°; IR (KBr, ν, cm-1): 3383 (NH), 2908 (-CH2-), 1668 (-C=O), 1585 (-C=N), 1521 (NO2), 815 (aryl-CH), 788 (F); 1H NMR(CDCl3-d1, D2O exchange, 300 MHz): δ 6.41–7.60 (m, 9H, Ar-CH), δ 7.00 (s, 1H, CONH), δ 4.00 (m, 1H, Ar-NH), δ 3.95 (m, 2H, methylene-H), δ 0.9 (methyl-CH); 13C NMR (CDCl3-d1): δ 173.0 (CONH), δ 168.7 (CN), δ 151.3 (Ar-CF), δ 143.2 (Ar- CNH), δ 134.0 (Ar-C), δ 115.1–131.1 (Ar-CH) δ 57.6 (CH2), δ 13.5 (CH3); anal: (calculated: C: 60.10, H: 04.73, N: 13.14; found: C: 59.88, H: 05.01, N: 13.11) %.
(4-fluoro-phenylamino)-acetic acid [1-(4-chlorophenyl)–phenyl-methylene]-hydrazide (4N): Molecular formula: C21H17ClFN3O; molecular weight: 381.83; yield: 96.02 %; m.p: 49°; IR (KBr, ν, cm-1): 3344 (NH), 2926 (-CH2-), 1651 (-C=O), 1583 (-C=N), 1518 (NO2), 844 (aryl-CH), 788 (F); 1H NMR(CDCl3-d1, D2O exchange, 300 MHz): δ 6.69–7.97 (m, 8H, Ar-CH), δ 7.01 (s, 1H, CONH), δ 4.00 (m, 1H, Ar-NH), δ 3.95 (m, 2H, methylene-H); 13C NMR (CDCl3-d1): δ 173.0 (CONH), δ 168.7 (CN), δ 151.3 (Ar-CF), δ 143.2 (Ar- CNH), δ 134.0 (Ar-C), δ 115.1–131.1 (Ar-CH), δ 57.6 (CH2), δ 13.5 (CH3); anal: (calculated: C: 61.69, H: 04.19, N: 13.70; found: C: 62.11, H: 04.19, N: 13.71) %.
Anticonvulsant screening:
The synthesized compounds were evaluated for their anticonvulsant activity under “The National Institute of Health (NIH) Anticonvulsant Drug Development (ADD) program preclinical Anticonvulsant Screening Project (ASP)”; Bethesda, USA. A battery of tests determines the anticonvulsant activity of the compounds. The synthesized compound were evaluated for their primary anticonvulsant activity by electrically induced seizures (Maximal Electroshock Seizure (MES), 6 Hz psychomotor seizure) test and chemically induced seizure (subcutaneous Metrazol (scMET)) test[16] against mice and was compared with the standard drugs phenytoin, carbamazepine, valproic acid and levetiracetam.
Conformational analysis and pharmacophore modelling:
The lead compounds 4E and 4H were selected for the modeling studies. In addition some inactive compounds (4A and 4K) were also chosen for the comparative structural analysis. All selected compounds were subjected to the conformational analysis and pharmacophore modeling studies with the following assumptions.
The compounds are assumed to act via inactivation of voltage gated sodium channels by binding to a hypothetical binding site. Two aryl rings (aromatic sextets) namely A and C in the ligands contribute to the hydrophobic interactions with the hypothetical binding site and are essential for activity. Such interactions are highly influenced by electronegative substituents such as bromo, chloro etc., on the rings.
The optimum interaction of these ligands with receptors also involves hydrogen bonding of pharmacophoric atoms with unshared electron pairs or protons (-CONH- , HBD) and an electron (pi) donor which are present in the linker (NH-CH2-CO-NH-N=C-) between the two aryl rings.
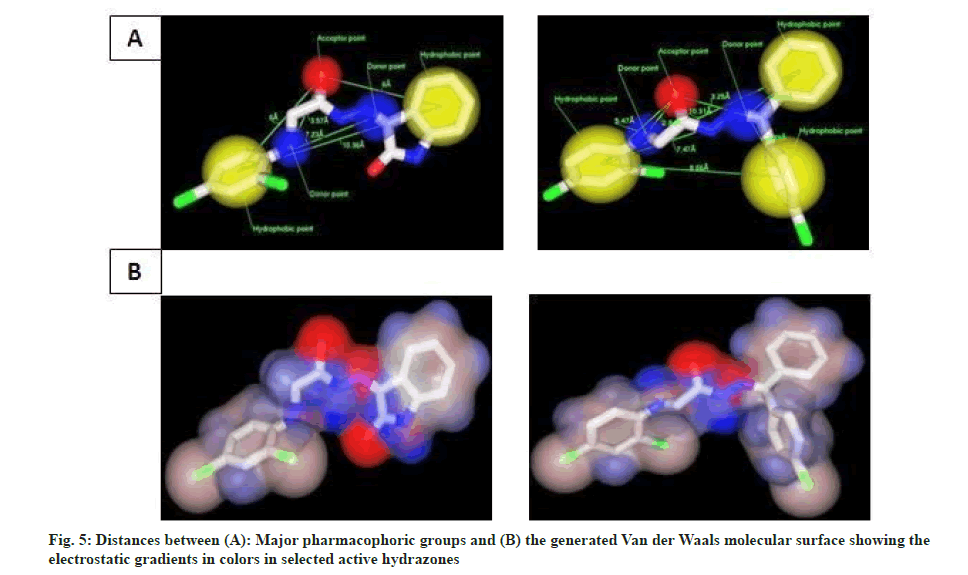
Initially, the conformational search was carried out to yield at least 10 low energy conformers. The selected conformers were then subjected to energy minimization by using Merck Molecular Force Field 94 (MMFF94) model available and the best aligning low energy conformer was selected for the manual pharmacophore modeling studies using Marvin. A pharmacophore model comprising all essential sites was generated manually to study their spatial relationships. The alignment, distances, molecular surface (electrostatic gradient maps) and the spatial relationship of pharmacophoric points are presented in fig. 5a and fig. 5b.
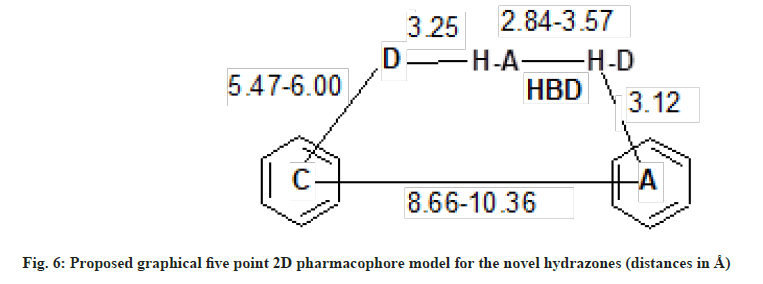
Based on the pharmacophore ensemble of active compound (4E) and its spatial arrangements along with distant constraints a simple five point Two- Dimensional (2D) pharmacophore model as shown in fig. 6 was generated which is in well agreement with our previously proposed four point pharmacophore model for semicarbazones[14] as shown in fig. 1.
Results and Discussion
Synthesis of aryl hydrazones was carried out by first esterification of the aryl amines (p-chloroaniline 2,4-dichloroaniline, p-nitroaniline and p-fluoroaniline) with ethylchloroaetate in presence of K2CO3, which gave the ethyl ester. The ethyl ester and hydrazine hydrate was refluxed with stirring in ethanol to give precipitates of hydrazide in good yield (78 %-81 %). Then refluxed the ethanolic solution of the hydrazides with appropriate carbonyl compounds which yielded the aryl hydrazones as shown in fig. 3 and fig. 4. The hydrazones were purified by recrystallization from appropriate solvent. IR, 1H-NMR, 13C NMR, mass spectra and elemental (CHN) data of all the synthesized compounds were consistent with the assigned structures. All the synthesized compounds were screened for anticonvulsant activity by MES, scMET and neurotoxicity (TOX) tests after Intraperitoneal (IP) injection to mice at doses of 30, 100 and 300 mg/kg (at “National Institutes of Health”, Maryland, USA). Also included the activity of the reference compounds phenytoin, carbamazepine and valproic acid. The result of anticonvulsant screening was obtained from NIH and is presented in Table 1.
| Compound code | Dose (IP; mg/kg) | MES | scMET | TOX | |||
|---|---|---|---|---|---|---|---|
| 0.5 h | 4 h | 0.5 h | 4 h | 0.5 h | 4 h | ||
| 4A | 30 | 0/1 | 0/1 | 0/1 | 0/1 | 0/4 | 0/2 |
| 100 | 0/3 | 0/3 | 0/1 | 0/1 | 0/8 | 0/4 | |
| 300 | 0/1 | 0/1 | 0/1 | 0/1 | 0/4 | 0/2 | |
| 4B | 30 | 0/1 | 0/1 | 0/1 | 0/1 | 0/4 | 0/2 |
| 100 | 0/3 | 0/3 | 0/1 | 0/1 | 0/8 | 0/4 | |
| 300 | 0/1 | 0/1 | 4/5 | 0/1 | 0/4 | 0/2 | |
| 4C | 30 | 0/1 | 0/1 | 0/1 | 0/1 | 0/4 | 0/2 |
| 100 | 0/3 | 0/3 | 0/1 | 0/1 | 0/8 | 0/4 | |
| 300 | 0/1 | 0/1 | 0/1 | 0/1 | 0/4 | 0/2 | |
| 4D | 30 | 0/1 | 0/1 | 0/1 | 0/1 | 0/4 | 0/2 |
| 100 | 0/3 | 0/3 | 0/1 | 0/1 | 0/8 | 0/4 | |
| 300 | 1/1 | 0/1 | 0/1 | 0/1 | 1/4 | 0/2 | |
| 4E | 30 | 0/1 | 0/1 | 0/1 | 0/1 | 0/4 | 0/2 |
| 100 | 1/3 | 0/3 | 0/1 | 0/1 | 0/8 | 0/4 | |
| 300 | 1/1 | 1/1 | 0/1 | 0/1 | 1/4 | 0/2 | |
| 4F | 30 | 0/1 | 0/1 | 0/1 | 0/1 | 0/4 | 0/2 |
| 100 | 0/3 | 1/3 | 0/1 | 0/1 | 0/8 | 0/4 | |
| 300 | 0/1 | 1/1 | 1/5 | 0/1 | 1/4 | 1/2 | |
| 4G | 30 | 0/1 | 0/1 | 0/1 | 0/1 | 0/4 | 0/2 |
| 100 | 0/3 | 1/3 | 1/3 | 0/1 | 0/8 | 0/4 | |
| 300 | 0/1 | 1/1 | 4/5 | 0/1 | 0/4 | 0/2 | |
| 4H | 30 | 0/1 | 0/1 | 0/1 | 0/1 | 0/4 | 0/2 |
| 100 | 0/3 | 1/3 | 0/1 | 0/1 | 0/8 | 0/4 | |
| 300 | 0/1 | 1/1 | 3/5 | 0/1 | 1/4 | 0/2 | |
| 4I | 30 | 0/1 | 0/1 | 0/1 | 0/1 | 0/4 | 0/2 |
| 100 | 0/3 | 0/3 | 0/1 | 0/1 | 0/8 | 0/4 | |
| 300 | 1/1 | 0/1 | 0/1 | 0/1 | 0/4 | 0/2 | |
| 4J | 30 | 0/1 | 0/1 | 0/1 | 0/1 | 0/4 | 0/2 |
| 100 | 0/3 | 1/3 | 0/1 | 0/1 | 0/8 | 0/4 | |
| 300 | 0/1 | 1/1 | 0/1 | 0/1 | 0/4 | 0/2 | |
| 4K | 30 | 0/1 | 0/1 | 0/1 | 0/1 | 0/4 | 0/2 |
| 100 | 0/3 | 0/3 | 0/1 | 0/1 | 0/8 | 0/4 | |
| 300 | 0/1 | 0/1 | 0/1 | 0/1 | 0/4 | 0/2 | |
| 4L | 30 | 0/1 | 0/1 | 0/1 | 0/1 | 0/4 | 0/2 |
| 100 | 1/3 | 0/3 | 0/1 | 0/1 | 0/8 | 0/4 | |
| 300 | 1/1 | 0/1 | 0/1 | 0/1 | 0/4 | 0/2 | |
| 30 | 0/1 | 0/1 | 0/1 | 0/1 | 0/4 | 0/2 | |
| 4M | 100 | 0/3 | 1/3 | 0/1 | 0/1 | 0/8 | 0/4 |
| 300 | 0/1 | 1/1 | 3/5 | 0/1 | 0/4 | 0/2 | |
| 4N | 30 | 0/1 | 0/1 | 0/1 | 0/1 | 0/4 | 0/2 |
| 100 | 0/3 | 0/3 | 0/1 | 0/1 | 0/8 | 0/4 | |
| 300 | 1/1 | 1/1 | 0/1 | 0/1 | 0/4 | 0/2 | |
| PHT | 30* | 100* | - | - | 100* | 100* | |
| CBZ | 30* | - | 100* | - | 100* | 300* | |
| VPA | - | - | 300* | - | - | - | |
Note: *The figures in the table indicate the minimum dose whereas bioactivity was demonstrated in 50 % or more of the mice. The animals were examined from 0.5 h to 4 h after the convulsive stimuli were applied. (-) indicates absence of activity at the maximum dose administered (300 mg/kg); (1/1) indicates protection in 100 percent animals while (0/1) indicates no protection at all
Table 1: Preliminary Anticonvulsant Screening Data of Hydrazones (4A To 4N) in Mice.
All the 14 newly synthesized hydrazones were screened for preliminary anticonvulsant activity in mice at the dose of 300, 100 and 30 mg/kg IP (Table 2). In general most of the compounds showed mild to good activity by protecting 25 % to 100 % tested animals.
In preliminary MES screening, compounds 4E, 4F, 4G, 4H, 4J,4L and 4M showed good activity at both 100 and 300 mg/kg and were more active than VPA with 33 %-100 % protection. Compound 4E, 4F, 4G, 4H, 4J, 4M and 4N have shown long duration of activity i.e. up to 4 h at 300 mg/kg. All the active compounds except 4D, 4E, 4F and 4H have exhibited neurotoxicity at their higher anticonvulsant dose i.e. 300 mg/kg. In scMET screening, most of the compounds were inactive except 4B, 4F, 4G, 4H and 4M and these active compounds showed protection ranging from 20 % to 80 %. One of the active compounds from the 60 Hz MES screening 4E (since 4E showed better result with maximum protection in MES screening) was further evaluated in 6 Hz MES screening at 100 mg/kg (Table 2). This compound exhibited 75 % protection up to 4 h without causing neurotoxicity. From the above results, compounds 4E and 4H has been identified as lead compounds as they have displayed excellent anticonvulsant activity in mice.
| Compound code | MES | TOX | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.25 h | 0.5 h | 1 h | 2 h | 4 h | 0.25 h | 0.5 h | 1 h | 2 h | 4 h | |
| 4E | 1/4 | 1/4 | 0/4 | 0/4 | 3/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| Levetiracetam | 0/4 | 2/4 | 4/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
Table 2: Anticonvulsant Screening Data of 4E in 6hz Mes Mice Model At 100 Mg/Kg (IP)
It can be summarized that the modifications made in the semicarbazone pharmacophore model resulted in the identification of two lead compound (4E and 4H) with excellent preliminary anticonvulsant profile with no neurotoxicity in MES and scMET mice model. Future studies on the molecules 4E and 4H and on its analogs, on advanced animal models (oral MES and quantitative MES) are essential to establish the extent of their potency and future prospective for the development of these molecules as novel antiepileptic agents.
Conflict of interests:
The authors declared no conflicts of interest.
References
- Eisa HM, Tantawy AS, El-Kerdawy MM. Synthesis of certain 2-aminoadamantane derivatives as potential antimicrobial agents. Pharmazie 1991;46(3):182-4.
[Google Scholar] [PubMed]
- Rollas S, Gulerman N, Erdeniz H. Synthesis and antimicrobial activity of some new hydrazones of 4-fluorobenzoic acid hydrazide and 3-acetyl-2,5-disubstituted-1,3,4-oxadiazolines. Farmaco 2002;57(2):171-4.
[Crossref] [Google Scholar] [PubMed]
- Vicini P, Zani F, Cozzini P, Doytchinova I. Hydrazones of 1,2-benzisothiazole hydrazides: Synthesis, antimicrobial activity and QSAR investigations. Eur J Med Chem 2002;37(7):553-64.
- Sah PP, Peoples SA. Isonicotinyl hydrazones as antitubercular agents and derivatives for identification of aldehydes and ketones. J Am Pharm Assoc 1954;43(9):513-24.
- Kaymakç?o?lu BK, Rollas S. Synthesis, characterization and evaluation of antituberculosis activity of some hydrazones. Farmaco 2002;57(7):595-9.
- Maccari R, Ottanà R, Vigorita MG. In vitro advanced antimycobacterial screening of isoniazid-related hydrazones, hydrazides and cyanoboranes: Part 14. Bioorg Med Chem Lett 2005;15(10):2509-13.
[Crossref] [Google Scholar] [PubMed]
- Loncle C, Brunel JM, Vidal N, Dherbomez M, Letourneux Y. Synthesis and antifungal activity of cholesterol-hydrazone derivatives. Eur J Med Chem 2004;39(12):1067-71.
[Crossref] [Google Scholar] [PubMed]
- Dimmock JR, Kumar P, Allen TM, Kao GY, Halleran S, Balzarini J, et al. Synthesis and cytotoxic evaluation of some carbohydrazones and thiocarbohydrazones of various unsaturated ketones and related Mannich bases. Pharmazie 1997;52(3):182-6.
[Google Scholar] [PubMed]
- Andreani A, Granaiola M, Leoni A, Locatelli A, Morigi R, Rambaldi M. Synthesis and antitubercular activity of imidazo [2,1-b] thiazoles. Eur J Med Chem 2001;36(9):743-6.
[Crossref] [Google Scholar] [PubMed]
- Parmar SS, Gupta AK, Gupta TK, Stenberg VI. Synthesis of substituted benzylidinohydrazines and their monoamine oxidase inhibitory and anticonvulsant properties. J Pharm Sci 1975;64(1):154-7.
- Kalsi R, Pande K, Bhalla TN, Barthwal JP, Gupta GP, Parmar SS. Anti-inflammatory activity of quinazolinoformazans. J Pharm Sci 1990;79(4):317-20.
[Crossref] [Google Scholar] [PubMed]
- Siddiqui N, Rana A, Khan SA, Bhat MA, Haque SE. Synthesis of benzothiazole semicarbazones as novel anticonvulsants: The role of hydrophobic domain. Bioorg Med Chem Lett 2007;17(15):4178-82.
[Crossref] [Google Scholar] [PubMed]
- Sridhar SK, Pandeya SN, Stables JP, Ramesh A. Anticonvulsant activity of hydrazones, Schiff and Mannich bases of isatin derivatives. Eur J Pharm Sci 2002;16(3):129-32.
[Crossref] [Google Scholar] [PubMed]
- Pandeya SN, Yogeeswari P, Stables JP. Synthesis and anticonvulsant activity of 4-bromophenyl substituted aryl semicarbazones. Eur J Med Chem 2000;35(10):879-86.
[Crossref] [Google Scholar] [PubMed]
- Salg?n-Gök?en U, Gökhan-Kelekçi N, Gökta? Ö, Köysal Y, K?l?ç E, I??k ?, et al. 1-Acylthiosemicarbazides, 1,2,4-triazole-5 (4H)-thiones, 1,3,4-thiadiazoles and hydrazones containing 5-methyl-2-benzoxazolinones: Synthesis, analgesic-anti-inflammatory and antimicrobial activities. Bioorg Med Chem 2007;15(17):5738-51.
[Crossref] [Google Scholar] [PubMed]
- Vogel HG, Müller G, Sandow J, Schölkens BA. Drug discovery and evaluation: Pharmacological assays. 3rd ed. Berlin: Springer publishers; 2002.